Ultra-High-Resolution CT of the Head and Neck with Deep Learning Reconstruction—Assessment of Image Quality and Radiation Exposure and Intraindividual Comparison with Normal-Resolution CT
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Image Acquisition
2.3. Subjective Image Evaluation
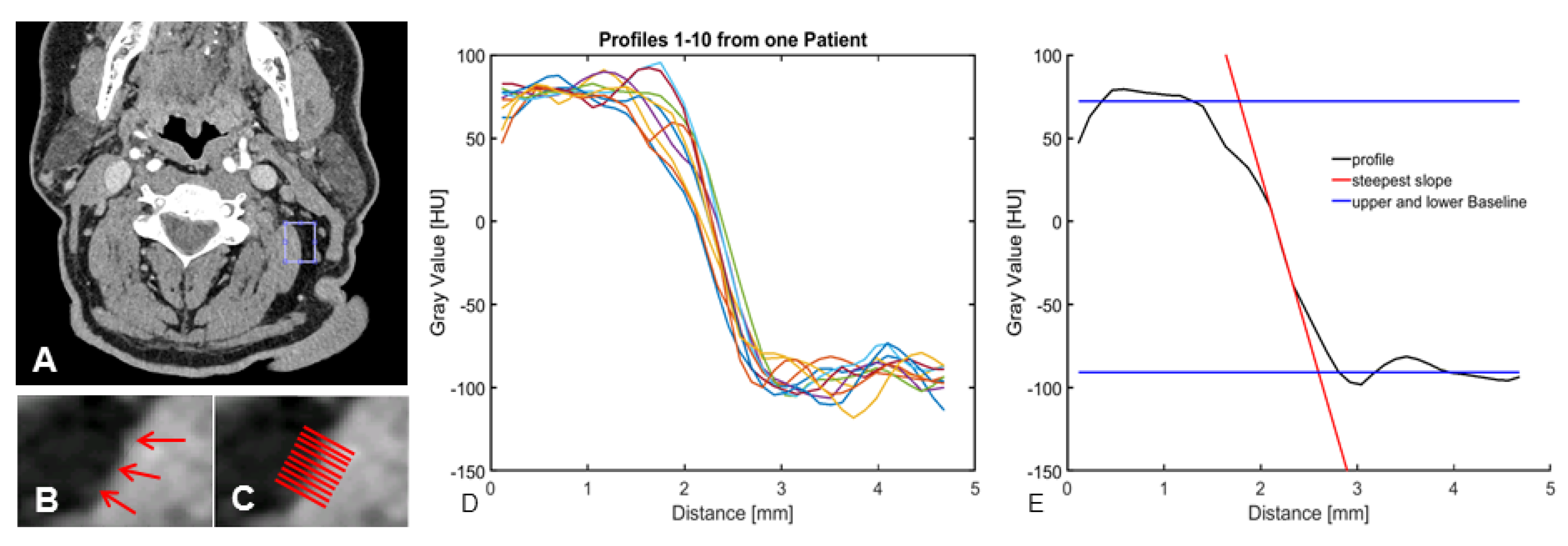
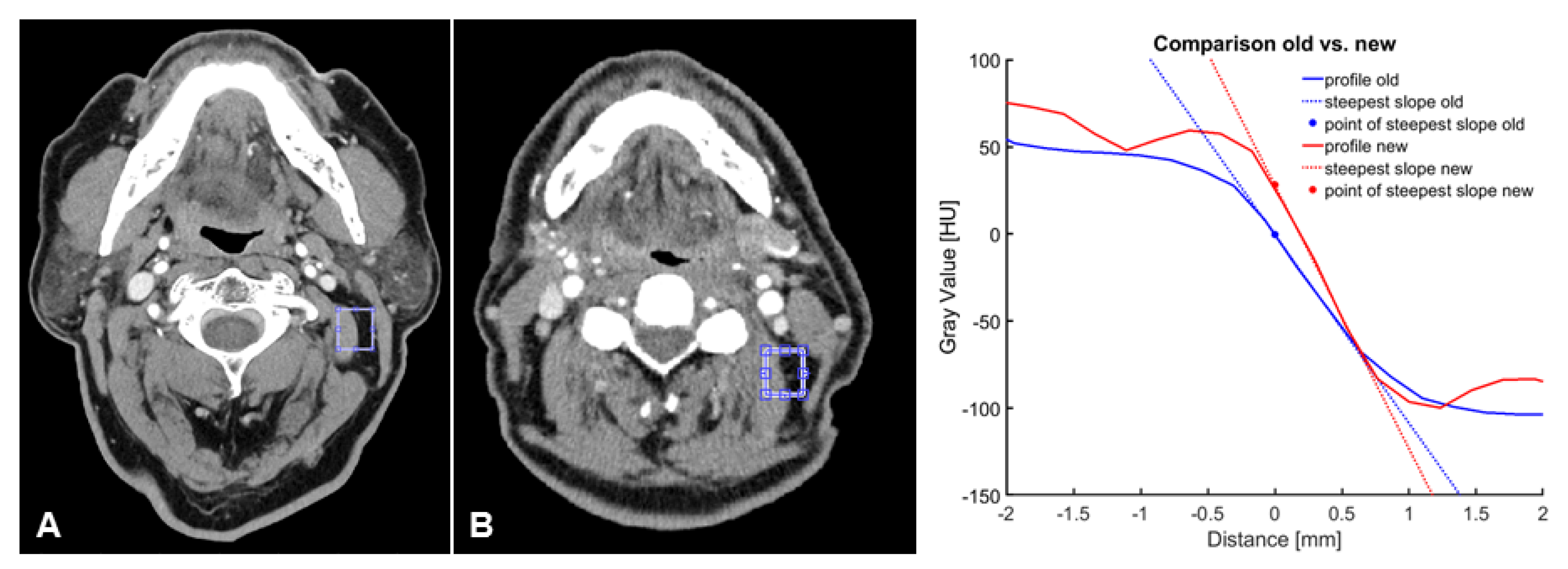
2.4. Objective Image Evaluation
2.5. Radiation Dose
2.6. Statistical Analysis
3. Results
3.1. Patient Cohort
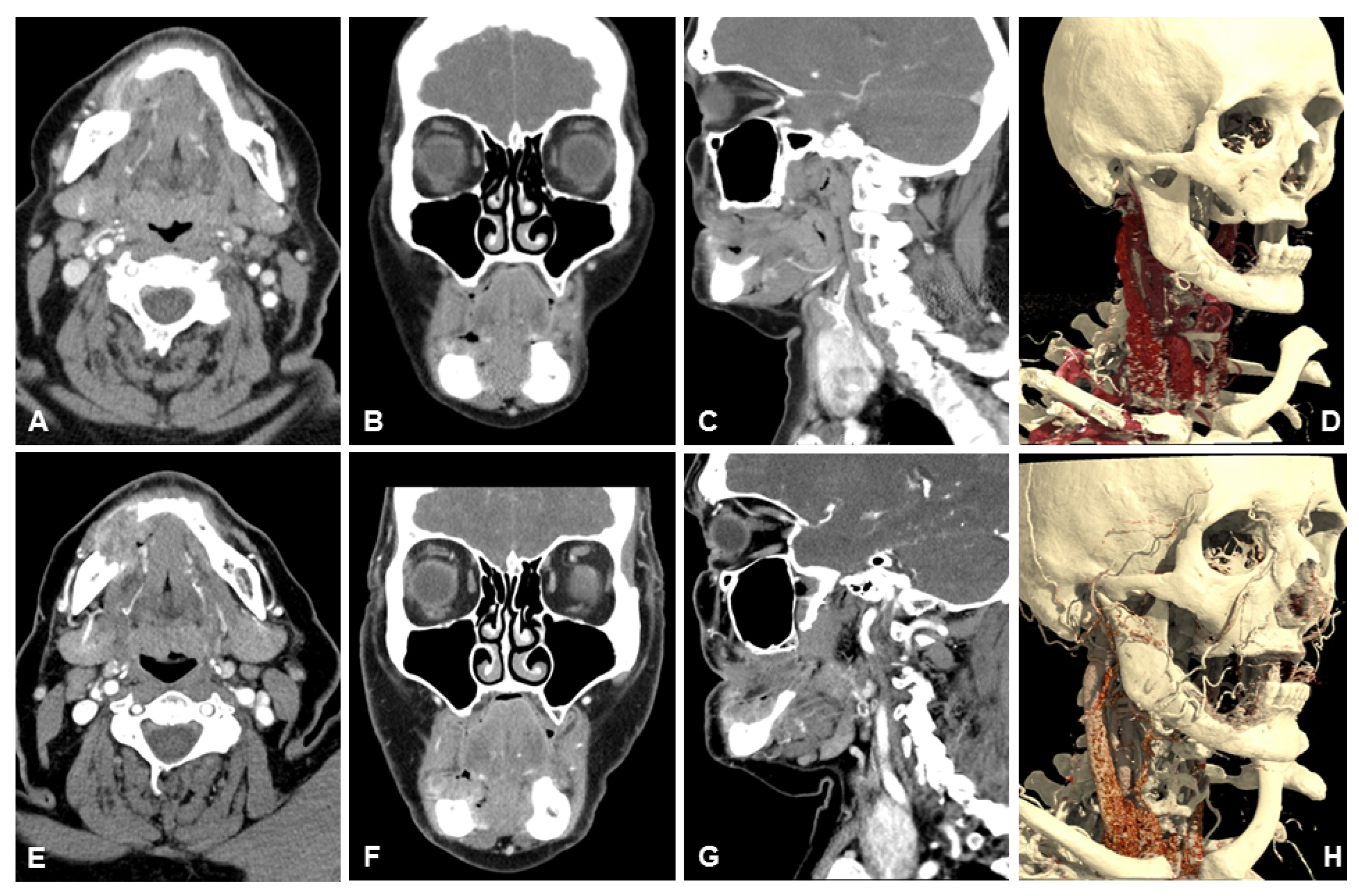
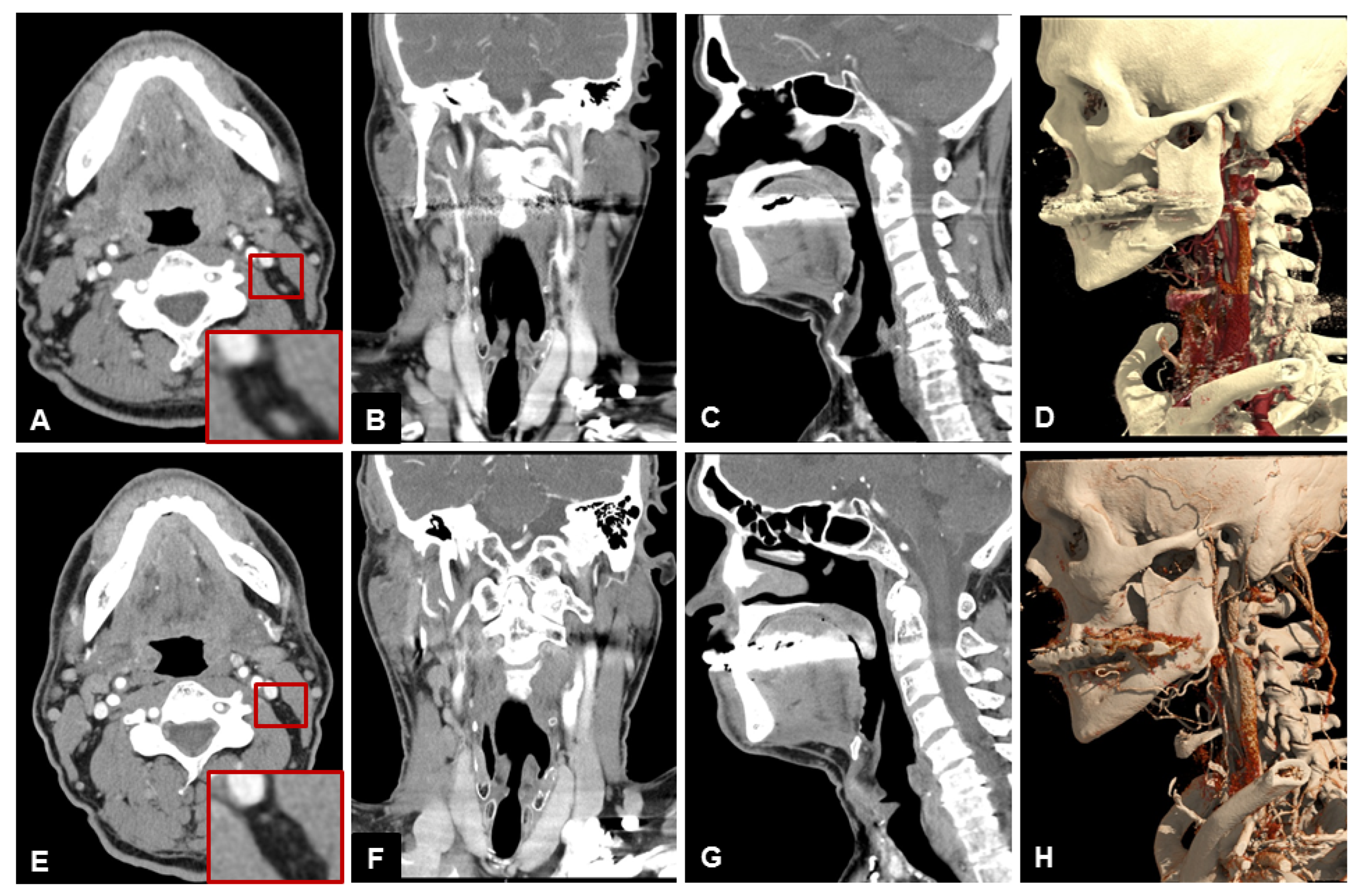
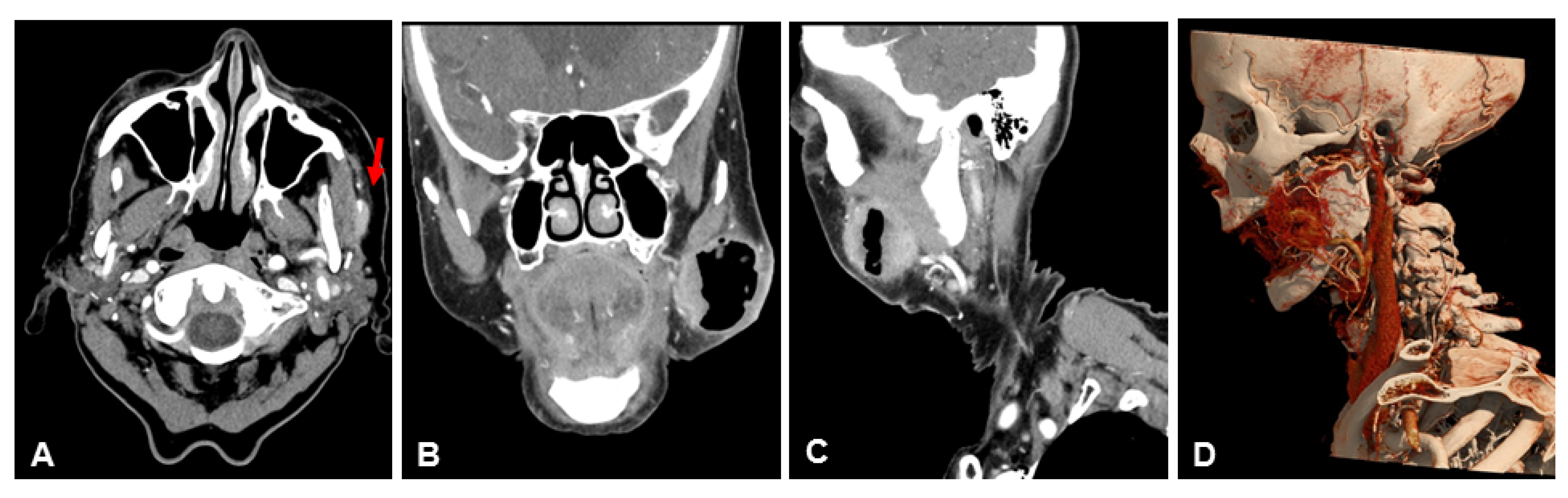
3.2. Subjective Image Quality
3.3. Objective Image Quality
3.4. Radiation Dose
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AEC | auto-exposure control |
| AiCE | Advanced intelligent Clear-IQ Engine |
| CNR | contrast to noise-ratio |
| CT | computed tomography |
| CTDIvol | Volume computed tomography dose index |
| DLP | dose length product |
| FBP | filtered back-projection |
| FoV | field of view |
| ICC | intraclass correlation |
| IR | iterative reconstruction |
| KV | kilovolt |
| MBIR | statistical model based iterative reconstruction |
| MRI | Magnetic resonance imaging |
| mSv | millisievert |
| NR | normal resolution |
| SNR | signal to noise-ratio |
| UHR | ultra-high resolution |
References
- Zanoni, D.K.; Patel, S.G.; Shah, J.P. Changes in the 8th Edition of the American Joint Committee on Cancer (AJCC) Staging of Head and Neck Cancer: Rationale and Implications. Curr. Oncol. Rep. 2019, 21, 52. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.J.; Harth, M.; Siebenhandl, P. Different imaging techniques in the head and neck: Assets and drawbacks. World J. Radiol. 2010, 2, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Junn, J.C.; Soderlund, K.A.; Glastonbury, C.M. Imaging of Head and Neck Cancer with CT, MRI, and US. Semin. Nucl. Med. 2021, 51, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Troeltzsch, D.; Shnayien, S.; Heiland, M.; Kreutzer, K.; Raguse, J.-D.; Hamm, B.; Niehues, S.M. Detectability of Head and Neck Cancer via New Computed Tomography Reconstruction Tools including Iterative Reconstruction and Metal Artifact Reduction. Diagnostics 2021, 11, 2154. [Google Scholar] [CrossRef]
- Lenga, L.; Lange, M.; Martin, S.S.; Albrecht, M.H.; Booz, C.; Yel, I.; Arendt, C.T.; Vogl, T.J.; Leithner, D. Head and neck single- and dual-energy CT: Differences in radiation dose and image quality of 2nd and 3rd generation dual-source CT. Br. J. Radiol. 2021, 94, 20210069. [Google Scholar] [CrossRef]
- Ucar, F.A.; Frenzel, M.; Abello Mercado, M.A.; Altmann, S.; Reder, S.; Brockmann, C.; Brockmann, M.A.; Othman, A.E. Feasibility of Ultra-High Resolution Supra-Aortic CT Angiography: An Assessment of Diagnostic Image Quality and Radiation Dose. Tomography 2021, 7, 711–720. [Google Scholar] [CrossRef]
- Roele, E.D.; Timmer, V.C.M.L.; Vaassen, L.A.A.; van Kroonenburgh, A.M.J.L.; Postma, A.A. Dual-Energy CT in Head and Neck Imaging. Curr. Radiol. Rep. 2017, 5, 19. [Google Scholar] [CrossRef]
- Oostveen, L.J.; Boedeker, K.L.; Brink, M.; Prokop, M.; de Lange, F.; Sechopoulos, I. Physical evaluation of an ultra-high-resolution CT scanner. Eur. Radiol. 2020, 30, 2552–2560. [Google Scholar] [CrossRef]
- Akagi, M.; Nakamura, Y.; Higaki, T.; Narita, K.; Honda, Y.; Zhou, J.; Yu, Z.; Akino, N.; Awai, K. Deep learning reconstruction improves image quality of abdominal ultra-high-resolution CT. Eur. Radiol. 2019, 29, 6163–6171. [Google Scholar] [CrossRef]
- Greffier, J.; Hamard, A.; Pereira, F.; Barrau, C.; Pasquier, H.; Beregi, J.P.; Frandon, J. Image quality and dose reduction opportunity of deep learning image reconstruction algorithm for CT: A phantom study. Eur. Radiol. 2020, 30, 3951–3959. [Google Scholar] [CrossRef]
- Bernard, A.; Comby, P.-O.; Lemogne, B.; Haioun, K.; Ricolfi, F.; Chevallier, O.; Loffroy, R. Deep learning reconstruction versus iterative reconstruction for cardiac CT angiography in a stroke imaging protocol: Reduced radiation dose and improved image quality. Quant. Imaging Med. Surg. 2021, 11, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Scholtz, J.-E.; Hüsers, K.; Kaup, M.; Albrecht, M.; Beeres, M.; Bauer, R.; Schulz, B.; Vogl, T.; Wichmann, J. Evaluation of image quality and dose reduction of 80 kVp neck computed tomography in patients with suspected peritonsillar abscess. Clin. Radiol. 2015, 70, e67–e73. [Google Scholar] [CrossRef] [PubMed]
- Geleijns, J.; Jessen, K.A.; Panzer, W.; Shrimpton, P.C.; Tosi, G. European Guidelines on Quality Criteria for Computed Tomography; European Commission: Brussels, Belgium, 2000; Available online: https://op.europa.eu/de/publication-detail/-/publication/d229c9e1-a967-49de-b169-59ee68605f1a (accessed on 29 July 2022).
- Malkus, A.; Szczykutowicz, T.P. A method to extract image noise level from patient images in CT. Med. Phys. 2017, 44, 2173–2184. [Google Scholar] [CrossRef] [PubMed]
- Dobbins, J.T., 3rd; Samei, E.; Ranger, N.T.; Chen, Y. Intercomparison of methods for image quality characterization. II. Noise power spectrum. Med. Phys. 2006, 33, 1466–1475. [Google Scholar] [CrossRef]
- Anam, C.; Arif, I.; Haryanto, F.; Lestari, F.P.; Widita, R.; Budi, W.S.; Sutanto, H.; Adi, K.; Fujibuchi, T.; Dougherty, G. An Improved Method of Automated Noise Measurement System in CT Images. J. Biomed. Phys. Eng. 2021, 11, 163–174. [Google Scholar] [CrossRef]
- Tian, X.; Samei, E. Accurate assessment and prediction of noise in clinical CT images. Med. Phys. 2015, 43, 475–482. [Google Scholar] [CrossRef]
- Guleng, A.; Bolstad, K.; Dalehaug, I.; Flatabø, S.; Aadnevik, D.; Pettersen, H.E.S. Spatial Distribution of Noise Reduction in Four Iterative Reconstruction Algorithms in CT—A Technical Evaluation. Diagnostics 2020, 10, 647. [Google Scholar] [CrossRef]
- Chun, M.; Choi, Y.H.; Kim, J.H. Automated measurement of CT noise in patient images with a novel structure coherence feature. Phys. Med. Biol. 2015, 60, 9107–9122. [Google Scholar] [CrossRef]
- Deak, P.D.; Smal, Y.; Kalender, W.A. Multisection CT Protocols: Sex- and Age-specific Conversion Factors Used to Determine Effective Dose from Dose-Length Product. Radiology 2010, 257, 158–166. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Boedeker, K. (Ed.) Aquilion Precision Ultra-High Resolution CT: Quantifying Diagnostic Image Quality; Canon Medical Systems Corporation: Otawara, Japan, 2018. [Google Scholar]
- Boedeker, K. (Ed.) AiCE Deep Learning Reconstruction: Bringing the Power of Ultra-High Resolution CT to Routine Imaging; Canon Medical Systems Corporation: Otawara, Japan, 2018. [Google Scholar]
- Vaishnav, J. White Paper on Canon‘s PureViSION Optics Technology for CT; Canon: Tokyo, Japan, 2019. [Google Scholar]
- Verdun, F.; Racine, D.; Ott, J.; Tapiovaara, M.; Toroi, P.; Bochud, F.; Veldkamp, W.; Schegerer, A.; Bouwman, R.; Giron, I.H.; et al. Image quality in CT: From physical measurements to model observers. Phys. Med. 2015, 31, 823–843. [Google Scholar] [CrossRef] [PubMed]
- Samei, E.; Richard, S. Assessment of the dose reduction potential of a model-based iterative reconstruction algorithm using a task-based performance metrology. Med. Phys. 2014, 42, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.E.; Bongers, M.N.; Zinsser, D.; Schabel, C.; Wichmann, J.L.; Arshid, R.; Notohamiprodjo, M.; Nikolaou, K.; Bamberg, F. Evaluation of reduced-dose CT for acute non-traumatic abdominal pain: Evaluation of diagnostic accuracy in comparison to standard-dose CT. Acta Radiol. 2017, 59, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Lell, M.M.; Kachelriess, M. Recent and Upcoming Technological Developments in Computed Tomography: High Speed, Low Dose, Deep Learning, Multienergy. Investig. Radiol. 2020, 55, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.S.; Maurer, M.; Storz, C.; Weiss, J.; Archid, R.; Bamberg, F.; Kim, J.H.; Nikolaou, K.; Othman, A.E. Effects of Radiation Dose Reduction on Diagnostic Accuracy of Abdominal CT in Young Adults with Suspected Acute Diverticulitis: A Retrospective Intraindividual Analysis. Acad. Radiol. 2018, 26, 782–790. [Google Scholar] [CrossRef]
- Higaki, T.; Nakamura, Y.; Zhou, J.; Yu, Z.; Nemoto, T.; Tatsugami, F.; Awai, K. Deep Learning Reconstruction at CT: Phantom Study of the Image Characteristics. Acad. Radiol. 2020, 27, 82–87. [Google Scholar] [CrossRef]
- Wang, H.; Li, L.-L.; Shang, J.; Song, J.; Liu, B. Application of deep learning image reconstruction in low-dose chest CT scan. Br. J. Radiol. 2022, 95, 20210380. [Google Scholar] [CrossRef]
- Dabli, D.; Loisy, M.; Frandon, J.; de Oliveira, F.; Meerun, A.M.; Guiu, B.; Beregi, J.-P.; Greffier, J. Comparison of image quality of two versions of deep-learning image reconstruction algorithm on a rapid kV-switching CT: A phantom study. Eur. Radiol. Exp. 2023, 7, 1. [Google Scholar] [CrossRef]
- Kubo, Y.; Ito, K.; Sone, M.; Nagasawa, H.; Onishi, Y.; Umakoshi, N.; Hasegawa, T.; Akimoto, T.; Kusumoto, M. Diagnostic Value of Model-Based Iterative Reconstruction Combined with a Metal Artifact Reduction Algorithm during CT of the Oral Cavity. Am. J. Neuroradiol. 2020, 41, 2132–2138. [Google Scholar] [CrossRef]
- Miyamoto, M.; Ohara, A.; Arai, T.; Koyanagi, M.; Watanabe, I.; Nakagawa, H.; Yokoyama, K.; Saito, K. Three-dimensional imaging of vocalizing larynx by ultra-high-resolution computed tomography. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 3159–3164. [Google Scholar] [CrossRef]
- Shen, H.; Dai, G.; Luo, M.; Duan, C.; Cai, W.; Liang, D.; Wang, X.; Zhu, D.; Li, W.; Qiu, J. Image Quality and Radiation Dose of CT Coronary Angiography with Automatic Tube Current Modulation and Strong Adaptive Iterative Dose Reduction Three-Dimensional (AIDR3D). PLoS ONE 2015, 10, e0142185. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Onishi, H.; Hori, M.; Nakamoto, A.; Ota, T.; Fukui, H.; Tatsumi, M.; Enchi, Y.; Sato, K.; Kaketaka, K.; et al. Visualization of small visceral arteries on abdominal CT angiography using ultra-high-resolution CT scanner. Jpn. J. Radiol. 2021, 39, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Digumarthy, S.R.; Muse, V.V.; Kambadakone, A.R.; Blake, M.A.; Tabari, A.; Hoi, Y.; Akino, N.; Angel, E.; Madan, R.; et al. Image Quality and Lesion Detection on Deep Learning Reconstruction and Iterative Reconstruction of Submillisievert Chest and Abdominal CT. AJR Am. J. Roentgenol. 2020, 214, 566–573. [Google Scholar] [CrossRef]
- Ong, C.K.; Chong, V.F. Imaging of perineural spread in head and neck tumours. Cancer Imaging 2010, 10, S92–S98. [Google Scholar] [CrossRef]
- Lee, K.J.; Abemayor, E.; Sayre, J.; Bhuta, S.; Kirsch, C. Determination of perineural invasion preoperatively on radiographic images. Otolaryngol. Neck Surg. 2008, 139, 275–280. [Google Scholar] [CrossRef]
- Warden, K.F.; Parmar, H.; Trobe, J. Perineural Spread of Cancer Along the Three Trigeminal Divisions. J. Neuro-Ophthalmol. 2009, 29, 300–307. [Google Scholar] [CrossRef]
- Waech, T.; Pazahr, S.; Guarda, V.; Rupp, N.J.; Broglie, M.A.; Morand, G.B. Measurement variations of MRI and CT in the assessment of tumor depth of invasion in oral cancer: A retrospective study. Eur. J. Radiol. 2020, 135, 109480. [Google Scholar] [CrossRef]
- Baba, A.; Hashimoto, K.; Kayama, R.; Yamauchi, H.; Ikeda, K.; Ojiri, H. Radiological approach for the newly incorporated T staging factor, depth of invasion (DOI), of the oral tongue cancer in the 8th edition of American Joint Committee on Cancer (AJCC) staging manual: Assessment of the necessity for elective neck dissection. Jpn. J. Radiol. 2020, 38, 821–832. [Google Scholar] [CrossRef]
- Baba, A.; Ojiri, H.; Ogane, S.; Hashimoto, K.; Inoue, T.; Takagiwa, M.; Goto, T.K. Usefulness of contrast-enhanced CT in the evaluation of depth of invasion in oral tongue squamous cell carcinoma: Comparison with MRI. Oral Radiol. 2020, 37, 86–94. [Google Scholar] [CrossRef]
- De las Heras Gala, H. Bekanntmachung der Aktualisierten Diagnostischen Referenzwerte für Diagnostische und Interventionelle Röntgenanwendungen. Bundesamt für Strahlenschutz. 2020. Available online: https://www.bfs.de/SharedDocs/Downloads/BfS/DE/fachinfo/ion/drw-roentgen.pdf?__blob=publicationFile&v=11 (accessed on 3 March 2023).
- German Guideline Program in Oncology (German Cancer Society, German Cancer Aid, AWMF): Oral Cavity Cancer, Long Version 3.0, Januar 2021, AWMF Registration Number: 007/100OL. Available online: https://www.leitlinienprogrammonkologie.de/leitlinien/mundhoehlenkarzinom/ (accessed on 25 August 2022).



| NR-CT | UHR-CT | |
|---|---|---|
| Focal spot size | 0.4 × 0.5 mm | 0.4 × 0.5 mm |
| Detector element size | 1.6 × 1.4 mm | 0.25 × 0.25 mm |
| Reconstruction Matrix | 512 | 1024 |
| Beam collimation | 0.5 mm × 32 | 0.25 mm × 160 |
| Pitch factor | 0.8 | 0.569 |
| Tube voltage | 120 kV | 120 kV |
| FOV | 240 mm | 240 mm |
| Grading | Image Noise | Image Sharpness | Artifacts | Diagnostic Acceptability |
|---|---|---|---|---|
| 1 | Unacceptable | Blurry | Present and affecting image interpretation | Unacceptable |
| 2 | Increased | Poorer than average | Present and affecting visualization of normal structures | Suboptimal |
| 3 | Average | Subtle lesion | Present but not affecting visualization of normal structures | Average |
| 4 | Less than average | Clearly visualized lesion, poor margin | None | Above average |
| 5 | Minimum or no noise | Clearly visualized lesion, clearly visualized margin | excellent |
| Assessed Parameter | UHR-CT Reader 1 (IQR) | UHR-CT Reader 2 (IQR) | ICC UHR-CT | NR-CT Reader 1 (IQR) | NR-CT Reader 2 (IQR) | ICC NR-CT | p-Value |
|---|---|---|---|---|---|---|---|
| Image noise | 5 (4.25–5) | 5 (5–5) | 0.93 | 3 (2–3) | 3 (2–3) | 0.93 | <0.000 |
| Image sharpness | 5 (5–5) | 5 (5–5) | 0.70 | 3 (2–3) | 3 (2–3) | 0.91 | <0.000 |
| Artifacts | 1 (1–3) | 1 (1–3) | 1.00 | 1 (1–1) | 1 (1–1) | 0.99 | <0.046 |
| Diagnostic acceptability | 4 (3–4.75) | 4 (4–5) | 0.91 | 2 (2–3) | 2 (2–3) | 0.96 | <0.000 |
| Skull base | 5 (5–5) | 5 (5–5) | 0.60 | 3 (2–3) | 3 (3–4) | 0.80 | <0.000 |
| Infratemporal fossa | 5 (5–5) | 5 (5–5) | 0.36 | 3 (3–3) | 3 (3–3) | 0.91 | <0.000 |
| Nasal cavity | 5 (5–5) | 5 (5–5) | 0.85 | 3 (3–3) | 3 (3–3) | 0.95 | <0.000 |
| Paranasal sinuses | 5 (5–5) | 5 (5–5) | 0.93 | 3 (3–4) | 3 (3–4) | 0.87 | <0.000 |
| Nasopharyngeal space | 5 (5–5) | 5 (4–5) | 0.71 | 3 (3–3) | 3 (3–3) | 0.89 | <0.000 |
| Oropharyngeal space | 4 (2–5) | 4 (3–4.5) | 0.97 | 2 (1–2) | 2 (1–2) | 0.98 | <0.000 |
| Hypopharyngeal space | 5 (4–5) | 5 (4–5) | 0.91 | 3 (3–3) | 3 (2.25–3) | 0.91 | <0.000 |
| Oral cavity and buccal mucosa | 2 (1–4) | 2 (1–4) | 0.99 | 1 (1–2) | 1 (1–2) | 0.97 | <0.019 |
| Floor of mouth | 5 (4.25–5) | 5 (4–5) | 0.98 | 3 (3–3) | 3 (2.25–3) | 0.89 | <0.000 |
| Lymph nodes Level I | 5 (5–5) | 5 (5–5) | 0.72 | 3 (3–3) | 3 (3–3) | 0.83 | <0.000 |
| Lymph nodes Level II-IV | 5 (5–5) | 5 (5–5) | 0.91 | 3 (3–3) | 3 (3–3) | 0.83 | <0.000 |
| Jugular fossa | 5 (4–5) | 5 (5–5) | 0.91 | 3 (3–3) | 3 (3–3) | 0.82 | <0.000 |
| Thyroid and upper mediastinum | 5 (4.25–5) | 5 (5–5) | 0.91 | 3 (3–3) | 3 (3–3) | 0.84 | <0.000 |
| Salivary glands | 4 (3–5) | 4 (3–5) | 0.94 | 2 (2–2.75) | 2 (2–3) | 0.86 | <0.000 |
| Carotid artery origin | 5 (3.25–5) | 5 (4–5) | 0.89 | 3 (3–4) | 3 (3–4) | 0.88 | <0.000 |
| Vertebral artery V1 | 5 (3–5) | 5 (4–5) | 0.97 | 3 (3–4) | 3 (2.25–4) | 0.91 | <0.000 |
| Carotid artery bifurcation | 5 (4–5) | 5 (4–5) | 0.97 | 3 (3–3.75) | 3 (3–3.75) | 0.96 | <0.000 |
| Vertebral artery V2 | 5 (4–5) | 5 (4–5) | 0.99 | 3 (2–3) | 3 (2–3) | 0.99 | <0.000 |
| Carotid artery C1/2 | 5 (5–5) | 5 (4.25–5) | 0.99 | 2 (2–3) | 3 (2–3) | 0.95 | <0.000 |
| Vertebral artery V3 | 5 (5–5) | 5 (4.25–5) | 0.97 | 2 (2–3) | 2 (2–3) | 0.96 | <0.000 |
| UHR-CT | NR-CT | p-Value | |
|---|---|---|---|
| SNR | 10.8 [10.2–11.3] | 8.8 [7.9–9.6] | <0.000 |
| Steepest slope [HU/mm] | −168.4 [−(177.8–159.4)] | −94.5 [−(100.1–89.0)] | <0.000 |
| Distance [mm] within the IQR | −0.56 [−(0.5–0.58)] | −0.97 [−(1.02–0.908)] | <0.000 |
| CNR | 26.1 [24.2–26.0] | 22.9 [20.9–24.9] | <0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altmann, S.; Abello Mercado, M.A.; Ucar, F.A.; Kronfeld, A.; Al-Nawas, B.; Mukhopadhyay, A.; Booz, C.; Brockmann, M.A.; Othman, A.E. Ultra-High-Resolution CT of the Head and Neck with Deep Learning Reconstruction—Assessment of Image Quality and Radiation Exposure and Intraindividual Comparison with Normal-Resolution CT. Diagnostics 2023, 13, 1534. https://doi.org/10.3390/diagnostics13091534
Altmann S, Abello Mercado MA, Ucar FA, Kronfeld A, Al-Nawas B, Mukhopadhyay A, Booz C, Brockmann MA, Othman AE. Ultra-High-Resolution CT of the Head and Neck with Deep Learning Reconstruction—Assessment of Image Quality and Radiation Exposure and Intraindividual Comparison with Normal-Resolution CT. Diagnostics. 2023; 13(9):1534. https://doi.org/10.3390/diagnostics13091534
Chicago/Turabian StyleAltmann, Sebastian, Mario A. Abello Mercado, Felix A. Ucar, Andrea Kronfeld, Bilal Al-Nawas, Anirban Mukhopadhyay, Christian Booz, Marc A. Brockmann, and Ahmed E. Othman. 2023. "Ultra-High-Resolution CT of the Head and Neck with Deep Learning Reconstruction—Assessment of Image Quality and Radiation Exposure and Intraindividual Comparison with Normal-Resolution CT" Diagnostics 13, no. 9: 1534. https://doi.org/10.3390/diagnostics13091534
APA StyleAltmann, S., Abello Mercado, M. A., Ucar, F. A., Kronfeld, A., Al-Nawas, B., Mukhopadhyay, A., Booz, C., Brockmann, M. A., & Othman, A. E. (2023). Ultra-High-Resolution CT of the Head and Neck with Deep Learning Reconstruction—Assessment of Image Quality and Radiation Exposure and Intraindividual Comparison with Normal-Resolution CT. Diagnostics, 13(9), 1534. https://doi.org/10.3390/diagnostics13091534






