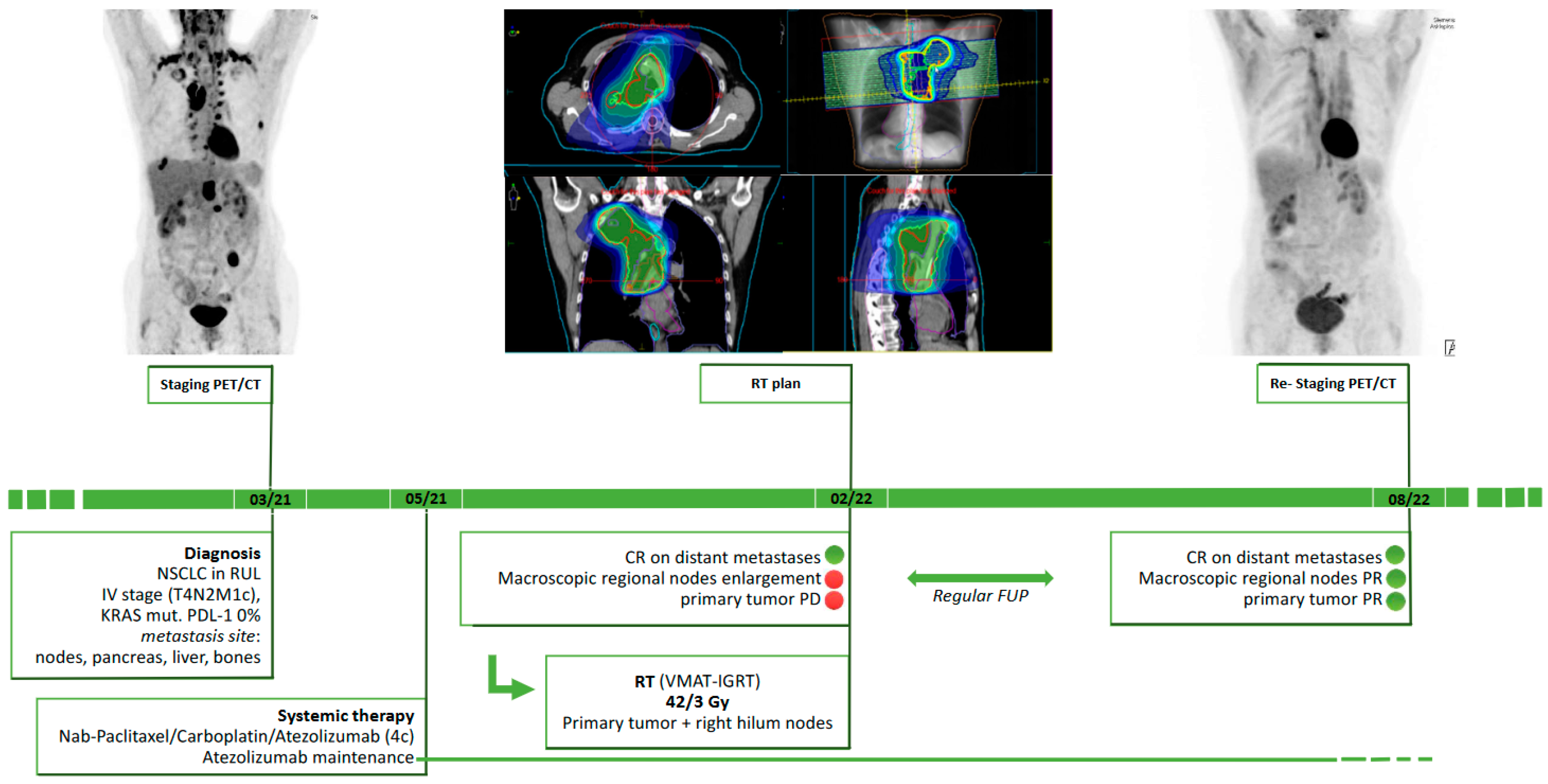
Positron Emission Tomography/Computed Tomography (PET/CT)-Based, Local Consolidative Radiotherapy after Chemoimmunotherapy in Metastatic, Non-Oncogene-Addicted Non-Small-Cell Lung Cancer (NSCLC)
Abstract
:Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- West, H.; McCleod, M.; Hussein, M.; Morabito, A.; Rittmeyer, A.; Conter, H.J.; Kopp, H.-G.; Daniel, D.; McCune, S.; Mekhail, T. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 924–937. [Google Scholar] [CrossRef] [PubMed]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009, 50, 122S–150S. [Google Scholar] [CrossRef] [PubMed]
- Shaverdian, N.; Lisberg, A.E.; Bornazyan, K.; Veruttipong, D.; Goldman, J.W.; Formenti, S.C.; Garon, E.B.; Lee, P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017, 18, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.M.G.; Li, X.; Gomez, D.R. Local Consolidative Therapy for Oligometastatic Non-Small Cell Lung Cancer. Cancers 2022, 14, 3977. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.R.; Blumenschein, G.R., Jr.; Lee, J.J.; Hernandez, M.; Ye, R.; Camidge, D.R.; Doebele, R.C.; Skoulidis, F.; Gaspar, L.E.; Gibbons, D.L. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: A multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016, 17, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.R.; Rimner, A.; Simone, C.B., II; Cho, B.J.; de Perrot, M.; Adjei, A.A.; Bueno, R.; Gill, R.R.; Harpole, D.H., Jr.; Hesdorffer, M. The use of radiation therapy for the treatment of malignant pleural mesothelioma: Expert opinion from the National Cancer Institute Thoracic Malignancy Steering Committee, International Association for the Study of Lung Cancer, and Mesothelioma Applied Research Foundation. J. Thorac. Oncol. 2019, 14, 1172–1183. [Google Scholar] [PubMed]
- Iyengar, P.; Wardak, Z.; Gerber, D.E.; Tumati, V.; Ahn, C.; Hughes, R.S.; Dowell, J.E.; Cheedella, N.; Nedzi, L.; Westover, K.D. Consolidative radiotherapy for limited metastatic non–small-cell lung cancer: A phase 2 randomized clinical trial. JAMA Oncol. 2018, 4, e173501. [Google Scholar] [CrossRef] [PubMed]
- De Ruysscher, D.; Wanders, R.; Hendriks, L.E.; van Baardwijk, A.; Reymen, B.; Houben, R.; Bootsma, G.; Pitz, C.; van Eijsden, L.; Dingemans, A.-M.C. Progression-free survival and overall survival beyond 5 years of NSCLC patients with synchronous oligometastases treated in a prospective phase II trial (NCT 01282450). J. Thorac. Oncol. 2018, 13, 1958–1961. [Google Scholar] [CrossRef] [PubMed]
- Gettinger, S.N.; Wurtz, A.; Goldberg, S.B.; Rimm, D.; Schalper, K.; Kaech, S.; Kavathas, P.; Chiang, A.; Lilenbaum, R.; Zelterman, D. Clinical features and management of acquired resistance to PD-1 axis inhibitors in 26 patients with advanced non–small cell lung cancer. J. Thorac. Oncol. 2018, 13, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Rheinheimer, S.; Heussel, C.-P.; Mayer, P.; Gaissmaier, L.; Bozorgmehr, F.; Winter, H.; Herth, F.J.; Muley, T.; Liersch, S.; Bischoff, H. Oligoprogressive non-small-cell lung cancer under treatment with PD-(L) 1 inhibitors. Cancers 2020, 12, 1046. [Google Scholar] [CrossRef] [PubMed]
- Käsmann, L.; Eze, C.; Manapov, F. Stereotactic Body Radiation Therapy (SBRT) Combined with Immune Check-Point Inhibition (ICI) in Advanced Lung Cancer: Which Metastatic Site Should Be Irradiated to Induce Immunogenic Cell Death? Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 225–226. [Google Scholar] [CrossRef] [PubMed]
- Theelen, W.S.; Chen, D.; Verma, V.; Hobbs, B.P.; Peulen, H.M.; Aerts, J.G.; Bahce, I.; Niemeijer, A.L.N.; Chang, J.Y.; de Groot, P.M. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Respir. Med. 2021, 9, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.; Menon, H.; Chen, D.; Verma, V.; Tang, C.; Altan, M.; Hess, K.; De Groot, P.; Nguyen, Q.-N.; Varghese, R. Pembrolizumab with or without radiation therapy for metastatic non-small cell lung cancer: A randomized phase I/II trial. J. Immunother. Cancer 2020, 8, e001001. [Google Scholar] [CrossRef] [PubMed]
- Theelen, W.S.M.E.; Peulen, H.M.U.; Lalezari, F.; van der Noort, V.; de Vries, J.F.; Aerts, J.G.J.V.; Dumoulin, D.W.; Bahce, I.; Niemeijer, A.-L.N.; de Langen, A.J.; et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non–Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical TrialPembrolizumab Alone vs After Stereotactic Body Radiotherapy in Patients With Advanced NSCLCPembrolizumab Alone vs After Stereotactic Body Radiotherapy in Patients With Advanced NSCLC. JAMA Oncol. 2019, 5, 1276–1282. [Google Scholar] [PubMed]
- Pazos, M.; Eze, C.; Kahnert, K.; Delius, M.; Tufman, A.; Alba-Alejandre, I.; Unterrainer, M.; Neumann, J.; Kirchner, T.; Manapov, F. Novel Multimodal Management of Post-Partum Synchronous Metastatic Pulmonary EBV-Associated Lymphoepithelioma-Like Carcinoma (LELC)—A Case Report. Diagnostics 2021, 11, 2072. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Zhang, Q.; Feng, S.; Li, C.; Wang, L.; Zhao, X.; Yang, Z.; Li, Z.; Luo, H.; Liu, R. Safety and Efficacy of PD-1/PD-L1 inhibitors combined with radiotherapy in patients with non-small-cell lung cancer: A systematic review and meta-analysis. Cancer Med. 2021, 10, 1222–1239. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Rengan, R.; Lee, S.; Santana-Davila, R.; Goulart, B.H.; Martins, R.; Baik, C.; Kalemkerian, G.P.; Hassan, K.A.; Schneider, B.J. A pilot study of atezolizumab plus hypofractionated image guided radiation therapy for the treatment of advanced non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Schild, S.E.; Wang, X.; Bestvina, C.M.; Williams, T.; Masters, G.; Singh, A.K.; Stinchcombe, T.E.; Salama, J.K.; Wolf, S.; Zemla, T. Alliance A082002-a randomized phase II/III trial of modern immunotherapy-based systemic therapy with or without SBRT for PD-L1-negative, advanced non-small cell lung cancer. Clin. Lung Cancer 2022, 23, e317–e320. [Google Scholar] [CrossRef] [PubMed]
- Belluomini, L.; Dionisi, V.; Palmerio, S.; Vincenzi, S.; Avancini, A.; Casali, M.; Riva, S.T.; Menis, J.; Mazzarotto, R.; Pilotto, S. Study Design and Rationale for Espera Trial: A Multicentre, Randomized, Phase II Clinical Trial Evaluating the Potential Efficacy of Adding SBRT to Pembrolizumab-Pemetrexed Maintenance in Responsive or Stable Advanced Non-Squamous NSCLC After Chemo-Immunotherapy Induction. Clin. Lung Cancer 2022, 23, e269–e272. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelini, L.; Pazos, M.; Käsmann, L.; Manapov, F. Positron Emission Tomography/Computed Tomography (PET/CT)-Based, Local Consolidative Radiotherapy after Chemoimmunotherapy in Metastatic, Non-Oncogene-Addicted Non-Small-Cell Lung Cancer (NSCLC). Diagnostics 2023, 13, 1538. https://doi.org/10.3390/diagnostics13091538
Angelini L, Pazos M, Käsmann L, Manapov F. Positron Emission Tomography/Computed Tomography (PET/CT)-Based, Local Consolidative Radiotherapy after Chemoimmunotherapy in Metastatic, Non-Oncogene-Addicted Non-Small-Cell Lung Cancer (NSCLC). Diagnostics. 2023; 13(9):1538. https://doi.org/10.3390/diagnostics13091538
Chicago/Turabian StyleAngelini, Lucia, Montserrat Pazos, Lukas Käsmann, and Farkhad Manapov. 2023. "Positron Emission Tomography/Computed Tomography (PET/CT)-Based, Local Consolidative Radiotherapy after Chemoimmunotherapy in Metastatic, Non-Oncogene-Addicted Non-Small-Cell Lung Cancer (NSCLC)" Diagnostics 13, no. 9: 1538. https://doi.org/10.3390/diagnostics13091538




