A Systematic Review and Meta-Analysis Comparing the Diagnostic Accuracy Tests of COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Search Strategy, Eligibility Criteria, and Data Extraction
2.3. Statistical Analysis
3. Results
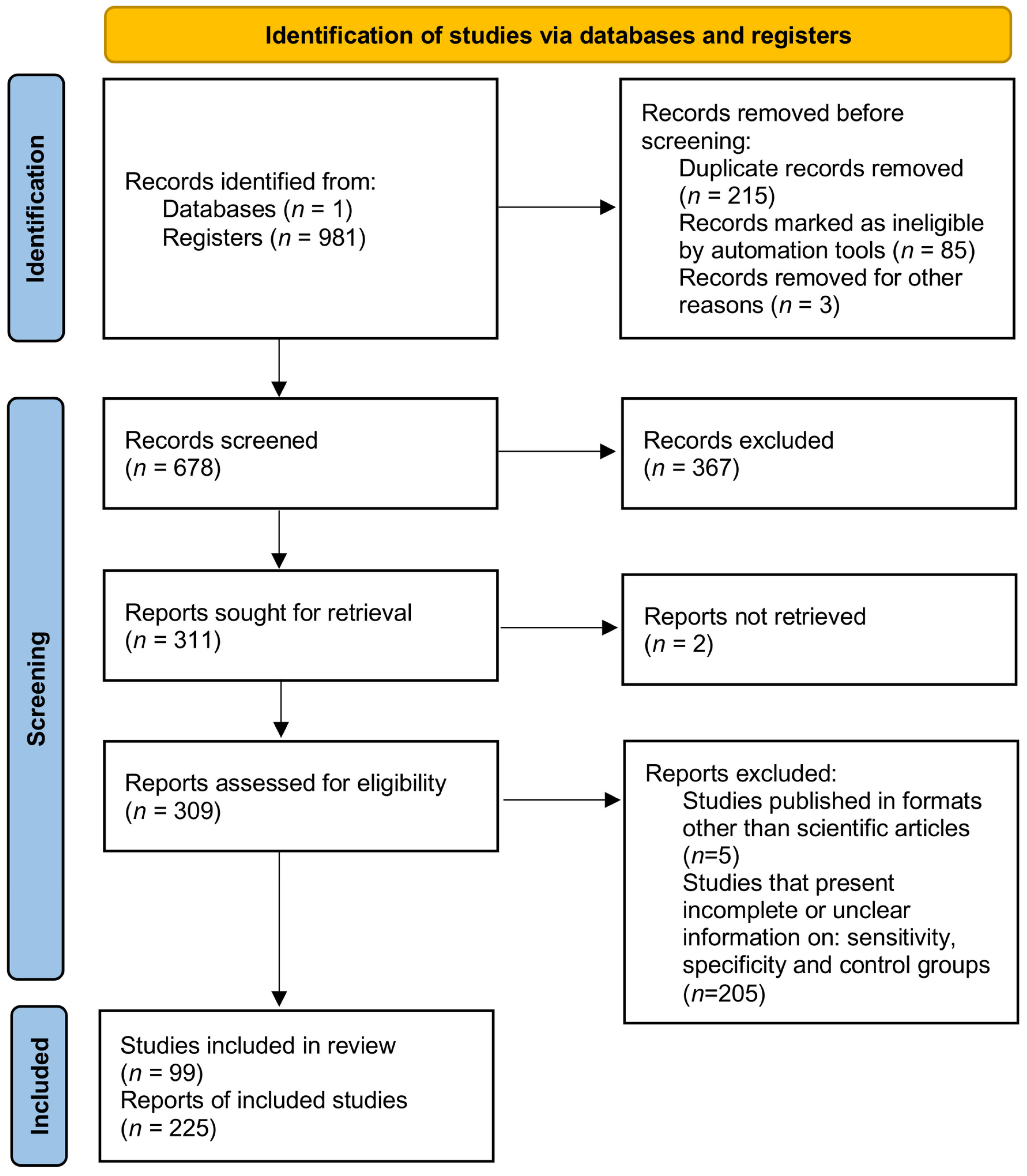
3.1. Data Sources and Study Selection
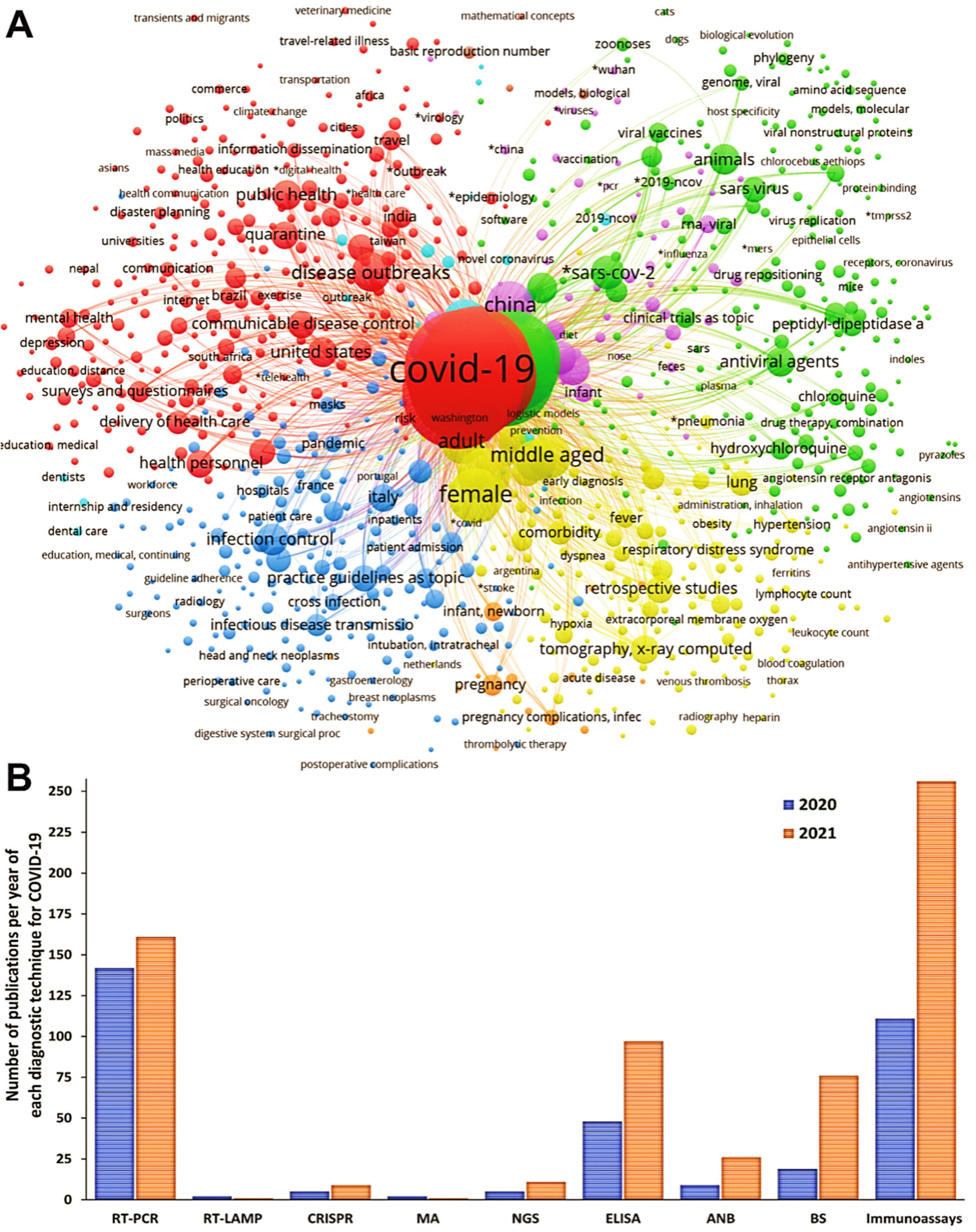
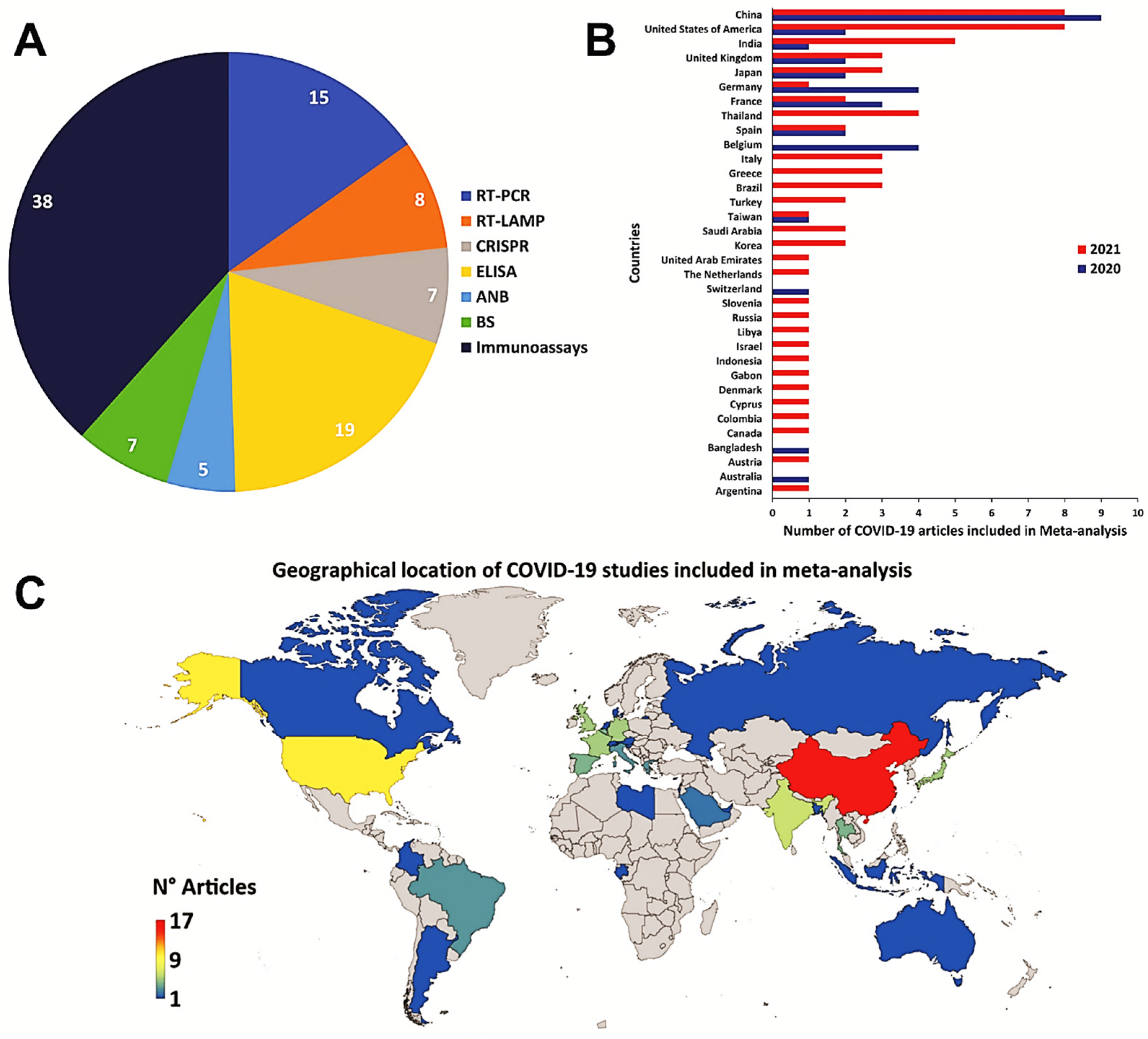
3.2. Meta-Analysis of the Diagnostic Techniques for COVID-19
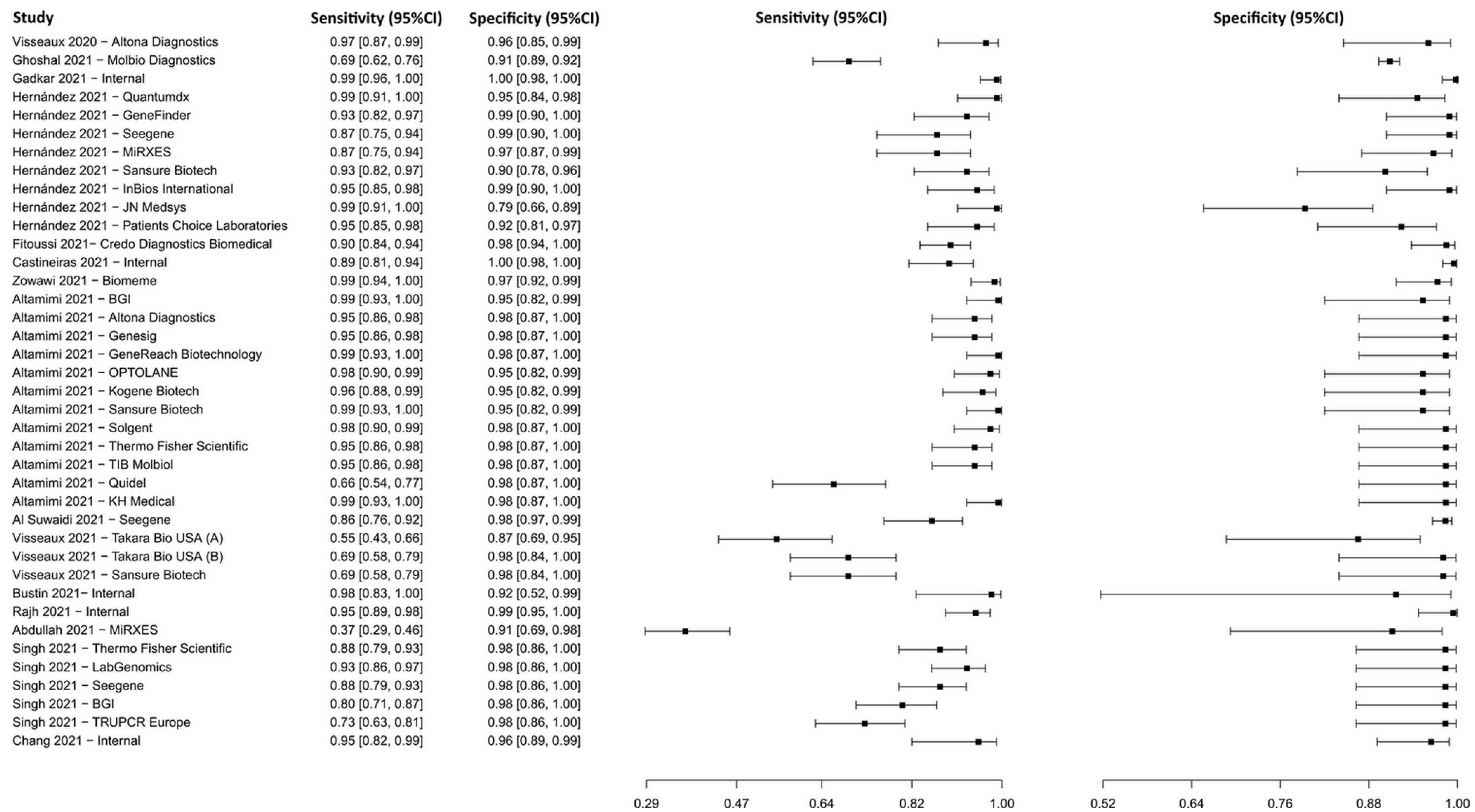
3.2.1. Reverse Transcription–Polymerase Chain Reaction
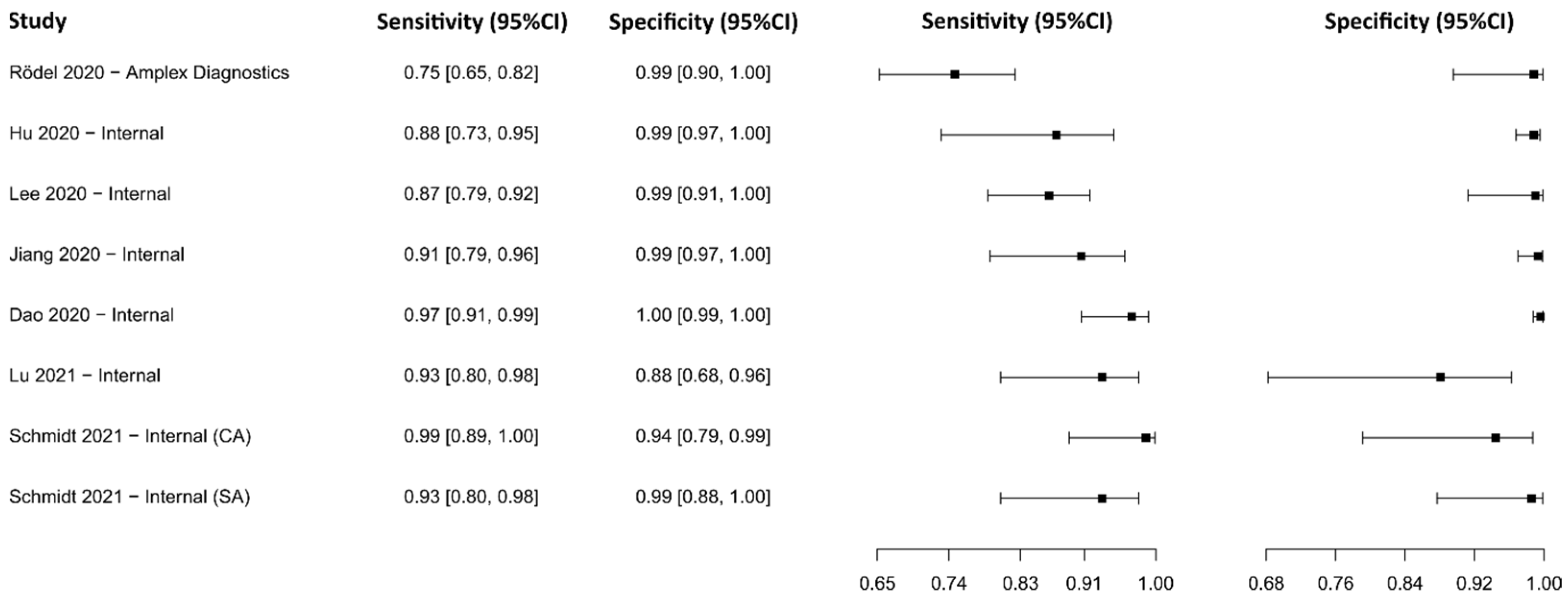
3.2.2. Reverse Transcriptase Loop-Mediated Isothermal Amplification
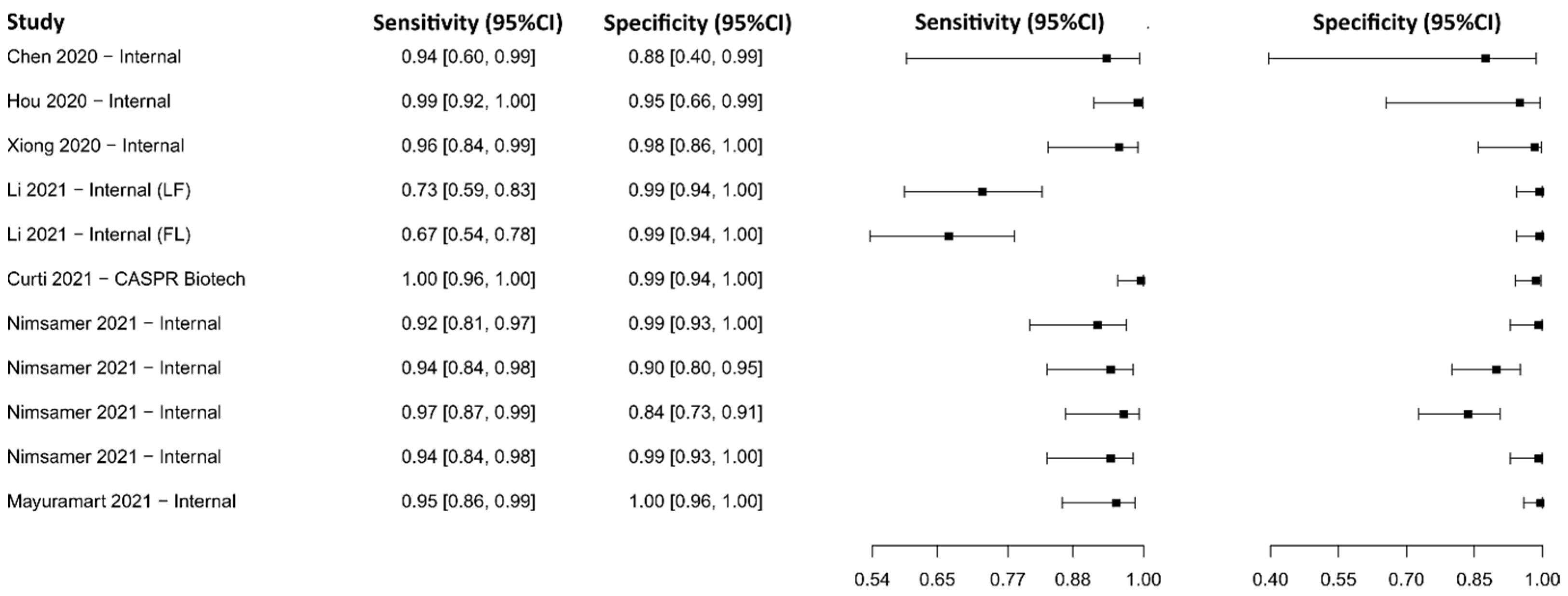
3.2.3. Clustered Regularly Interspaced Short Palindromic Repeats
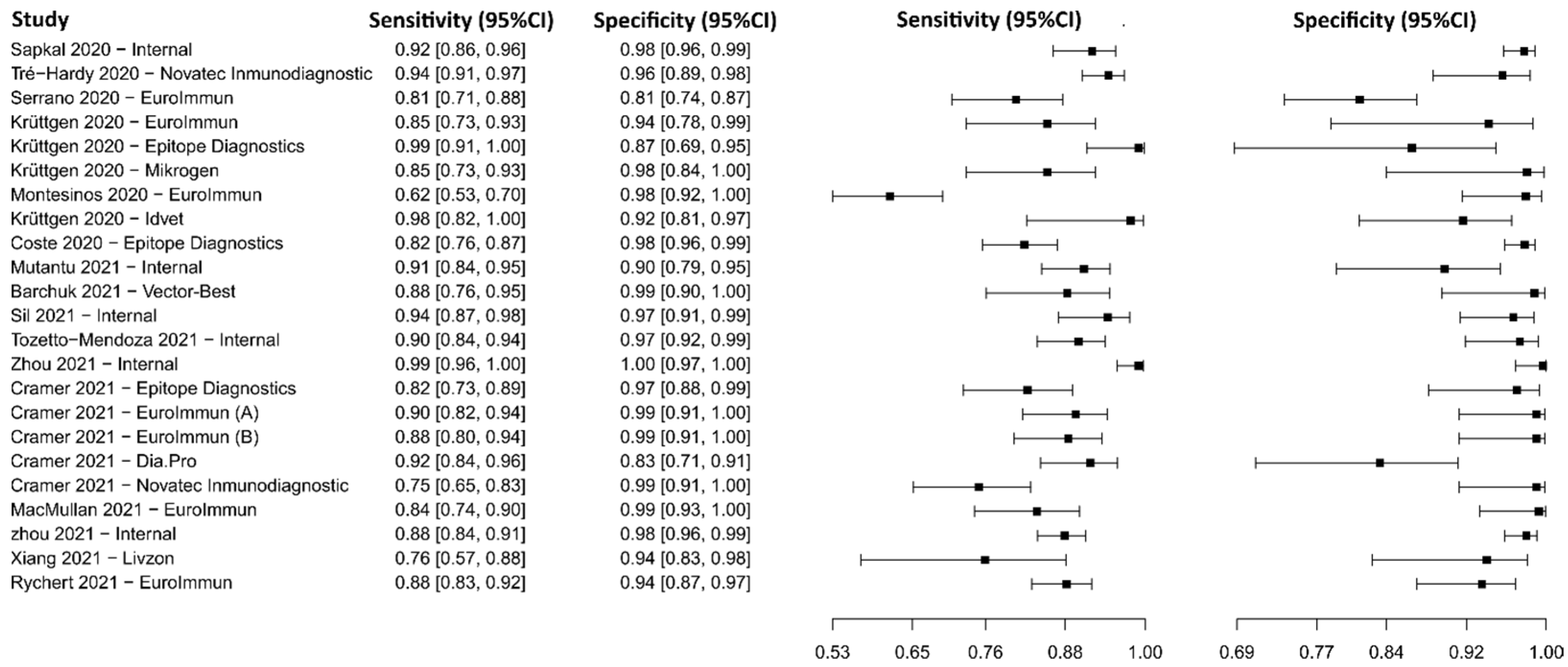
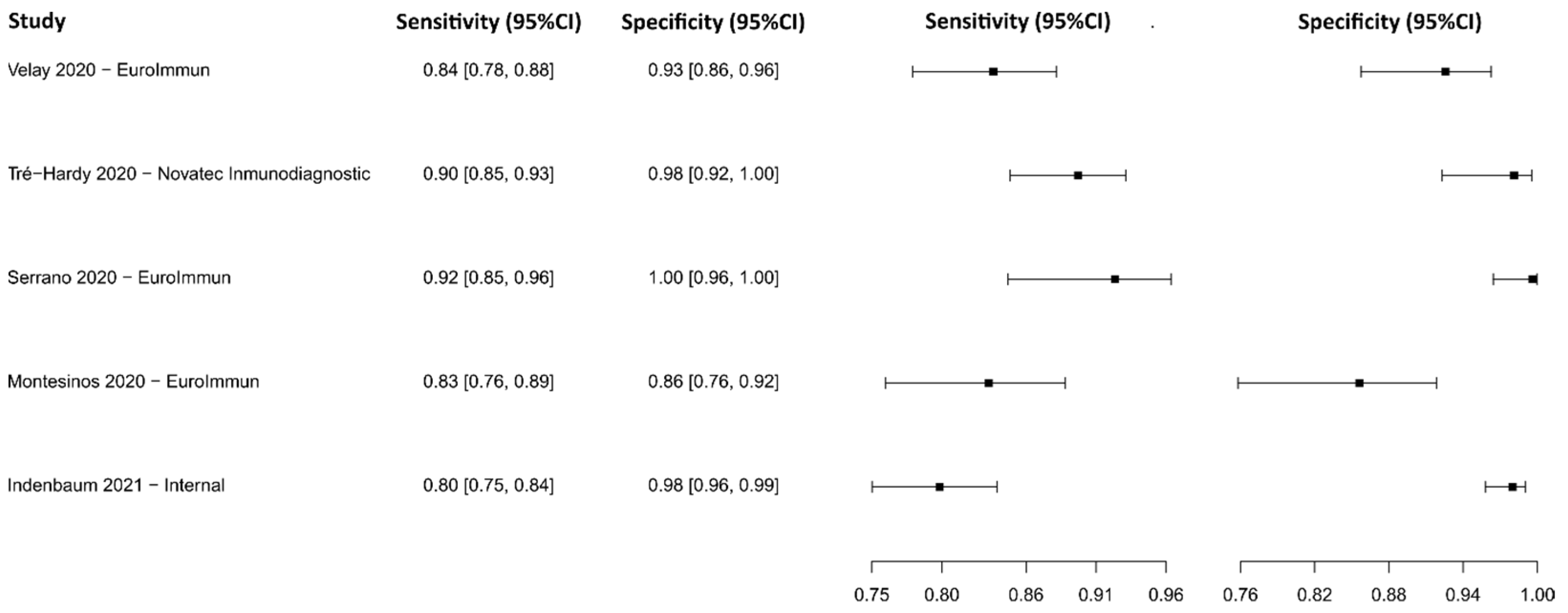
3.2.4. Enzyme-Linked Immunosorbent Assay for IgG
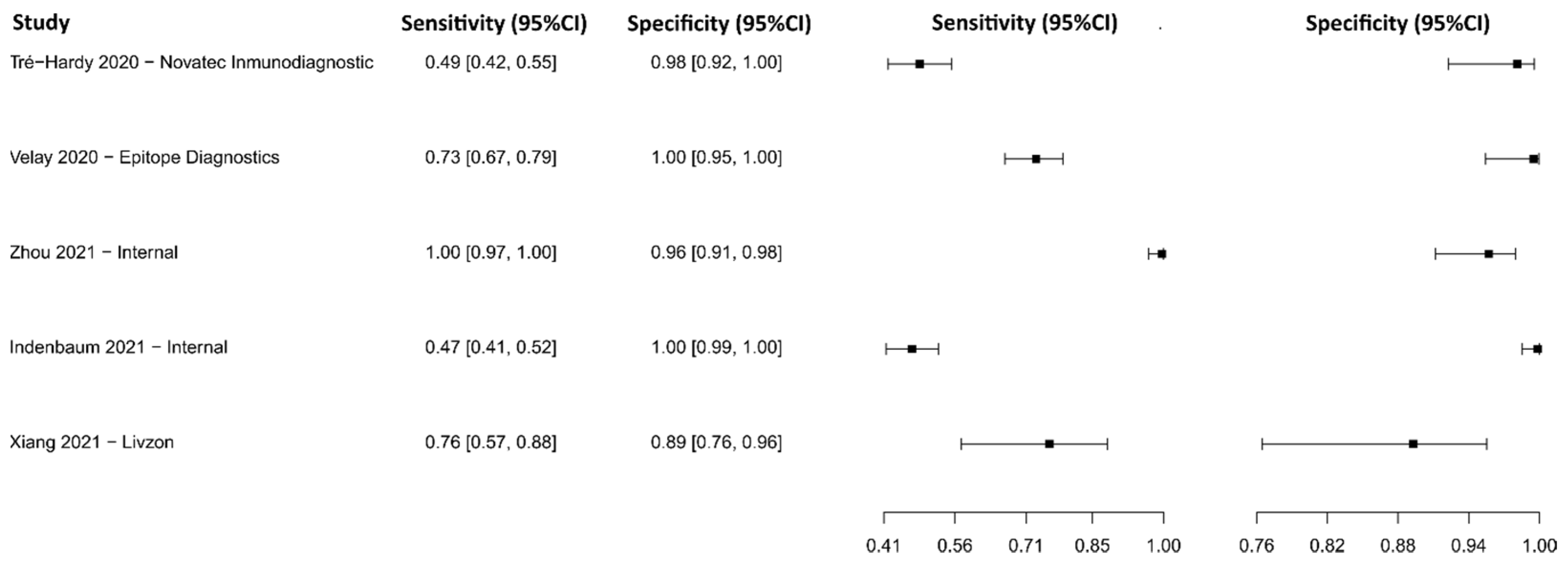
3.2.5. Enzyme-Linked Immunosorbent Assay for IgM
3.2.6. Enzyme-Linked Immunosorbent Assay for IgA
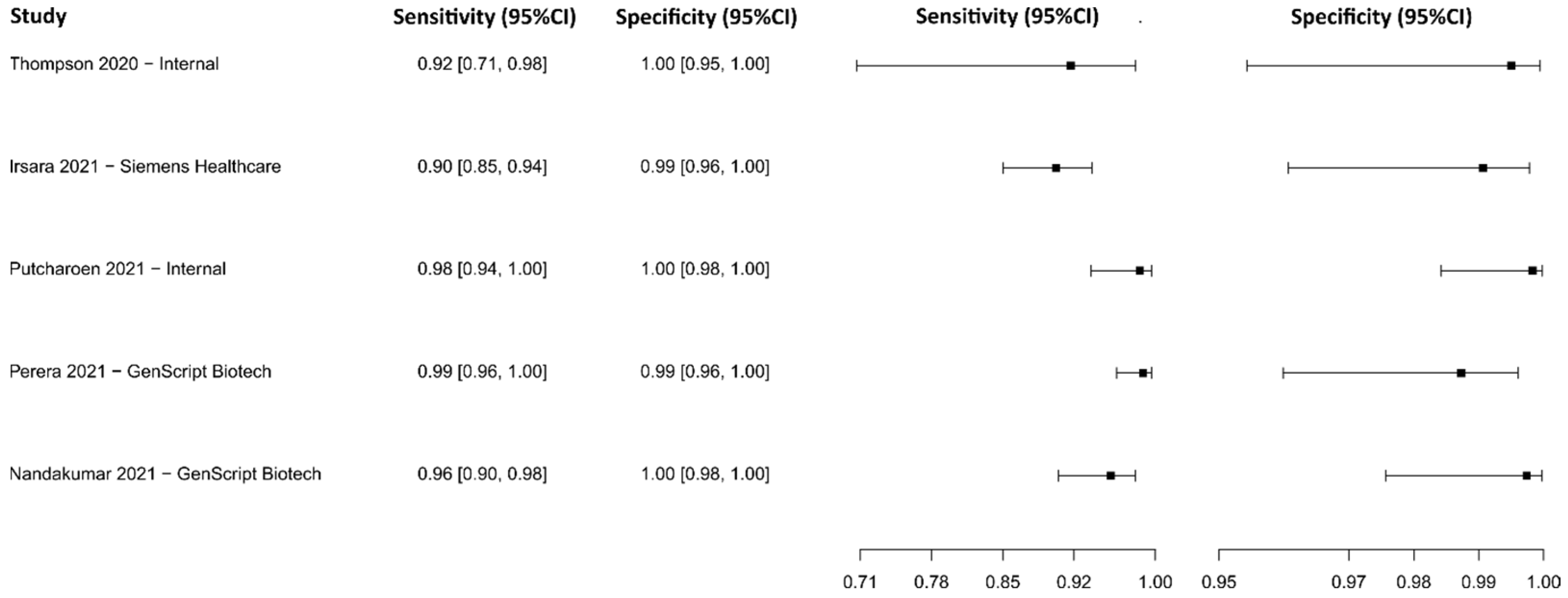
3.2.7. Antiviral Neutralization Bioassay
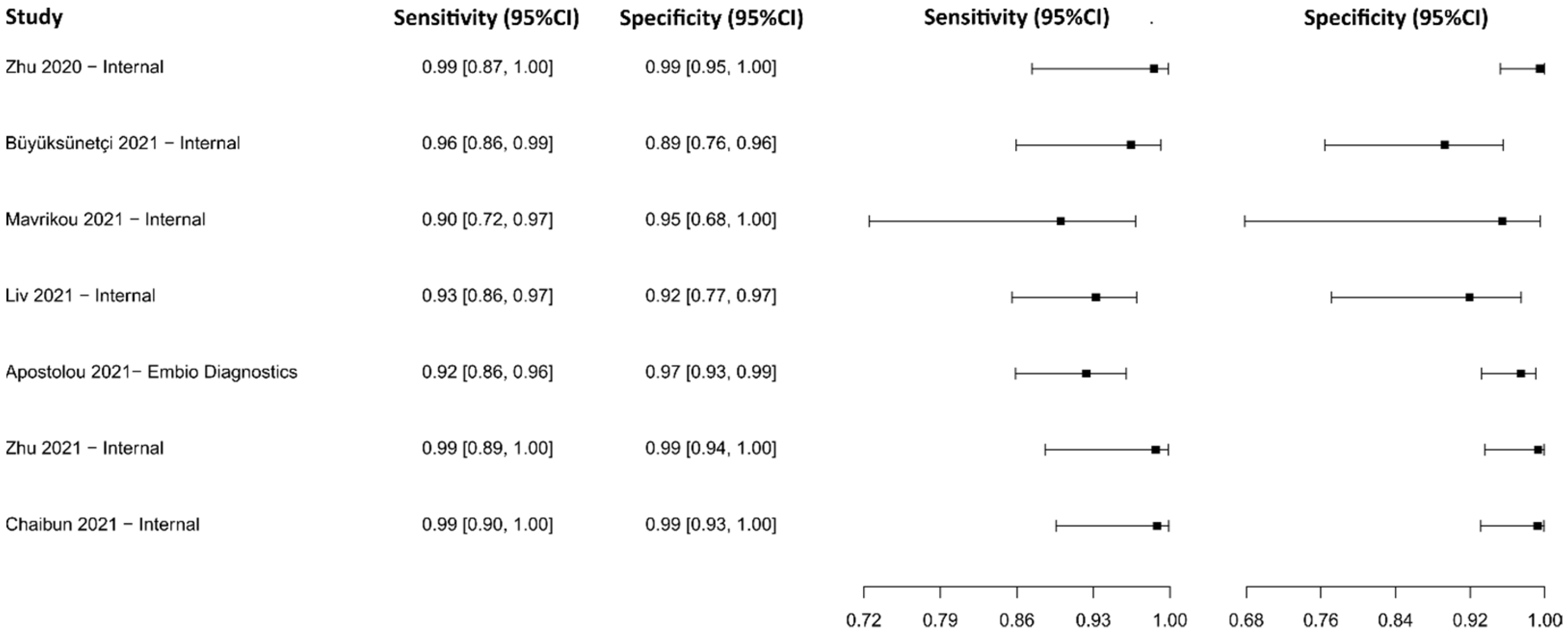
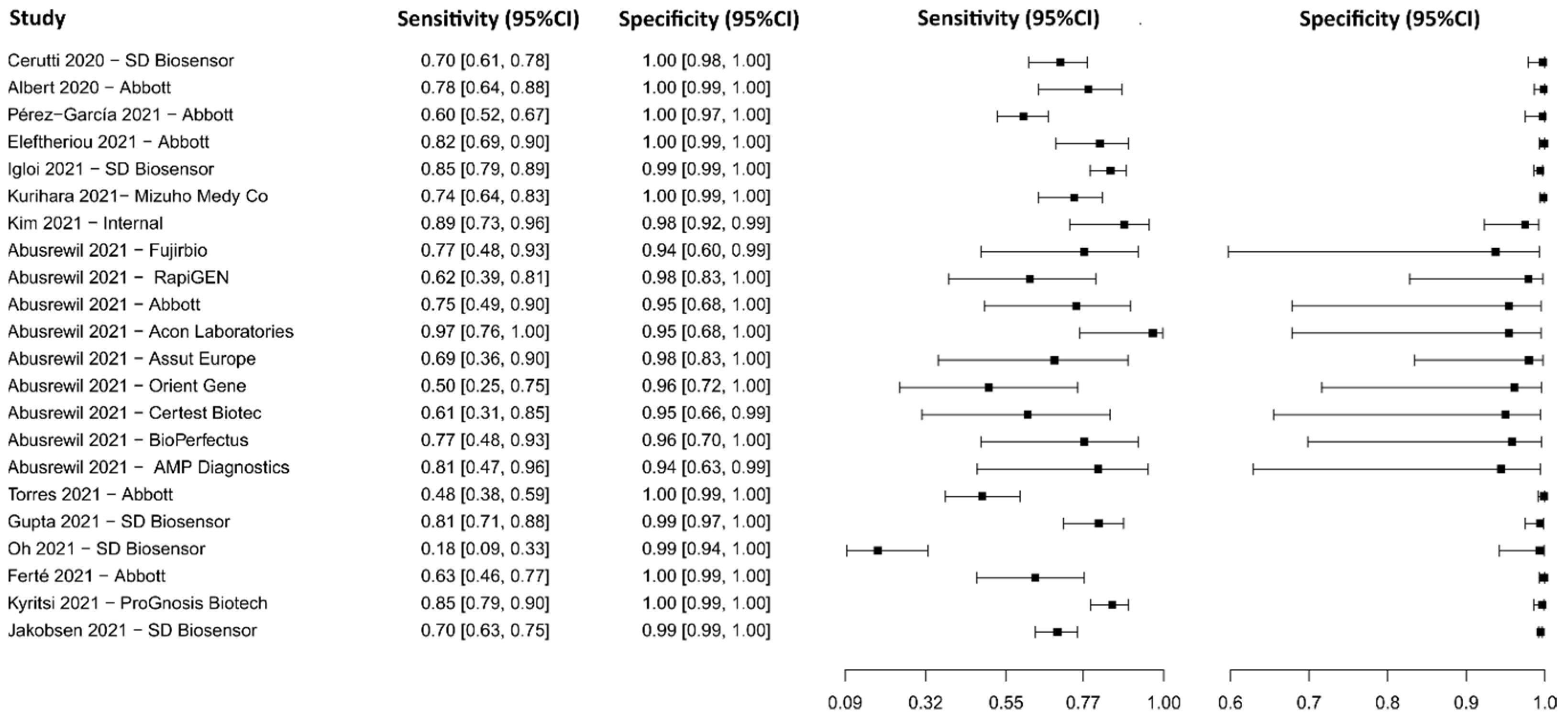
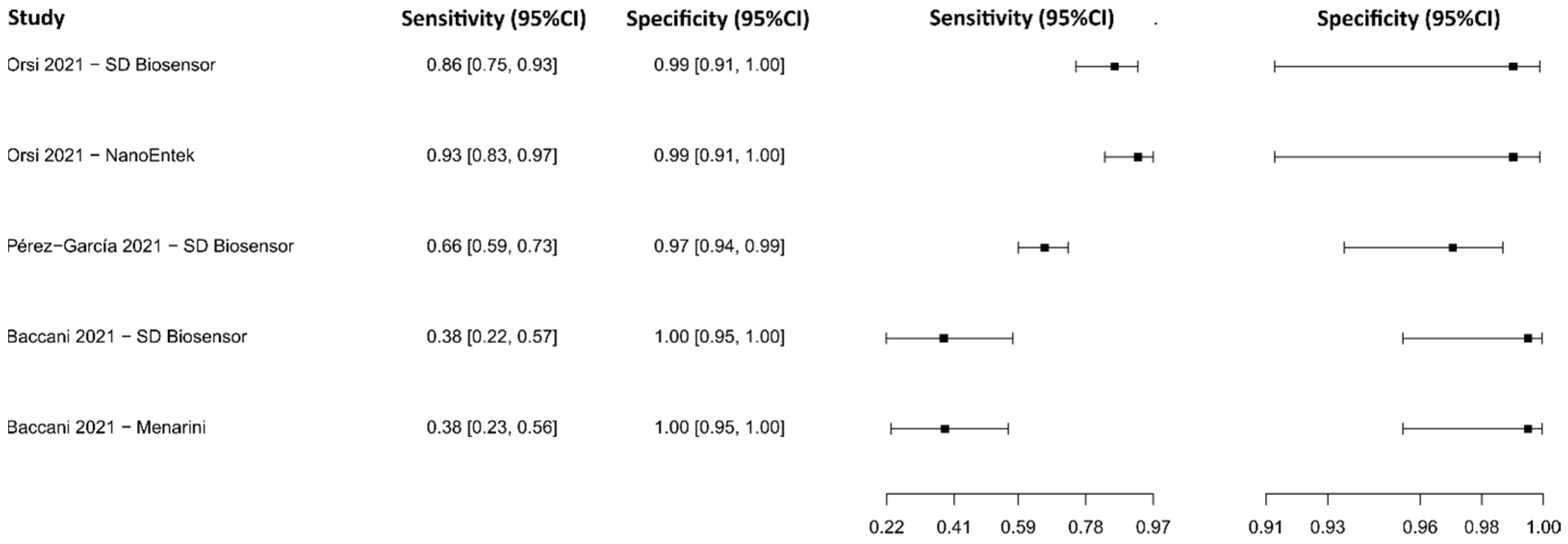
3.2.8. Biosensors
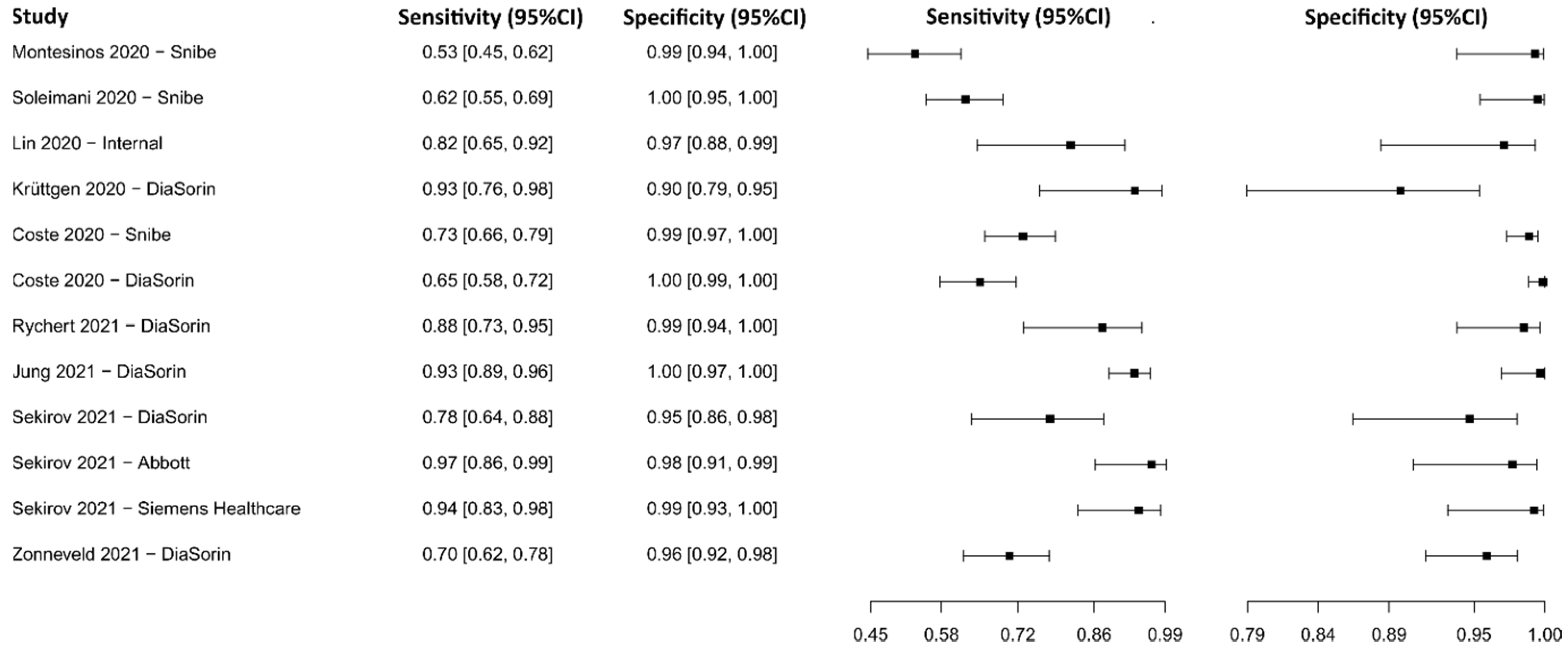
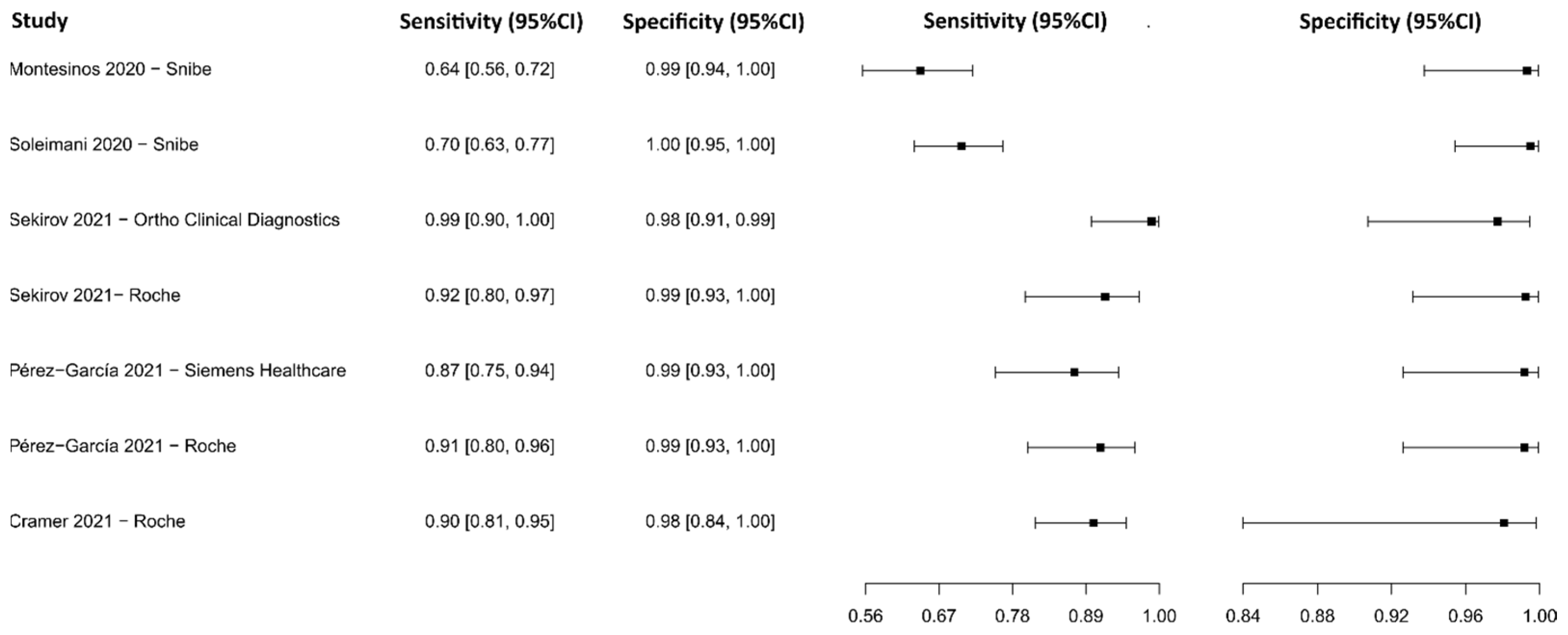
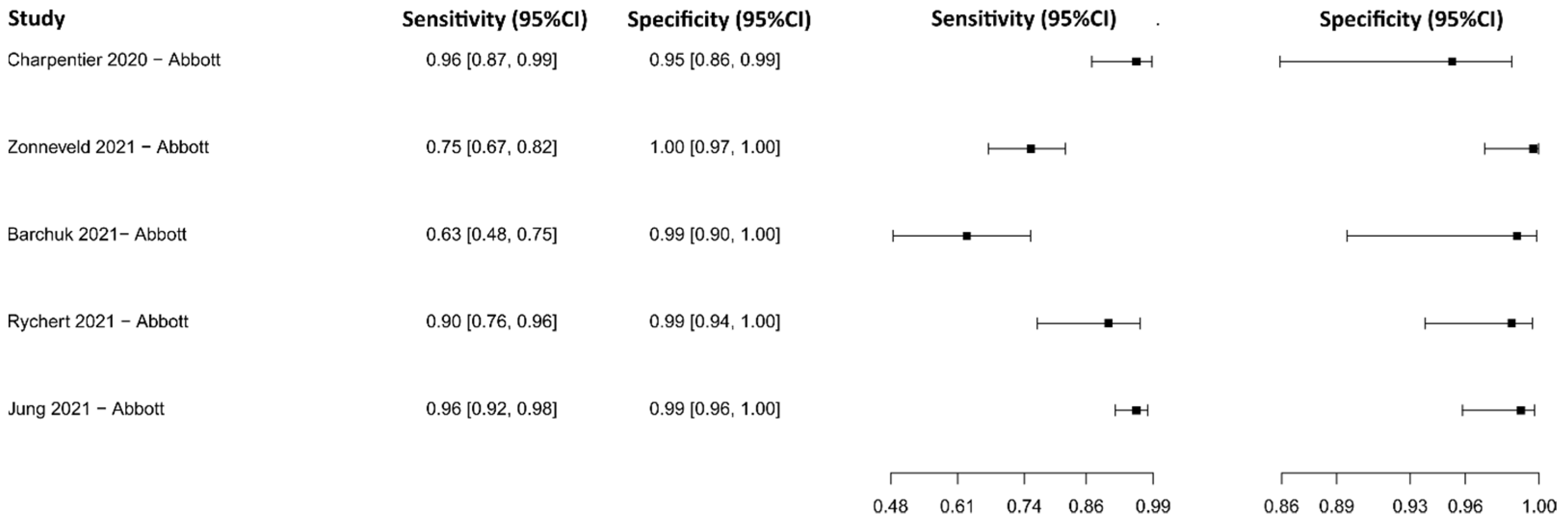
3.2.9. Chemiluminescence Immunoassay for IgG
3.2.10. Chemiluminescence Immunoassay for IgM
3.2.11. Chemiluminescence Immunoassay for IgM-IgG
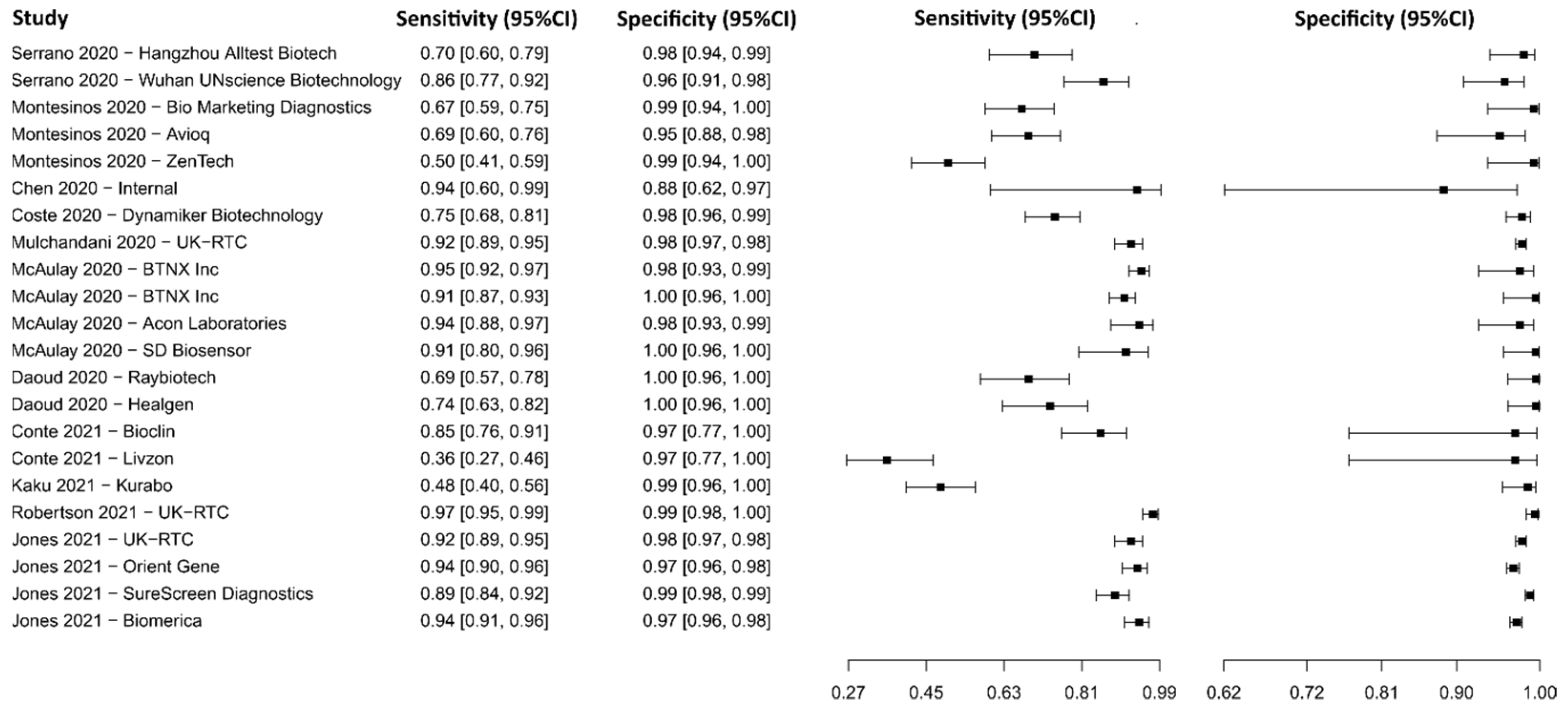
3.2.12. Lateral Flow Immunoassay for IgG
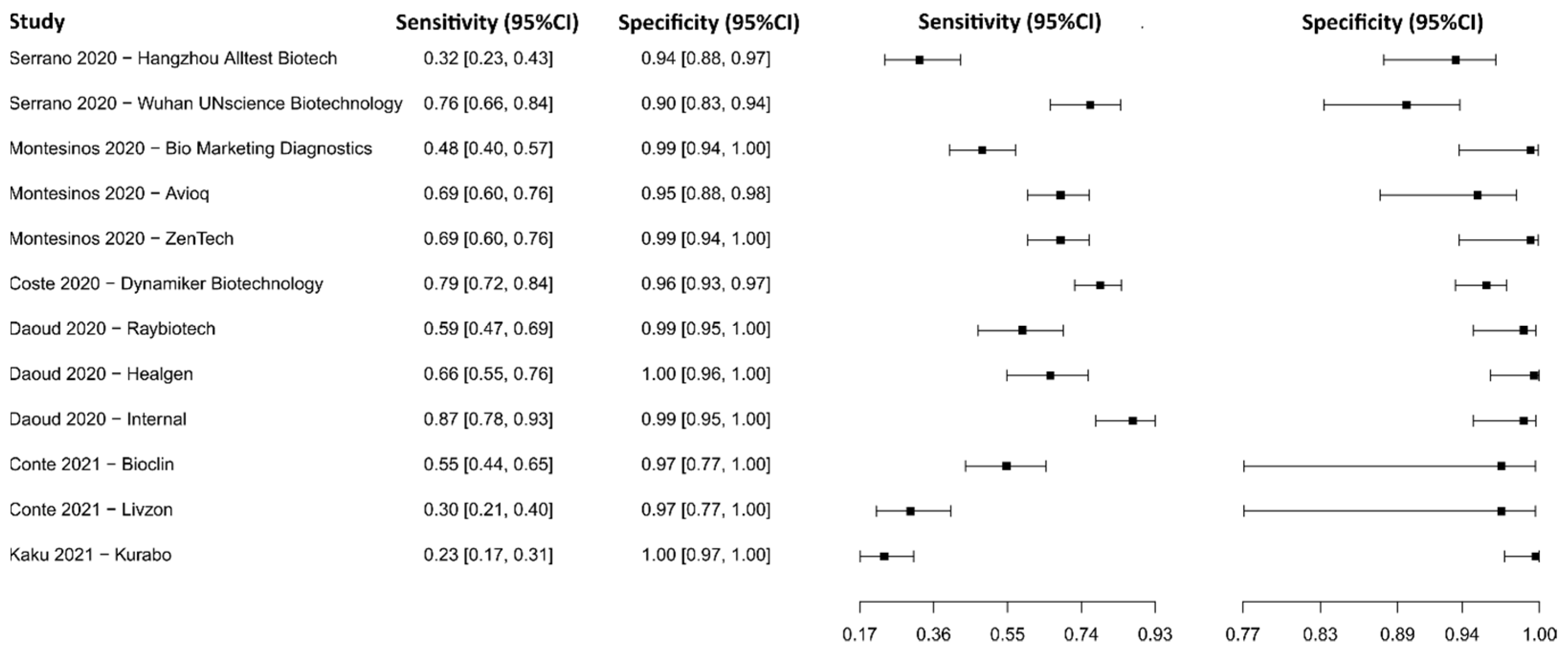
3.2.13. Lateral Flow Immunoassay for IgM
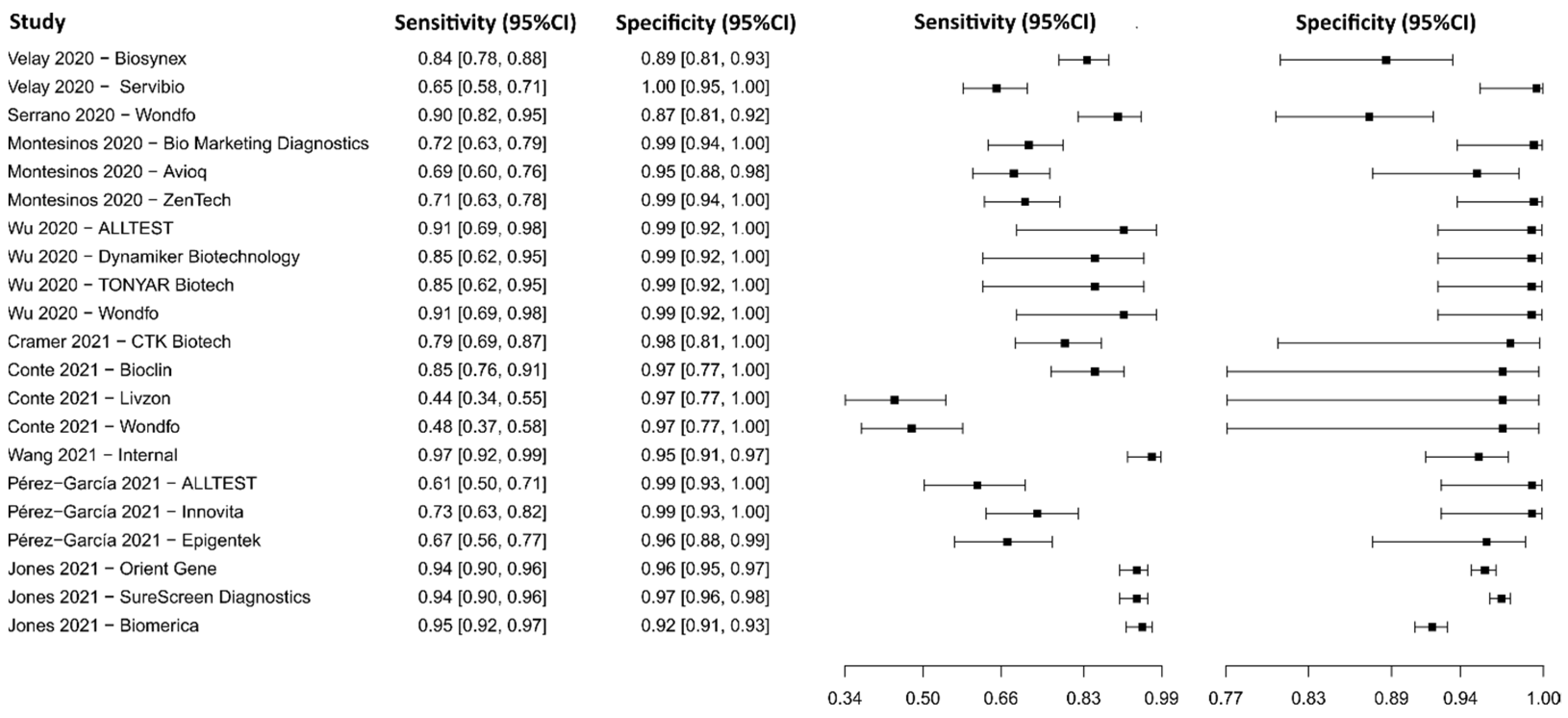
3.2.14. Lateral Flow Immunoassay for IgM-IgG
3.2.15. Lateral Flow Immunoassay for N Protein
3.2.16. Chemiluminescent Microparticle Immunoassay
3.2.17. Fluorescence Immunoassay
3.2.18. Other Techniques
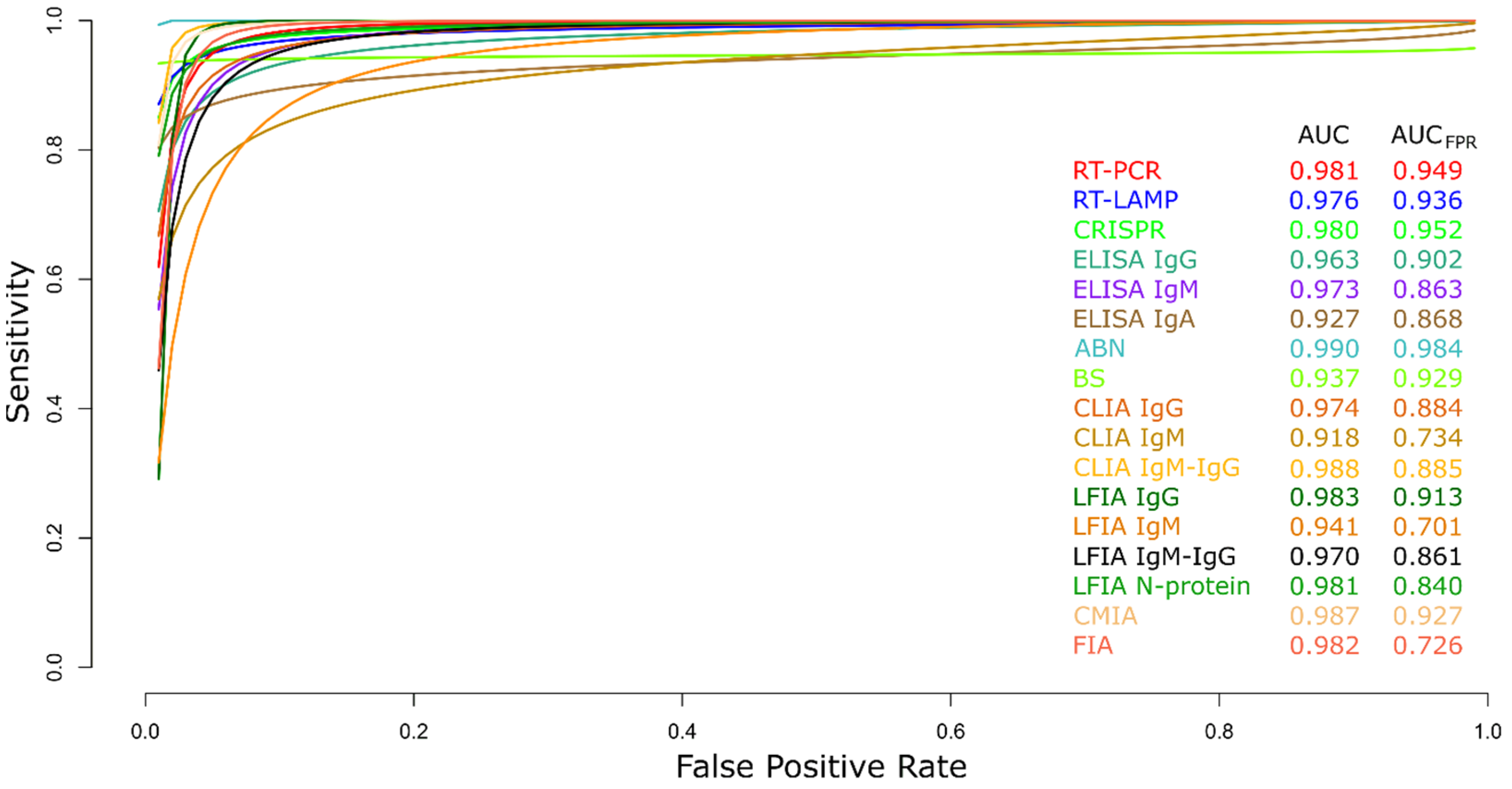
3.2.19. Summary ROC Curves (sROC)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE2 | Angiotensin-converting enzyme 2 |
| ANB | Antiviral neutralization bioassay |
| AUC | Area Under the Curve |
| AUCFPR | Area Under the Curve Restricted to The False Positive Rates |
| BS | Biosensors |
| CI | Confidence interval |
| CLIA | Chemiluminescence immunoassay |
| CMIA | Chemiluminescent microparticle immunoassay |
| COVID-19 | Coronavirus disease |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| DOR | Diagnostic Likelihood Ratio |
| ECLIA | Electrochemiluminescence immunoassay |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| FIA | Fluorescence immunoassay |
| FN | False negatives |
| FP | False positives |
| IgA | Immunoglobulin A |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| INPLASY | International Platform of Registered Systematic Review and Meta-analysis Protocols |
| LFIA | Lateral flow immunoassay |
| LR− | Negative Likelihood Ratio |
| LR+ | Positive Likelihood Ratio |
| MA | Microarrays |
| MeSH | Medical Subject Headings |
| NCBI | National Center for Biotechnology Information |
| NGS | Next-generation sequencing |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PRNT | Plaque Reduction Neutralization Test |
| ROC | Receiver Operating Characteristics |
| RT-LAMP | Reverse transcriptase loop-mediated isothermal amplification |
| RT-PCR | Reverse transcription–polymerase chain reaction |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| Se | Sensibility |
| Sp | Specificity |
| sROC | Summary Receiver Operating Characteristics |
| TN | True negatives |
| TP | True positives |
| WHO | World Health Organization |
References
- Goh, G.K.-M.; Dunker, A.K.; Foster, J.A.; Uversky, V.N. Computational, Experimental, and Clinical Evidence of a Specific but Peculiar Evolutionary Nature of (COVID-19) SARS-CoV-2. J. Proteome Res. 2022, 21, 874–890. [Google Scholar] [CrossRef] [PubMed]
- Labib, S.M.; Browning, M.H.E.M.; Rigolon, A.; Helbich, M.; James, P. Nature’s Contributions in Coping with a Pandemic in the 21st Century: A Narrative Review of Evidence during COVID-19. Sci. Total Environ. 2022, 833, 155095. [Google Scholar] [CrossRef] [PubMed]
- Stiti, M.; Castanet, G.; Corber, A.; Alden, M.; Berrocal, E. Transition from Saliva Droplets to Solid Aerosols in the Context of COVID-19 Spreading. Environ. Res. 2022, 204, 112072. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Sun, J.; Cao, X.; Wang, X.; Chen, X.; Xu, H.; Zhao, J.; Fu, Y.-X.; Peng, H. Non-Adjuvanted Interferon-Armed RBD Protein Nasal Drops Protect Airway Infection from SARS-CoV-2. Cell Discov. 2022, 8, 43. [Google Scholar] [CrossRef]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 13 February 2023).
- Ranjan, P.; Singhal, A.; Yadav, S.; Kumar, N.; Murali, S.; Sanghi, S.K.; Khan, R. Rapid Diagnosis of SARS-CoV-2 Using Potential Point-of-Care Electrochemical Immunosensor: Toward the Future Prospects. Int. Rev. Immunol. 2021, 40, 126–142. [Google Scholar] [CrossRef]
- Xu, H.; Xie, C.; Li, P.; Ji, Z.; Sun, J.; Hu, B.; Li, X.; Fang, M. Demographic, Virological Characteristics and Prognosis of Asymptomatic COVID-19 Patients in South China. Front. Med. 2022, 9, 830942. [Google Scholar] [CrossRef]
- Sah, P.; Fitzpatrick, M.C.; Zimmer, C.F.; Abdollahi, E.; Juden-Kelly, L.; Moghadas, S.M.; Singer, B.H.; Galvani, A.P. Asymptomatic SARS-CoV-2 Infection: A Systematic Review and Meta-Analysis. Proc. Natl. Acad. Sci. USA 2021, 118, e2109229118. [Google Scholar] [CrossRef]
- Bartsch, S.M.; Ferguson, M.C.; McKinnell, J.A.; O’Shea, K.J.; Wedlock, P.T.; Siegmund, S.S.; Lee, B.Y. The Potential Health Care Costs and Resource Use Associated With COVID-19 In The United States. Health Aff. 2020, 39, 927–935. [Google Scholar] [CrossRef]
- Umakanthan, S.; Chattu, V.K.; Ranade, A.V.; Das, D.; Basavarajegowda, A.; Bukelo, M. A Rapid Review of Recent Advances in Diagnosis, Treatment and Vaccination for COVID-19. AIMS Public Health 2021, 8, 137–153. [Google Scholar] [CrossRef]
- SeyedAlinaghi, S.; Afsahi, A.; MohsseniPour, M.; Behnezhad, F.; Salehi, M.; Barzegary, A.; Mirzapour, P.; Mehraeen, E.; Dadras, O. Late Complications of COVID-19; a Systematic Review of Current Evidence. Arch. Acad. Emerg. Med. 2021, 9, e14. [Google Scholar] [CrossRef]
- da Rosa Mesquita, R.; Francelino Silva Junior, L.C.; Santos Santana, F.M.; de Oliveira, T.F.; Campos Alcântara, R.; Monteiro Arnozo, G.; da Silva Filho, E.R.; dos Santos, A.G.G.; da Cunha, E.J.O.; de Aquino, S.H.S.; et al. Clinical Manifestations of COVID-19 in the General Population: Systematic Review. Wien. Klin. Wochenschr. 2021, 133, 377–382. [Google Scholar] [CrossRef]
- Eskian, M.; Rezaei, N. Clinical Manifestations of COVID-19. In Coronavirus Disease—COVID-19; Springer: Heidelberg, Germany, 2021; pp. 179–196. [Google Scholar]
- Badraoui, R.; Alrashedi, M.M.; El-May, M.V.; Bardakci, F. Acute Respiratory Distress Syndrome: A Life Threatening Associated Complication of SARS-CoV-2 Infection Inducing COVID-19. J. Biomol. Struct. Dyn. 2021, 39, 6842–6851. [Google Scholar] [CrossRef]
- Ball, L.; Silva, P.L.; Giacobbe, D.R.; Bassetti, M.; Zubieta-Calleja, G.R.; Rocco, P.R.M.; Pelosi, P. Understanding the Pathophysiology of Typical Acute Respiratory Distress Syndrome and Severe COVID-19. Expert Rev. Respir. Med. 2022, 16, 437–446. [Google Scholar] [CrossRef]
- Rajput, A.; Sajid, M.; Tanvi; Shekhar, C.; Aggarwal, R. Optimal Control Strategies on COVID-19 Infection to Bolster the Efficacy of Vaccination in India. Sci. Rep. 2021, 11, 20124. [Google Scholar] [CrossRef]
- Nguyen, K.H.; Nguyen, K.; Corlin, L.; Allen, J.D.; Chung, M. Changes in COVID-19 Vaccination Receipt and Intention to Vaccinate by Socioeconomic Characteristics and Geographic Area, United States, January 6–March 29, 2021. Ann. Med. 2021, 53, 1419–1428. [Google Scholar] [CrossRef]
- Evans, N.G.; Berger, Z.D.; Phelan, A.L.; Silverman, R.D. COVID-19, Equity, and Inclusiveness. BMJ 2021, 373, n1631. [Google Scholar] [CrossRef]
- Christie, A.; Mbaeyi, S.A.; Walensky, R.P. CDC Interim Recommendations for Fully Vaccinated People. JAMA 2021, 325, 1501. [Google Scholar] [CrossRef]
- Desai, A.D.; Lavelle, M.; Boursiquot, B.C.; Wan, E.Y. Long-Term Complications of COVID-19. Am. J. Physiol.-Cell Physiol. 2022, 322, C1–C11. [Google Scholar] [CrossRef]
- Hanna, G.; Bankler, S.; Schandl, A.; Roël, M.; Hedman, A.; Andersson Franko, M.; Lyngå, P.; Geborek, A.; Joelsson-Alm, E.; Söderberg, M.; et al. The Role of Ventilatory Support for Long-term Outcomes after Critical Infection with COVID-19: A Prospective Cohort Study. Clin. Respir. J. 2022, 16, 63–71. [Google Scholar] [CrossRef]
- Chen, B.; Liu, M.; Huang, C. Current Diagnostic and Therapeutic Strategies for COVID-19. J. Pharm. Anal. 2021, 11, 129–137. [Google Scholar] [CrossRef]
- dos Santos, M.; Ferreira, A.V.F.; da Silva, J.O.; Nogueira, L.M.; Machado, J.M.; Francisco, M.F.C.; da Paz, M.C.; Giunchetti, R.C.; Galdino, A.S. Patents Related to Pathogenic Human Coronaviruses. Recent Pat. Biotechnol. 2021, 15, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Udugama, B.; Kadhiresan, P.; Kozlowski, H.N.; Malekjahani, A.; Osborne, M.; Li, V.Y.C.; Chen, H.; Mubareka, S.; Gubbay, J.B.; Chan, W.C.W. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano 2020, 14, 3822–3835. [Google Scholar] [CrossRef] [PubMed]
- Venter, M.; Richter, K. Towards Effective Diagnostic Assays for COVID-19: A Review. J. Clin. Pathol. 2020, 73, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Tahamtan, A.; Ardebili, A. Real-Time RT-PCR in COVID-19 Detection: Issues Affecting the Results. Expert Rev. Mol. Diagn. 2020, 20, 453–454. [Google Scholar] [CrossRef]
- Dolatshahi, Z.; Nargesi, S.; Sadeghifar, J.; Mezginejad, F.; Jafari, A.; Bazyar, M.; Ghafourian, S.; Sani’ee, N. Economic Evaluation of Laboratory Diagnostic Test Types in COVID-19 Epidemic: A Systematic Review. Int. J. Surg. 2022, 105, 106820. [Google Scholar] [CrossRef]
- Spick, M.; Lewis, H.M.; Wilde, M.J.; Hopley, C.; Huggett, J.; Bailey, M.J. Systematic Review with Meta-Analysis of Diagnostic Test Accuracy for COVID-19 by Mass Spectrometry. Metabolism 2022, 126, 154922. [Google Scholar] [CrossRef]
- Peroni, L.A.; Toscaro, J.M.; Canateli, C.; Tonoli, C.C.C.; de Olivera, R.R.; Benedetti, C.E.; Coimbra, L.D.; Pereira, A.B.; Marques, R.E.; Proença-Modena, J.L.; et al. Serological Testing for COVID-19, Immunological Surveillance, and Exploration of Protective Antibodies. Front. Immunol. 2021, 12, 635701. [Google Scholar] [CrossRef]
- Makoah, N.A.; Tipih, T.; Litabe, M.M.; Brink, M.; Sempa, J.B.; Goedhals, D.; Burt, F.J. A Systematic Review and Meta-Analysis of the Sensitivity of Antibody Tests for the Laboratory Confirmation of COVID-19. Future Virol. 2022, 17, 119–139. [Google Scholar] [CrossRef]
- Guo, J.; Ge, J.; Guo, Y. Recent Advances in Methods for the Diagnosis of Corona Virus Disease 2019. J. Clin. Lab. Anal. 2022, 36, e24178. [Google Scholar] [CrossRef]
- Nuccetelli, M.; Pieri, M.; Gisone, F.; Bernardini, S. Combined Anti-SARS-CoV-2 IgA, IgG, and IgM Detection as a Better Strategy to Prevent Second Infection Spreading Waves. Immunol. Investig. 2022, 51, 233–245. [Google Scholar] [CrossRef]
- Peeling, R.W.; Olliaro, P.L.; Boeras, D.I.; Fongwen, N. Scaling up COVID-19 Rapid Antigen Tests: Promises and Challenges. Lancet Infect. Dis. 2021, 21, e290–e295. [Google Scholar] [CrossRef]
- Khalid, M.F.; Selvam, K.; Jeffry, A.J.N.; Salmi, M.F.; Najib, M.A.; Norhayati, M.N.; Aziah, I. Performance of Rapid Antigen Tests for COVID-19 Diagnosis: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 110. [Google Scholar] [CrossRef]
- Yamayoshi, S.; Sakai-Tagawa, Y.; Koga, M.; Akasaka, O.; Nakachi, I.; Koh, H.; Maeda, K.; Adachi, E.; Saito, M.; Nagai, H.; et al. Comparison of Rapid Antigen Tests for COVID-19. Viruses 2020, 12, 1420. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Fiorini, N.; Leaman, R.; Lipman, D.J.; Lu, Z. How User Intelligence Is Improving PubMed. Nat. Biotechnol. 2018, 36, 937–945. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Citation-Based Clustering of Publications Using CitNetExplorer and VOSviewer. Scientometrics 2017, 111, 1053–1070. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.W.; Choi, S.H.; Huh, J.; Park, S.H. Systematic Review and Meta-Analysis of Studies Evaluating Diagnostic Test Accuracy: A Practical Review for Clinical Researchers-Part II. Statistical Methods of Meta-Analysis. Korean J. Radiol. 2015, 16, 1188. [Google Scholar] [CrossRef]
- Shim, S.R.; Kim, S.-J.; Lee, J. Diagnostic Test Accuracy: Application and Practice Using R Software. Epidemiol. Health 2019, 41, e2019007. [Google Scholar] [CrossRef]
- Shreffler, J.; Huecker, M.R. Diagnostic Testing Accuracy: Sensitivity, Specificity, Predictive Values and Likelihood Ratios. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Huang, Y.; Yin, J.; Samawi, H. Methods Improving the Estimate of Diagnostic Odds Ratio. Commun. Stat. Simul. Comput. 2018, 47, 353–366. [Google Scholar] [CrossRef]
- Reitsma, J.B.; Glas, A.S.; Rutjes, A.W.S.; Scholten, R.J.P.M.; Bossuyt, P.M.; Zwinderman, A.H. Bivariate Analysis of Sensitivity and Specificity Produces Informative Summary Measures in Diagnostic Reviews. J. Clin. Epidemiol. 2005, 58, 982–990. [Google Scholar] [CrossRef]
- Visseaux, B.; Collin, G.; Houhou-Fidouh, N.; Le Hingrat, Q.; Ferré, V.M.; Damond, F.; Ichou, H.; Descamps, D.; Charpentier, C. Evaluation of Three Extraction-Free SARS-CoV-2 RT-PCR Assays: A Feasible Alternative Approach with Low Technical Requirements. J. Virol. Methods 2021, 291, 114086. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.; Garg, A.; Vasanth, S.; Arya, A.K.; Pandey, A.; Tejan, N.; Patel, V.; Singh, V.P. Assessing a Chip Based Rapid RTPCR Test for SARS CoV-2 Detection (TrueNat Assay): A Diagnostic Accuracy Study. PLoS ONE 2021, 16, e0257834. [Google Scholar] [CrossRef] [PubMed]
- Gadkar, V.J.; Goldfarb, D.M.; Young, V.; Watson, N.; Al-Rawahi, G.N.; Srigley, J.A.; Tilley, P. Development and Validation of a New Triplex Real-Time Quantitative Reverse Transcriptase-PCR Assay for the Clinical Detection of SARS-CoV-2. Mol. Cell. Probes 2021, 58, 101744. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; Florez, C.; Castañeda, S.; Ballesteros, N.; Martínez, D.; Castillo, A.; Muñoz, M.; Gomez, S.; Rico, A.; Pardo, L.; et al. Evaluation of the Diagnostic Performance of Nine Commercial RT-PCR Kits for the Detection of SARS-CoV-2 in Colombia. J. Med. Virol. 2021, 93, 5618–5622. [Google Scholar] [CrossRef]
- Fitoussi, F.; Dupont, R.; Tonen-Wolyec, S.; Bélec, L. Performances of the VitaPCRTM SARS-CoV-2 Assay during the Second Wave of the COVID-19 Epidemic in France. J. Med. Virol. 2021, 93, 4351–4357. [Google Scholar] [CrossRef]
- Castineiras, T.M.P.P.; Nascimento, É.R.D.S.; Faffe, D.S.; Galliez, R.M.; Mariani, D.; de Carvalho Leitão, I.; de Melo, M.G.M.; Ferreira, O.C.; Tanuri, A.; UFRJ COVID-19 Working Group. Performance of an Alternative RT-PCR Procedure Using Residual Sample from the PanbioTM Ag COVID-19 Test. Braz. J. Infect. Dis. 2021, 25, 101630. [Google Scholar] [CrossRef]
- Zowawi, H.M.; Alenazi, T.H.; AlOmaim, W.S.; Wazzan, A.; Alsufayan, A.; Hasanain, R.A.; Aldibasi, O.S.; Althawadi, S.; Altamimi, S.A.; Mutabagani, M.; et al. Portable RT-PCR System: A Rapid and Scalable Diagnostic Tool for COVID-19 Testing. J. Clin. Microbiol. 2021, 59, e03004-20. [Google Scholar] [CrossRef]
- Altamimi, A.M.; Obeid, D.A.; Alaifan, T.A.; Taha, M.T.; Alhothali, M.T.; Alzahrani, F.A.; Albarrag, A.M. Assessment of 12 Qualitative RT-PCR Commercial Kits for the Detection of SARS-CoV-2. J. Med. Virol. 2021, 93, 3219–3226. [Google Scholar] [CrossRef]
- Al Suwaidi, H.; Senok, A.; Varghese, R.; Deesi, Z.; Khansaheb, H.; Pokasirakath, S.; Chacko, B.; Abufara, I.; Loney, T.; Alsheikh-Ali, A. Saliva for Molecular Detection of SARS-CoV-2 in School-Age Children. Clin. Microbiol. Infect. 2021, 27, 1330–1335. [Google Scholar] [CrossRef]
- Bustin, S.; Coward, A.; Sadler, G.; Teare, L.; Nolan, T. CoV2-ID, a MIQE-Compliant Sub-20-Min 5-Plex RT-PCR Assay Targeting SARS-CoV-2 for the Diagnosis of COVID-19. Sci. Rep. 2020, 10, 22214. [Google Scholar] [CrossRef]
- Rajh, E.; Šket, T.; Praznik, A.; Sušjan, P.; Šmid, A.; Urbančič, D.; Mlinarič-Raščan, I.; Kogovšek, P.; Demšar, T.; Milavec, M.; et al. Robust Saliva-Based RNA Extraction-Free One-Step Nucleic Acid Amplification Test for Mass SARS-CoV-2 Monitoring. Molecules 2021, 26, 6617. [Google Scholar] [CrossRef]
- Abdullah, M.; Sudrajat, D.G.; Muzellina, V.N.; Kurniawan, J.; Rizka, A.; Utari, A.P.; Pribadi, R.R.; Idrus, M.F.; Yusra, Y.; Meilany, S.; et al. The Value of Anal Swab RT-PCR for COVID-19 Diagnosis in Adult Indonesian Patients. BMJ Open Gastroenterol. 2021, 8, e000590. [Google Scholar] [CrossRef]
- Singh, J.; Yadav, A.K.; Pakhare, A.; Kulkarni, P.; Lokhande, L.; Soni, P.; Dadheech, M.; Gupta, P.; Masarkar, N.; Maurya, A.K.; et al. Comparative Analysis of the Diagnostic Performance of Five Commercial COVID-19 QRT PCR Kits Used in India. Sci. Rep. 2021, 11, 22013. [Google Scholar] [CrossRef]
- Chang, P.-L.; Lin, C.-Y.; Chen, C.-P.; Lin, Y.-C.; Hu, H.-C.; Cheng, S.-H.; Cheng, C.-Y. Clinical Validation of an Automated Reverse Transcription-Insulated Isothermal PCR Assay for the Detection of Severe Acute Respiratory Syndrome Coronavirus 2. J. Microbiol. Immunol. Infect. 2021, 54, 522–526. [Google Scholar] [CrossRef]
- Visseaux, B.; Le Hingrat, Q.; Collin, G.; Ferré, V.; Storto, A.; Ichou, H.; Bouzid, D.; Poey, N.; de Montmollin, E.; Descamps, D.; et al. Evaluation of the RealStar® SARS-CoV-2 RT-PCR Kit RUO Performances and Limit of Detection. J. Clin. Virol. 2020, 129, 104520. [Google Scholar] [CrossRef]
- Rödel, J.; Egerer, R.; Suleyman, A.; Sommer-Schmid, B.; Baier, M.; Henke, A.; Edel, B.; Löffler, B. Use of the VariplexTM SARS-CoV-2 RT-LAMP as a Rapid Molecular Assay to Complement RT-PCR for COVID-19 Diagnosis. J. Clin. Virol. 2020, 132, 104616. [Google Scholar] [CrossRef]
- Hu, X.; Deng, Q.; Li, J.; Chen, J.; Wang, Z.; Zhang, X.; Fang, Z.; Li, H.; Zhao, Y.; Yu, P.; et al. Development and Clinical Application of a Rapid and Sensitive Loop-Mediated Isothermal Amplification Test for SARS-CoV-2 Infection. mSphere 2020, 5, e00808-20. [Google Scholar] [CrossRef]
- Lee, J.Y.H.; Best, N.; McAuley, J.; Porter, J.L.; Seemann, T.; Schultz, M.B.; Sait, M.; Orlando, N.; Mercoulia, K.; Ballard, S.A.; et al. Validation of a Single-Step, Single-Tube Reverse Transcription Loop-Mediated Isothermal Amplification Assay for Rapid Detection of SARS-CoV-2 RNA. J. Med. Microbiol. 2020, 69, 1169–1178. [Google Scholar] [CrossRef]
- Jiang, M.; Pan, W.; Arasthfer, A.; Fang, W.; Ling, L.; Fang, H.; Daneshnia, F.; Yu, J.; Liao, W.; Pei, H.; et al. Development and Validation of a Rapid, Single-Step Reverse Transcriptase Loop-Mediated Isothermal Amplification (RT-LAMP) System Potentially to Be Used for Reliable and High-Throughput Screening of COVID-19. Front. Cell. Infect. Microbiol. 2020, 10, 331. [Google Scholar] [CrossRef]
- Dao Thi, V.L.; Herbst, K.; Boerner, K.; Meurer, M.; Kremer, L.P.; Kirrmaier, D.; Freistaedter, A.; Papagiannidis, D.; Galmozzi, C.; Stanifer, M.L.; et al. A Colorimetric RT-LAMP Assay and LAMP-Sequencing for Detecting SARS-CoV-2 RNA in Clinical Samples. Sci. Transl. Med. 2020, 12, eabc7075. [Google Scholar] [CrossRef]
- Lu, R.; Wu, X.; Wan, Z.; Li, Y.; Jin, X.; Zhang, C. A Novel Reverse Transcription Loop-Mediated Isothermal Amplification Method for Rapid Detection of SARS-CoV-2. Int. J. Mol. Sci. 2020, 21, 2826. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Berghaus, S.; Blessing, F.; Wenzel, F.; Herbeck, H.; Blessing, J.; Schierack, P.; Rödiger, S.; Roggenbuck, D. A Semi-Automated, Isolation-Free, High-Throughput SARS-CoV-2 Reverse Transcriptase (RT) Loop-Mediated Isothermal Amplification (LAMP) Test. Sci. Rep. 2021, 11, 21385. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, Y.; Chen, Y.; Yang, Z.; Wu, H.; Zhou, Z.; Li, J.; Ping, J.; He, L.; Shen, H.; et al. Contamination-Free Visual Detection of SARS-CoV-2 with CRISPR/Cas12a: A Promising Method in the Point-of-Care Detection. Biosens. Bioelectron. 2020, 169, 112642. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Zeng, W.; Yang, M.; Chen, W.; Ren, L.; Ai, J.; Wu, J.; Liao, Y.; Gou, X.; Li, Y.; et al. Development and Evaluation of a Rapid CRISPR-Based Diagnostic for COVID-19. PLoS Pathog. 2020, 16, e1008705. [Google Scholar] [CrossRef]
- Xiong, E.; Jiang, L.; Tian, T.; Hu, M.; Yue, H.; Huang, M.; Lin, W.; Jiang, Y.; Zhu, D.; Zhou, X. Simultaneous Dual-Gene Diagnosis of SARS-CoV-2 Based on CRISPR/Cas9-Mediated Lateral Flow Assay. Angew. Chem. Int. Ed. Engl. 2021, 60, 5307–5315. [Google Scholar] [CrossRef]
- Li, S.; Huang, J.; Ren, L.; Jiang, W.; Wang, M.; Zhuang, L.; Zheng, Q.; Yang, R.; Zeng, Y.; Luu, L.D.W.; et al. A One-Step, One-Pot CRISPR Nucleic Acid Detection Platform (CRISPR-Top): Application for the Diagnosis of COVID-19. Talanta 2021, 233, 122591. [Google Scholar] [CrossRef]
- Curti, L.A.; Primost, I.; Valla, S.; Ibañez Alegre, D.; Olguin Perglione, C.; Repizo, G.D.; Lara, J.; Parcerisa, I.; Palacios, A.; Llases, M.E.; et al. Evaluation of a Lyophilized CRISPR-Cas12 Assay for a Sensitive, Specific, and Rapid Detection of SARS-CoV-2. Viruses 2021, 13, 420. [Google Scholar] [CrossRef]
- Nimsamer, P.; Mayuramart, O.; Rattanaburi, S.; Chantaravisoot, N.; Saengchoowong, S.; Puenpa, J.; Poovorawan, Y.; Payungporn, S. Comparative Performance of CRISPR-Cas12a Assays for SARS-CoV-2 Detection Tested with RNA Extracted from Clinical Specimens. J. Virol. Methods 2021, 290, 114092. [Google Scholar] [CrossRef]
- Mayuramart, O.; Nimsamer, P.; Rattanaburi, S.; Chantaravisoot, N.; Khongnomnan, K.; Chansaenroj, J.; Puenpa, J.; Suntronwong, N.; Vichaiwattana, P.; Poovorawan, Y.; et al. Detection of Severe Acute Respiratory Syndrome Coronavirus 2 and Influenza Viruses Based on CRISPR-Cas12a. Exp. Biol. Med. 2021, 246, 400–405. [Google Scholar] [CrossRef]
- Sapkal, G.; Shete-Aich, A.; Jain, R.; Yadav, P.D.; Sarkale, P.; Lakra, R.; Baradkar, S.; Deshpande, G.R.; Mali, D.; Tilekar, B.N.; et al. Development of Indigenous IgG ELISA for the Detection of Anti-SARS-CoV-2 IgG. Indian J. Med. Res. 2020, 151, 444–449. [Google Scholar] [CrossRef]
- Tré-Hardy, M.; Wilmet, A.; Beukinga, I.; Favresse, J.; Dogné, J.-M.; Douxfils, J.; Blairon, L. Analytical and Clinical Validation of an ELISA for Specific SARS-CoV-2 IgG, IgA, and IgM Antibodies. J. Med. Virol. 2021, 93, 803–811. [Google Scholar] [CrossRef]
- Serrano, M.M.; Rodríguez, D.N.; Palop, N.T.; Arenas, R.O.; Córdoba, M.M.; Mochón, M.D.O.; Cardona, C.G. Comparison of Commercial Lateral Flow Immunoassays and ELISA for SARS-CoV-2 Antibody Detection. J. Clin. Virol. 2020, 129, 104529. [Google Scholar] [CrossRef]
- Krüttgen, A.; Cornelissen, C.G.; Dreher, M.; Hornef, M.; Imöhl, M.; Kleines, M. Comparison of Four New Commercial Serologic Assays for Determination of SARS-CoV-2 IgG. J. Clin. Virol. 2020, 128, 104394. [Google Scholar] [CrossRef]
- Montesinos, I.; Gruson, D.; Kabamba, B.; Dahma, H.; Van den Wijngaert, S.; Reza, S.; Carbone, V.; Vandenberg, O.; Gulbis, B.; Wolff, F.; et al. Evaluation of Two Automated and Three Rapid Lateral Flow Immunoassays for the Detection of Anti-SARS-CoV-2 Antibodies. J. Clin. Virol. 2020, 128, 104413. [Google Scholar] [CrossRef]
- Krüttgen, A.; Cornelissen, C.G.; Dreher, M.; Hornef, M.W.; Imöhl, M.; Kleines, M. Determination of SARS-CoV-2 Antibodies with Assays from Diasorin, Roche and IDvet. J. Virol. Methods 2021, 287, 113978. [Google Scholar] [CrossRef]
- Coste, A.T.; Jaton, K.; Papadimitriou-Olivgeris, M.; Greub, G.; Croxatto, A. Comparison of SARS-CoV-2 Serological Tests with Different Antigen Targets. J. Clin. Virol. 2021, 134, 104690. [Google Scholar] [CrossRef]
- Mutantu, P.N.; Ngwe Tun, M.M.; Nabeshima, T.; Yu, F.; Mukadi, P.K.; Tanaka, T.; Tashiro, M.; Fujita, A.; Kanie, N.; Oshiro, R.; et al. Development and Evaluation of Quantitative Immunoglobulin G Enzyme-Linked Immunosorbent Assay for the Diagnosis of Coronavirus Disease 2019 Using Truncated Recombinant Nucleocapsid Protein as Assay Antigen. Int. J. Environ. Res. Public Health 2021, 18, 9630. [Google Scholar] [CrossRef]
- Barchuk, A.; Shirokov, D.; Sergeeva, M.; Tursun Zade, R.; Dudkina, O.; Tychkova, V.; Barabanova, L.; Skougarevskiy, D.; Danilenko, D. Evaluation of the Performance of SARS-CoV-2 Antibody Assays for a Longitudinal Population-Based Study of COVID-19 Spread in St. Petersburg, Russia. J. Med. Virol. 2021, 93, 5846–5852. [Google Scholar] [CrossRef]
- Sil, B.K.; Jahan, N.; Haq, M.A.; Oishee, M.J.; Ali, T.; Khandker, S.S.; Kobatake, E.; Mie, M.; Khondoker, M.U.; Jamiruddin, M.R.; et al. Development and Performance Evaluation of a Rapid In-House ELISA for Retrospective Serosurveillance of SARS-CoV-2. PLoS ONE 2021, 16, e0246346. [Google Scholar] [CrossRef]
- Tozetto-Mendoza, T.R.; Kanunfre, K.A.; Vilas-Boas, L.S.; Sanchez Espinoza, E.P.; Paião, H.G.O.; Rocha, M.C.; de Paula, A.V.; de Oliveira, M.S.; Zampelli, D.B.; Vieira, J.M.; et al. Nucleoprotein-Based ELISA for Detection of SARS-COV-2 IgG Antibodies: Could an Old Assay Be Suitable for Serodiagnosis of the New Coronavirus? J. Virol. Methods 2021, 290, 114064. [Google Scholar] [CrossRef]
- Zhou, C.; Bu, G.; Sun, Y.; Ren, C.; Qu, M.; Gao, Y.; Zhu, Y.; Wang, L.; Sun, L.; Liu, Y. Evaluation of Serum IgM and IgG Antibodies in COVID-19 Patients by Enzyme Linked Immunosorbent Assay. J. Med. Virol. 2021, 93, 2857–2866. [Google Scholar] [CrossRef] [PubMed]
- Cramer, A.; Goodman, N.; Cross, T.; Gant, V.; Dziadzio, M. Analytical Evaluation and Critical Appraisal of Early Commercial SARS-CoV-2 Immunoassays for Routine Use in a Diagnostic Laboratory. Br. J. Biomed. Sci. 2021, 78, 141–146. [Google Scholar] [CrossRef] [PubMed]
- MacMullan, M.A.; Ibrayeva, A.; Trettner, K.; Deming, L.; Das, S.; Tran, F.; Moreno, J.R.; Casian, J.G.; Chellamuthu, P.; Kraft, J.; et al. ELISA Detection of SARS-CoV-2 Antibodies in Saliva. Sci. Rep. 2020, 10, 20818. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Chen, Z.; Zhou, J.; Tian, D.; Ran, X.; Zhang, Z.; Shi, S.; Xiao, D.; Zhou, Y. Comparative Analysis of the Main Haematological Indexes and RNA Detection for the Diagnosis of SARS-CoV-2 Infection. BMC Infect. Dis. 2020, 20, 779. [Google Scholar] [CrossRef] [PubMed]
- Rychert, J.; Couturier, M.R.; Elgort, M.; Lozier, B.K.; La’ulu, S.; Genzen, J.R.; Straseski, J.A.; Delgado, J.C.; Slev, P.R. Evaluation of 3 SARS-CoV-2 IgG Antibody Assays and Correlation with Neutralizing Antibodies. J. Appl. Lab. Med. 2021, 6, 614–624. [Google Scholar] [CrossRef]
- Indenbaum, V.; Koren, R.; Katz-Likvornik, S.; Yitzchaki, M.; Halpern, O.; Regev-Yochay, G.; Cohen, C.; Biber, A.; Feferman, T.; Cohen Saban, N.; et al. Testing IgG Antibodies against the RBD of SARS-CoV-2 Is Sufficient and Necessary for COVID-19 Diagnosis. PLoS ONE 2020, 15, e0241164. [Google Scholar] [CrossRef]
- Velay, A.; Gallais, F.; Benotmane, I.; Wendling, M.J.; Danion, F.; Collange, O.; De Sèze, J.; Schmidt-Mutter, C.; Schneider, F.; Bilbault, P.; et al. Evaluation of the Performance of SARS-CoV-2 Serological Tools and Their Positioning in COVID-19 Diagnostic Strategies. Diagn Microbiol. Infect. Dis. 2020, 98, 115181. [Google Scholar] [CrossRef]
- Nandakumar, V.; Profaizer, T.; Lozier, B.K.; Elgort, M.G.; Larragoite, E.T.; Williams, E.S.C.P.; Solis-Leal, A.; Lopez, J.B.; Berges, B.K.; Planelles, V.; et al. Evaluation of a Surrogate Enzyme-Linked Immunosorbent Assay-Based Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) CPass Neutralization Antibody Detection Assay and Correlation with Immunoglobulin G Commercial Serology Assays. Arch. Pathol. Lab. Med. 2021, 145, 1212–1220. [Google Scholar] [CrossRef]
- Perera, R.A.P.M.; Ko, R.; Tsang, O.T.Y.; Hui, D.S.C.; Kwan, M.Y.M.; Brackman, C.J.; To, E.M.W.; Yen, H.-L.; Leung, K.; Cheng, S.M.S.; et al. Evaluation of a SARS-CoV-2 Surrogate Virus Neutralization Test for Detection of Antibody in Human, Canine, Cat, and Hamster Sera. J. Clin. Microbiol. 2021, 59, e02504-20. [Google Scholar] [CrossRef]
- Putcharoen, O.; Wacharapluesadee, S.; Chia, W.N.; Paitoonpong, L.; Tan, C.W.; Suwanpimolkul, G.; Jantarabenjakul, W.; Ruchisrisarod, C.; Wanthong, P.; Sophonphan, J.; et al. Early Detection of Neutralizing Antibodies against SARS-CoV-2 in COVID-19 Patients in Thailand. PLoS ONE 2021, 16, e0246864. [Google Scholar] [CrossRef]
- Irsara, C.; Egger, A.E.; Prokop, W.; Nairz, M.; Loacker, L.; Sahanic, S.; Pizzini, A.; Sonnweber, T.; Holzer, B.; Mayer, W.; et al. Clinical Validation of the Siemens Quantitative SARS-CoV-2 Spike IgG Assay (SCOVG) Reveals Improved Sensitivity and a Good Correlation with Virus Neutralization Titers. Clin. Chem. Lab. Med. 2021, 59, 1453–1462. [Google Scholar] [CrossRef]
- Thompson, C.P.; Grayson, N.E.; Paton, R.S.; Bolton, J.S.; Lourenço, J.; Penman, B.S.; Lee, L.N.; Odon, V.; Mongkolsapaya, J.; Chinnakannan, S.; et al. Detection of Neutralising Antibodies to SARS-CoV-2 to Determine Population Exposure in Scottish Blood Donors between March and May 2020. Eurosurveillance 2020, 25, 2000685. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, X.; Han, L.; Chen, T.; Wang, L.; Li, H.; Li, S.; He, L.; Fu, X.; Chen, S.; et al. Multiplex Reverse Transcription Loop-Mediated Isothermal Amplification Combined with Nanoparticle-Based Lateral Flow Biosensor for the Diagnosis of COVID-19. Biosens. Bioelectron. 2020, 166, 112437. [Google Scholar] [CrossRef]
- Büyüksünetçi, Y.T.; Çitil, B.E.; Tapan, U.; Anık, Ü. Development and Application of a SARS-CoV-2 Colorimetric Biosensor Based on the Peroxidase-Mimic Activity of γ-Fe2O3 Nanoparticles. Mikrochim. Acta 2021, 188, 335. [Google Scholar] [CrossRef]
- Mavrikou, S.; Tsekouras, V.; Hatziagapiou, K.; Paradeisi, F.; Bakakos, P.; Michos, A.; Koutsoukou, A.; Konstantellou, E.; Lambrou, G.I.; Koniari, E.; et al. Clinical Application of the Novel Cell-Based Biosensor for the Ultra-Rapid Detection of the SARS-CoV-2 S1 Spike Protein Antigen: A Practical Approach. Biosensors 2021, 11, 224. [Google Scholar] [CrossRef]
- Liv, L.; Çoban, G.; Nakiboğlu, N.; Kocagöz, T. A Rapid, Ultrasensitive Voltammetric Biosensor for Determining SARS-CoV-2 Spike Protein in Real Samples. Biosens. Bioelectron. 2021, 192, 113497. [Google Scholar] [CrossRef]
- Apostolou, T.; Kyritsi, M.; Vontas, A.; Loizou, K.; Hadjilouka, A.; Speletas, M.; Mouchtouri, V.; Hadjichristodoulou, C. Development and Performance Characteristics Evaluation of a New Bioelectric Recognition Assay (BERA) Method for Rapid Sars-CoV-2 Detection in Clinical Samples. J. Virol. Methods 2021, 293, 114166. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, X.; Li, S.; Luo, W.; Zhang, X.; Wang, C.; Chen, Q.; Yu, S.; Tai, J.; Wang, Y. Rapid, Ultrasensitive, and Highly Specific Diagnosis of COVID-19 by CRISPR-Based Detection. ACS Sens. 2021, 6, 881–888. [Google Scholar] [CrossRef]
- Chaibun, T.; Puenpa, J.; Ngamdee, T.; Boonapatcharoen, N.; Athamanolap, P.; O’Mullane, A.P.; Vongpunsawad, S.; Poovorawan, Y.; Lee, S.Y.; Lertanantawong, B. Rapid Electrochemical Detection of Coronavirus SARS-CoV-2. Nat. Commun. 2021, 12, 802. [Google Scholar] [CrossRef]
- Soleimani, R.; Khourssaji, M.; Gruson, D.; Rodriguez-Villalobos, H.; Berghmans, M.; Belkhir, L.; Yombi, J.-C.; Kabamba-Mukadi, B. Clinical Usefulness of Fully Automated Chemiluminescent Immunoassay for Quantitative Antibody Measurements in COVID-19 Patients. J. Med. Virol. 2021, 93, 1465–1477. [Google Scholar] [CrossRef]
- Lin, D.; Liu, L.; Zhang, M.; Hu, Y.; Yang, Q.; Guo, J.; Dai, Y.; Xu, Y.; Cai, Y.; Chen, X.; et al. Evaluations of the Serological Test in the Diagnosis of 2019 Novel Coronavirus (SARS-CoV-2) Infections during the COVID-19 Outbreak. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2271–2277. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Shin, S.; Nam, M.; Hong, Y.J.; Roh, E.Y.; Park, K.U.; Song, E.Y. Performance Evaluation of Three Automated Quantitative Immunoassays and Their Correlation with a Surrogate Virus Neutralization Test in Coronavirus Disease 19 Patients and Pre-Pandemic Controls. J. Clin. Lab. Anal. 2021, 35, e23921. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Barakauskas, V.E.; Simons, J.; Cook, D.; Bates, B.; Burns, L.; Masud, S.; Charles, M.; McLennan, M.; Mak, A.; et al. SARS-CoV-2 Serology: Validation of High-Throughput Chemiluminescent Immunoassay (CLIA) Platforms and a Field Study in British Columbia. J. Clin. Virol. 2021, 142, 104914. [Google Scholar] [CrossRef] [PubMed]
- Zonneveld, R.; Jurriaans, S.; van Gool, T.; Hofstra, J.J.; Hekker, T.A.M.; Defoer, P.; Broekhuizen-van Haaften, P.E.; Wentink-Bonnema, E.M.; Boonkamp, L.; Teunissen, C.E.; et al. Head-to-Head Validation of Six Immunoassays for SARS-CoV-2 in Hospitalized Patients. J. Clin. Virol. 2021, 139, 104821. [Google Scholar] [CrossRef]
- Pérez-García, F.; Pérez-Tanoira, R.; Iglesias, M.E.; Romanyk, J.; Arroyo, T.; Gómez-Herruz, P.; González, R.; Lapeña García, S.; Cuadros-González, J. Comparative Evaluation of Six Immunoassays for the Detection of Antibodies against SARS-CoV-2. J. Virol. Methods 2021, 289, 114047. [Google Scholar] [CrossRef]
- Jones, H.E.; Mulchandani, R.; Taylor-Phillips, S.; Ades, A.E.; Shute, J.; Perry, K.R.; Chandra, N.L.; Brooks, T.; Charlett, A.; Hickman, M.; et al. Accuracy of Four Lateral Flow Immunoassays for Anti SARS-CoV-2 Antibodies: A Head-to-Head Comparative Study. EBioMedicine 2021, 68, 103414. [Google Scholar] [CrossRef]
- Robertson, L.J.; Moore, J.S.; Blighe, K.; Ng, K.Y.; Quinn, N.; Jennings, F.; Warnock, G.; Sharpe, P.; Clarke, M.; Maguire, K.; et al. Evaluation of the IgG Antibody Response to SARS CoV-2 Infection and Performance of a Lateral Flow Immunoassay: Cross-Sectional and Longitudinal Analysis over 11 Months. BMJ Open 2021, 11, e048142. [Google Scholar] [CrossRef]
- Kaku, N.; Nishimura, F.; Shigeishi, Y.; Tachiki, R.; Sakai, H.; Sasaki, D.; Ota, K.; Sakamoto, K.; Kosai, K.; Hasegawa, H.; et al. Performance of Anti-SARS-CoV-2 Antibody Testing in Asymptomatic or Mild COVID-19 Patients: A Retrospective Study in Outbreak on a Cruise Ship. PLoS ONE 2021, 16, e0257452. [Google Scholar] [CrossRef]
- Daoud, Z.; McLeod, J.; Stockman, D.L. Higher Sensitivity Provided by the Combination of Two Lateral Flow Immunoassay Tests for the Detection of COVID-19 Immunoglobulins. Front. Cell. Infect. Microbiol. 2020, 10, 479. [Google Scholar] [CrossRef]
- McAulay, K.; Bryan, A.; Greninger, A.L.; Grill, F.; Lake, D.; Kaleta, E.J.; Grys, T.E. Retrospective Clinical Evaluation of 4 Lateral Flow Assays for the Detection of SARS-CoV-2 IgG. Diagn. Microbiol. Infect. Dis. 2020, 98, 115161. [Google Scholar] [CrossRef]
- Mulchandani, R.; Jones, H.E.; Taylor-Phillips, S.; Shute, J.; Perry, K.; Jamarani, S.; Brooks, T.; Charlett, A.; Hickman, M.; Oliver, I.; et al. Accuracy of UK Rapid Test Consortium (UK-RTC) “AbC-19 Rapid Test” for Detection of Previous SARS-CoV-2 Infection in Key Workers: Test Accuracy Study. BMJ 2020, 371, m4262. [Google Scholar] [CrossRef]
- Conte, D.D.; Carvalho, J.M.A.; de Souza Luna, L.K.; Faíco-Filho, K.S.; Perosa, A.H.; Bellei, N. Comparative Analysis of Three Point-of-Care Lateral Flow Immunoassays for Detection of Anti-SARS-CoV-2 Antibodies: Data from 100 Healthcare Workers in Brazil. Braz. J. Microbiol. 2021, 52, 1161–1165. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Z.; Zhai, X.; Li, Y.; Lin, L.; Zhao, H.; Bian, L.; Li, P.; Yu, L.; Wu, Y.; et al. Rapid and Sensitive Detection of Anti-SARS-CoV-2 IgG, Using Lanthanide-Doped Nanoparticles-Based Lateral Flow Immunoassay. Anal. Chem. 2020, 92, 7226–7231. [Google Scholar] [CrossRef]
- Wang, C.; Shi, D.; Wan, N.; Yang, X.; Liu, H.; Gao, H.; Zhang, M.; Bai, Z.; Li, D.; Dai, E.; et al. Development of Spike Protein-Based Fluorescence Lateral Flow Assay for the Simultaneous Detection of SARS-CoV-2 Specific IgM and IgG. Analyst 2021, 146, 3908–3917. [Google Scholar] [CrossRef]
- Wu, J.-L.; Tseng, W.-P.; Lin, C.-H.; Lee, T.-F.; Chung, M.-Y.; Huang, C.-H.; Chen, S.-Y.; Hsueh, P.-R.; Chen, S.-C. Four Point-of-Care Lateral Flow Immunoassays for Diagnosis of COVID-19 and for Assessing Dynamics of Antibody Responses to SARS-CoV-2. J. Infect. 2020, 81, 435–442. [Google Scholar] [CrossRef]
- Cerutti, F.; Burdino, E.; Milia, M.G.; Allice, T.; Gregori, G.; Bruzzone, B.; Ghisetti, V. Urgent Need of Rapid Tests for SARS CoV-2 Antigen Detection: Evaluation of the SD-Biosensor Antigen Test for SARS-CoV-2. J. Clin. Virol. 2020, 132, 104654. [Google Scholar] [CrossRef]
- Albert, E.; Torres, I.; Bueno, F.; Huntley, D.; Molla, E.; Fernández-Fuentes, M.Á.; Martínez, M.; Poujois, S.; Forqué, L.; Valdivia, A.; et al. Field Evaluation of a Rapid Antigen Test (PanbioTM COVID-19 Ag Rapid Test Device) for COVID-19 Diagnosis in Primary Healthcare Centres. Clin. Microbiol. Infect. 2021, 27, 472.e7–472.e10. [Google Scholar] [CrossRef]
- Pérez-García, F.; Romanyk, J.; Moya Gutiérrez, H.; Labrador Ballestero, A.; Pérez Ranz, I.; González Arroyo, J.; González Ventosa, V.; Pérez-Tanoira, R.; Domingo Cruz, C.; Cuadros-González, J. Comparative Evaluation of Panbio and SD Biosensor Antigen Rapid Diagnostic Tests for COVID-19 Diagnosis. J. Med. Virol. 2021, 93, 5650–5654. [Google Scholar] [CrossRef]
- Eleftheriou, I.; Dasoula, F.; Dimopoulou, D.; Lebessi, E.; Serafi, E.; Spyridis, N.; Tsolia, M. Real-Life Evaluation of a COVID-19 Rapid Antigen Detection Test in Hospitalized Children. J. Med. Virol. 2021, 93, 6040–6044. [Google Scholar] [CrossRef]
- Igloi, Z.; Velzing, J.; van Beek, J.; van de Vijver, D.; Aron, G.; Ensing, R.; Benschop, K.; Han, W.; Boelsums, T.; Koopmans, M.; et al. Clinical Evaluation of Roche SD Biosensor Rapid Antigen Test for SARS-CoV-2 in Municipal Health Service Testing Site, the Netherlands. Emerg. Infect. Dis. 2021, 27, 1323–1329. [Google Scholar] [CrossRef]
- Kurihara, Y.; Kiyasu, Y.; Akashi, Y.; Takeuchi, Y.; Narahara, K.; Mori, S.; Takeshige, T.; Notake, S.; Ueda, A.; Nakamura, K.; et al. The Evaluation of a Novel Digital Immunochromatographic Assay with Silver Amplification to Detect SARS-CoV-2. J. Infect. Chemother. 2021, 27, 1493–1497. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, J.; Bal, J.; Seo, S.K.; Chong, C.-K.; Lee, J.H.; Park, H. Development and Clinical Evaluation of an Immunochromatography-Based Rapid Antigen Test (GenBodyTM COVAG025) for COVID-19 Diagnosis. Viruses 2021, 13, 796. [Google Scholar] [CrossRef] [PubMed]
- Abusrewil, Z.; Alhudiri, I.M.; Kaal, H.H.; El Meshri, S.E.; Ebrahim, F.O.; Dalyoum, T.; Efrefer, A.A.; Ibrahim, K.; Elfghi, M.B.; Abusrewil, S.; et al. Time Scale Performance of Rapid Antigen Testing for SARS-CoV-2: Evaluation of 10 Rapid Antigen Assays. J. Med. Virol. 2021, 93, 6512–6518. [Google Scholar] [CrossRef] [PubMed]
- Torres, I.; Poujois, S.; Albert, E.; Colomina, J.; Navarro, D. Evaluation of a Rapid Antigen Test (PanbioTM COVID-19 Ag Rapid Test Device) for SARS-CoV-2 Detection in Asymptomatic Close Contacts of COVID-19 Patients. Clin. Microbiol. Infect. 2021, 27, 636.e1–636.e4. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Khurana, S.; Das, R.; Srigyan, D.; Singh, A.; Mittal, A.; Singh, P.; Soneja, M.; Kumar, A.; Singh, A.K.; et al. Rapid Chromatographic Immunoassay-Based Evaluation of COVID-19: A Cross-Sectional, Diagnostic Test Accuracy Study & Its Implications for COVID-19 Management in India. Indian J. Med. Res. 2021, 153, 126–131. [Google Scholar] [CrossRef]
- Oh, S.M.; Jeong, H.; Chang, E.; Choe, P.G.; Kang, C.K.; Park, W.B.; Kim, T.S.; Kwon, W.Y.; Oh, M.D.; Kim, N.J. Clinical Application of the Standard Q COVID-19 Ag Test for the Detection of SARS-CoV-2 Infection. J. Korean Med. Sci. 2021, 36, e101. [Google Scholar] [CrossRef]
- Ferté, T.; Ramel, V.; Cazanave, C.; Lafon, M.-E.; Bébéar, C.; Malvy, D.; Georges-Walryck, A.; Dehail, P. Accuracy of COVID-19 Rapid Antigenic Tests Compared to RT-PCR in a Student Population: The StudyCov Study. J. Clin. Virol. 2021, 141, 104878. [Google Scholar] [CrossRef]
- Kyritsi, M.; Vontas, A.; Voulgaridi, I.; Matziri, A.; Komnos, A.; Babalis, D.; Papadogoulas, A.; Oikonomou, A.; Mouchtouri, V.A.; Speletas, M.; et al. Rapid Test Ag 2019-NCoV (PROGNOSIS, BIOTECH, Larissa, Greece); Performance Evaluation in Hospital Setting with Real Time RT-PCR. Int. J. Environ. Res. Public Health 2021, 18, 9151. [Google Scholar] [CrossRef]
- Jakobsen, K.K.; Jensen, J.S.; Todsen, T.; Tolsgaard, M.G.; Kirkby, N.; Lippert, F.; Vangsted, A.-M.; Martel, C.J.-M.; Klokker, M.; von Buchwald, C. Accuracy and Cost Description of Rapid Antigen Test Compared with Reverse Transcriptase-Polymerase Chain Reaction for SARS-CoV-2 Detection. Dan. Med. J. 2021, 68, A03210217. [Google Scholar]
- Charpentier, C.; Ichou, H.; Damond, F.; Bouvet, E.; Chaix, M.-L.; Ferré, V.; Delaugerre, C.; Mahjoub, N.; Larrouy, L.; Le Hingrat, Q.; et al. Performance Evaluation of Two SARS-CoV-2 IgG/IgM Rapid Tests (Covid-Presto and NG-Test) and One IgG Automated Immunoassay (Abbott). J. Clin. Virol. 2020, 132, 104618. [Google Scholar] [CrossRef]
- Orsi, A.; Pennati, B.M.; Bruzzone, B.; Ricucci, V.; Ferone, D.; Barbera, P.; Arboscello, E.; Dentone, C.; Icardi, G. On-Field Evaluation of a Ultra-Rapid Fluorescence Immunoassay as a Frontline Test for SARS-CoV-2 Diagnostic. J. Virol. Methods 2021, 295, 114201. [Google Scholar] [CrossRef]
- Baccani, I.; Morecchiato, F.; Chilleri, C.; Cervini, C.; Gori, E.; Matarrese, D.; Bassetti, A.; Bonizzoli, M.; Mencarini, J.; Antonelli, A.; et al. Evaluation of Three Immunoassays for the Rapid Detection of SARS-CoV-2 Antigens. Diagn Microbiol. Infect. Dis. 2021, 101, 115434. [Google Scholar] [CrossRef]
- MacMullan, M.A.; Chellamuthu, P.; Mades, A.; Das, S.; Turner, F.; Slepnev, V.I.; Ibrayeva, A. Detection of SARS-CoV-2 Antibodies in Oral Fluid Obtained Using a Rapid Collection Device. J. Clin. Microbiol. 2021, 59, e02510-20. [Google Scholar] [CrossRef]
- Mullins, K.E.; Merrill, V.; Ward, M.; King, B.; Rock, P.; Caswell, M.; Ahlman, M.; Harris, A.D.; Christenson, R. Validation of COVID-19 Serologic Tests and Large Scale Screening of Asymptomatic Healthcare Workers. Clin. Biochem. 2021, 90, 23–27. [Google Scholar] [CrossRef]
- Hirotsu, Y.; Maejima, M.; Shibusawa, M.; Nagakubo, Y.; Hosaka, K.; Amemiya, K.; Sueki, H.; Hayakawa, M.; Mochizuki, H.; Tsutsui, T.; et al. Comparison of Automated SARS-CoV-2 Antigen Test for COVID-19 Infection with Quantitative RT-PCR Using 313 Nasopharyngeal Swabs, Including from Seven Serially Followed Patients. Int. J. Infect. Dis. 2020, 99, 397–402. [Google Scholar] [CrossRef]
- Asai, N.; Sakanashi, D.; Ohashi, W.; Nakamura, A.; Kawamoto, Y.; Miyazaki, N.; Ohno, T.; Yamada, A.; Chida, S.; Shibata, Y.; et al. Efficacy and Validity of Automated Quantitative Chemiluminescent Enzyme Immunoassay for SARS-CoV-2 Antigen Test from Saliva Specimen in the Diagnosis of COVID-19. J. Infect. Chemother. 2021, 27, 1039–1042. [Google Scholar] [CrossRef]
- Paul, D.; Gupta, A.; Rooge, S.; Gupta, E. Performance Evaluation of Automated Chemiluminescence Immunoassay Based Antigen Detection—Moving towards More Reliable Ways to Predict SARS-CoV-2 Infection. J. Virol. Methods 2021, 298, 114299. [Google Scholar] [CrossRef]
- Candia-Puma, M.A.; Machaca-Luque, L.Y.; Roque-Pumahuanca, B.M.; Galdino, A.S.; Giunchetti, R.C.; Coelho, E.A.F.; Chávez-Fumagalli, M.A. Accuracy of Diagnostic Tests for the Detection of Chagas Disease: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 2752. [Google Scholar] [CrossRef]
- Chávez-Fumagalli, M.A.; Shrivastava, P.; Aguilar-Pineda, J.A.; Nieto-Montesinos, R.; del Carpio, G.D.; Peralta-Mestas, A.; Caracela-Zeballos, C.; Valdez-Lazo, G.; Fernandez-Macedo, V.; Pino-Figueroa, A.; et al. Diagnosis of Alzheimer’s Disease in Developed and Developing Countries: Systematic Review and Meta-Analysis of Diagnostic Test Accuracy. J. Alzheimer’s Dis. Rep. 2021, 5, 15–30. [Google Scholar] [CrossRef]
- Sachs, J.D.; Karim, S.S.A.; Aknin, L.; Allen, J.; Brosbøl, K.; Colombo, F.; Barron, G.C.; Espinosa, M.F.; Gaspar, V.; Gaviria, A.; et al. The Lancet Commission on Lessons for the Future from the COVID-19 Pandemic. Lancet 2022, 400, 1224–1280. [Google Scholar] [CrossRef]
- Singh, S.; Mcnab, C.; Olson, R.M.; Bristol, N.; Nolan, C.; Bergstrøm, E.; Bartos, M.; Mabuchi, S.; Panjabi, R.; Karan, A.; et al. How an Outbreak Became a Pandemic: A Chronological Analysis of Crucial Junctures and International Obligations in the Early Months of the COVID-19 Pandemic. Lancet 2021, 398, 2109–2124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, S.; Hu, T.; Fu, X.; Wang, X.; Hu, Y.; Halloran, B.; Li, Z.; Cui, Y.; Liu, H.; et al. Human Mobility and COVID-19 Transmission: A Systematic Review and Future Directions. Ann. GIS 2022, 28, 501–514. [Google Scholar] [CrossRef]
- Gao, W.; Lv, J.; Pang, Y.; Li, L.-M. Role of Asymptomatic and Pre-Symptomatic Infections in Covid-19 Pandemic. BMJ 2021, 375, n2342. [Google Scholar] [CrossRef] [PubMed]
- Laccourreye, O.; Cohen, R.; Couloigner, V. Keys for Analysis of Diagnostic and Serologic Tests for CoV-2. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2020, 137, 323–324. [Google Scholar] [CrossRef]
- Kwok, H.F. The Significance of Advanced COVID-19 Diagnostic Testing in Pandemic Control Measures. Int. J. Biol. Sci. 2022, 18, 4610–4617. [Google Scholar] [CrossRef]
- Carpenter, C.R.; Mudd, P.A.; West, C.P.; Wilber, E.; Wilber, S.T. Diagnosing COVID-19 in the Emergency Department: A Scoping Review of Clinical Examinations, Laboratory Tests, Imaging Accuracy, and Biases. Acad. Emerg. Med. 2020, 27, 653–670. [Google Scholar] [CrossRef]
- Guglielmi, G. The Explosion of New Coronavirus Tests That Could Help to End the Pandemic. Nature 2020, 583, 506–509. [Google Scholar] [CrossRef]
- Vandenberg, O.; Martiny, D.; Rochas, O.; van Belkum, A.; Kozlakidis, Z. Considerations for Diagnostic COVID-19 Tests. Nat. Rev. Microbiol. 2021, 19, 171–183. [Google Scholar] [CrossRef]
- Jeong, S.; González-Grandío, E.; Navarro, N.; Pinals, R.L.; Ledesma, F.; Yang, D.; Landry, M.P. Extraction of Viral Nucleic Acids with Carbon Nanotubes Increases SARS-CoV-2 Quantitative Reverse Transcription Polymerase Chain Reaction Detection Sensitivity. ACS Nano 2021, 15, 10309–10317. [Google Scholar] [CrossRef]
- El Jaddaoui, I.; Allali, M.; Raoui, S.; Sehli, S.; Habib, N.; Chaouni, B.; Al Idrissi, N.; Benslima, N.; Maher, W.; Benrahma, H.; et al. A Review on Current Diagnostic Techniques for COVID-19. Expert Rev. Mol. Diagn. 2021, 21, 141–160. [Google Scholar] [CrossRef]
- Teymouri, M.; Mollazadeh, S.; Mortazavi, H.; Naderi Ghale-noie, Z.; Keyvani, V.; Aghababaei, F.; Hamblin, M.R.; Abbaszadeh-Goudarzi, G.; Pourghadamyari, H.; Hashemian, S.M.R.; et al. Recent Advances and Challenges of RT-PCR Tests for the Diagnosis of COVID-19. Pathol.—Res. Pract. 2021, 221, 153443. [Google Scholar] [CrossRef]
- Rahimi, H.; Salehiabar, M.; Barsbay, M.; Ghaffarlou, M.; Kavetskyy, T.; Sharafi, A.; Davaran, S.; Chauhan, S.C.; Danafar, H.; Kaboli, S.; et al. CRISPR Systems for COVID-19 Diagnosis. ACS Sens. 2021, 6, 1430–1445. [Google Scholar] [CrossRef]
- Jiang, C.; Li, X.; Ge, C.; Ding, Y.; Zhang, T.; Cao, S.; Meng, L.; Lu, S. Molecular Detection of SARS-CoV-2 Being Challenged by Virus Variation and Asymptomatic Infection. J. Pharm. Anal. 2021, 11, 257–264. [Google Scholar] [CrossRef]
- Abbasi, J. The Promise and Peril of Antibody Testing for COVID-19. JAMA 2020, 323, 1881. [Google Scholar] [CrossRef]
- Afzal, A. Molecular Diagnostic Technologies for COVID-19: Limitations and Challenges. J. Adv. Res. 2020, 26, 149–159. [Google Scholar] [CrossRef]
- Mohit, E.; Rostami, Z.; Vahidi, H. A Comparative Review of Immunoassays for COVID-19 Detection. Expert Rev. Clin. Immunol. 2021, 17, 573–599. [Google Scholar] [CrossRef]
- Ong, D.S.Y.; Fragkou, P.C.; Schweitzer, V.A.; Chemaly, R.F.; Moschopoulos, C.D.; Skevaki, C. How to Interpret and Use COVID-19 Serology and Immunology Tests. Clin. Microbiol. Infect. 2021, 27, 981–986. [Google Scholar] [CrossRef]
- Zhang, J.J.Y.; Lee, K.S.; Ong, C.W.; Chan, M.Y.; Ang, L.W.; Leo, Y.S.; Chen, M.I.; Lye, D.C.B.; Young, B.E. Diagnostic Performance of COVID-19 Serological Assays during Early Infection: A Systematic Review and Meta-analysis of 11 516 Samples. Influenza Other Respir. Viruses 2021, 15, 529–538. [Google Scholar] [CrossRef]
- Yong, S.E.F.; Anderson, D.E.; Wei, W.E.; Pang, J.; Chia, W.N.; Tan, C.W.; Teoh, Y.L.; Rajendram, P.; Toh, M.P.H.S.; Poh, C.; et al. Connecting Clusters of COVID-19: An Epidemiological and Serological Investigation. Lancet Infect. Dis. 2020, 20, 809–815. [Google Scholar] [CrossRef]
- Neagu, M.; Constantin, C.; Surcel, M. Testing Antigens, Antibodies, and Immune Cells in COVID-19 as a Public Health Topic—Experience and Outlines. Int. J. Environ. Res. Public Health 2021, 18, 13173. [Google Scholar] [CrossRef]
- Combadière, B. Immunité Adaptative Contre Le Virus SARS-CoV-2. Médecine Sci. 2020, 36, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zeng, W.; He, H.; Zhao, D.; Jiang, D.; Zhou, P.; Cheng, L.; Li, Y.; Ma, X.; Jin, T. Serum IgA, IgM, and IgG Responses in COVID-19. Cell. Mol. Immunol. 2020, 17, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Wei, H.; Li, Q.; Liu, L.; Li, B. Evaluation and Comparison of Serological Methods for COVID-19 Diagnosis. Front. Mol. Biosci. 2021, 8, 682405. [Google Scholar] [CrossRef]
- Batra, R.; Olivieri, L.G.; Rubin, D.; Vallari, A.; Pearce, S.; Olivo, A.; Prostko, J.; Nebbia, G.; Douthwaite, S.; Rodgers, M.; et al. A Comparative Evaluation between the Abbott PanbioTM COVID-19 IgG/IgM Rapid Test Device and Abbott ArchitectTM SARS CoV-2 IgG Assay. J. Clin. Virol. 2020, 132, 104645. [Google Scholar] [CrossRef] [PubMed]
- Malik, Y.A. Properties of Coronavirus and SARS-CoV-2. Malays. J. Pathol. 2020, 42, 3–11. [Google Scholar]
- Zeng, W.; Liu, G.; Ma, H.; Zhao, D.; Yang, Y.; Liu, M.; Mohammed, A.; Zhao, C.; Yang, Y.; Xie, J.; et al. Biochemical Characterization of SARS-CoV-2 Nucleocapsid Protein. Biochem. Biophys. Res. Commun. 2020, 527, 618–623. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Wang, H.; Li, X.; Zhang, S.; Xu, Y.; Wei, W. Serum SARS-COV-2 Nucleocapsid Protein: A Sensitivity and Specificity Early Diagnostic Marker for SARS-COV-2 Infection. Front. Cell. Infect. Microbiol. 2020, 10, 470. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, J.; Li, Q.; Hu, H.; Lu, J.; Chen, Z. Advances in Neutralization Assays for SARS-CoV-2. Scand. J. Immunol. 2021, 94, e13088. [Google Scholar] [CrossRef]
- Chen, X.; Pan, Z.; Yue, S.; Yu, F.; Zhang, J.; Yang, Y.; Li, R.; Liu, B.; Yang, X.; Gao, L.; et al. Disease Severity Dictates SARS-CoV-2-Specific Neutralizing Antibody Responses in COVID-19. Signal Transduct. Target. Ther. 2020, 5, 180. [Google Scholar] [CrossRef]
- Muruato, A.E.; Fontes-Garfias, C.R.; Ren, P.; Garcia-Blanco, M.A.; Menachery, V.D.; Xie, X.; Shi, P.-Y. A High-Throughput Neutralizing Antibody Assay for COVID-19 Diagnosis and Vaccine Evaluation. Nat. Commun. 2020, 11, 4059. [Google Scholar] [CrossRef]
- John, G.; Sahajpal, N.S.; Mondal, A.K.; Ananth, S.; Williams, C.; Chaubey, A.; Rojiani, A.M.; Kolhe, R. Next-Generation Sequencing (NGS) in COVID-19: A Tool for SARS-CoV-2 Diagnosis, Monitoring New Strains and Phylodynamic Modeling in Molecular Epidemiology. Curr. Issues Mol. Biol. 2021, 43, 845–867. [Google Scholar] [CrossRef]
- Yoo, S.M.; Choi, J.H.; Lee, S.Y.; Yoo, N.C. Applications of DNA Microarray in Disease Diagnostics. J. Microbiol. Biotechnol. 2009, 19, 635–646. [Google Scholar] [CrossRef]
- Xu, M.; Wang, D.; Wang, H.; Zhang, X.; Liang, T.; Dai, J.; Li, M.; Zhang, J.; Zhang, K.; Xu, D.; et al. COVID-19 Diagnostic Testing: Technology Perspective. Clin. Transl. Med. 2020, 10, e158. [Google Scholar] [CrossRef]
- Irungbam, M.; Chitkara, A.; Singh, V.K.; Sonkar, S.C.; Dubey, A.; Bansal, A.; Shrivastava, R.; Goswami, B.; Manchanda, V.; Saxena, S.; et al. Evaluation of Performance of Detection of Immunoglobulin G and Immunoglobulin M Antibody Against Spike Protein of SARS-CoV-2 by a Rapid Kit in a Real-Life Hospital Setting. Front. Microbiol. 2022, 13, 802292. [Google Scholar] [CrossRef]
- Pieri, M.; Ciotti, M.; Carlozzi, N.; Frassanito, M.L.; Meloni, A.; Cistera, A.; Turchetti, G.; Niscola, S.; Labate, G.; Calugi, G.; et al. SARS-CoV-2 Infection Serology Validation of Different Methods: Usefulness of IgA in the Early Phase of Infection. Clin. Chim. Acta 2020, 511, 28–32. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Ganeshkumar, P. Systematic Reviews and Meta-Analysis: Understanding the Best Evidence in Primary Healthcare. J. Fam. Med. Prim. Care 2013, 2, 9. [Google Scholar] [CrossRef]
- Tatsioni, A. Challenges in Systematic Reviews of Diagnostic Technologies. Ann. Intern. Med. 2005, 142, 1048. [Google Scholar] [CrossRef]
- de França, N.C.; Campêlo, G.L.; de França, J.M.S.; Vale, E.G.; Badagnan, T.F. A Decomposition Analysis for Socioeconomic Inequalities in Health Status Associated with the COVID-19 Diagnosis and Related Symptoms during Brazil’s First Wave of Infections. Economía 2021, 22, 251–264. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilca-Alosilla, J.J.; Candia-Puma, M.A.; Coronel-Monje, K.; Goyzueta-Mamani, L.D.; Galdino, A.S.; Machado-de-Ávila, R.A.; Giunchetti, R.C.; Ferraz Coelho, E.A.; Chávez-Fumagalli, M.A. A Systematic Review and Meta-Analysis Comparing the Diagnostic Accuracy Tests of COVID-19. Diagnostics 2023, 13, 1549. https://doi.org/10.3390/diagnostics13091549
Vilca-Alosilla JJ, Candia-Puma MA, Coronel-Monje K, Goyzueta-Mamani LD, Galdino AS, Machado-de-Ávila RA, Giunchetti RC, Ferraz Coelho EA, Chávez-Fumagalli MA. A Systematic Review and Meta-Analysis Comparing the Diagnostic Accuracy Tests of COVID-19. Diagnostics. 2023; 13(9):1549. https://doi.org/10.3390/diagnostics13091549
Chicago/Turabian StyleVilca-Alosilla, Juan Jeferson, Mayron Antonio Candia-Puma, Katiusca Coronel-Monje, Luis Daniel Goyzueta-Mamani, Alexsandro Sobreira Galdino, Ricardo Andrez Machado-de-Ávila, Rodolfo Cordeiro Giunchetti, Eduardo Antonio Ferraz Coelho, and Miguel Angel Chávez-Fumagalli. 2023. "A Systematic Review and Meta-Analysis Comparing the Diagnostic Accuracy Tests of COVID-19" Diagnostics 13, no. 9: 1549. https://doi.org/10.3390/diagnostics13091549










