The Role of Ultrasound in the Diagnosis of Pulmonary Infection Caused by Intracellular, Fungal Pathogens and Mycobacteria: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
Study Selection and Data Extraction
3. Results
3.1. Study Selection and LUS Definitions
3.2. Primary Results
3.2.1. Intracellular Pathogens Lung Infection
3.2.2. Fungal Lung infection
3.2.3. Mycobacterium tuberculosis Lung Infection
4. Discussion
4.1. General Considerations
4.2. Intracellular Pathogens Lung Infection
4.3. Fungal Lung Infection
4.4. Mycobacterium tuberculosis Infection
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Joyner, C.; Herman, R.J.; Reid, J.M. Reflected ultrasound in the detection and localization of pleural effusion. JAMA 1967, 200, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.; Mézière, G.; Biderman, P.; Gepner, A.; Barrè, O. The comettail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am. J. Respir. Crit. Care Med. 1997, 156, 1640–1646. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Testa, A.; Silva, F.R.; Carbone, L.; Portale, G.; Silveri, N.G. Chest ultrasonography in lung contusion. Chest 2006, 130, 533–538. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chavez, M.A.; Shams, N.; Ellington, L.E.; Naithani, N.; Gilman, R.H.; Steinhoff, M.C.; Santosham, M.; Black, R.E.; Price, C.; Gross, M.; et al. Lung ultrasound for the diagnosis of pneumonia in adults: A systematic review and meta-analysis. Respir. Res. 2014, 15, 50. [Google Scholar] [CrossRef][Green Version]
- Iorio, G.; Capasso, M.; De Luca, G.; Prisco, S.; Mancusi, C.; Laganà, B.; Comune, V. Lung Ultrasound in the Diagnosis of Pneumonia in Children: Proposal for a New Diagnostic Algorithm. Peer J. 2015, 3, e1374. [Google Scholar] [CrossRef][Green Version]
- Ticinesi, A.; Lauretani, F.; Nouvenne, A.; Mori, G.; Chiussi, G.; Maggio, M.; Meschi, T. Lung ultrasound and chest x-ray for detecting pneumonia in an acute geriatric ward. Medicine 2016, 95, e4153. [Google Scholar] [CrossRef]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef][Green Version]
- Urbankowska, E.; Krenke, K.; Drobczyński, Ł.; Korczyński, P.; Urbankowski, T.; Krawiec, M.; Kraj, G.; Brzewski, M.; Kulus, M. Lung ultrasound in the diagnosis and monitoring of community acquired pneumonia in children. Respir. Med. 2015, 109, 1207–1212. [Google Scholar] [CrossRef][Green Version]
- Balk, D.S.; Lee, C.; Schafer, J.; Welwarth, J.; Hardin, J.; Novack, V.; Yarza, S.; Hoffmann, B. Lung ultrasound compared to chest-X-ray for diagnosis of pediatric pneumonia: A meta-analysis. Pediatr. Pulmonol. 2018, 53, 1130–1139. [Google Scholar] [CrossRef]
- Najgrodzka, P.; Buda, N.; Zamojska, A.; Marciniewicz, E.; Lewandowicz-Uszyńska, A. Lung Ultrasonography in the Diagnosis of Pneumonia in Children—A Metaanalysis and a Review of Pediatric Lung Imaging. Ultrasound Q. 2019, 35, 157–163. [Google Scholar] [CrossRef]
- Laursen, C.B.; Clive, A.; Hallifax, R.; Pietersen, P.I.; Asciak, R.; Davidsen, J.R.; Bhatnagar, R.; Bedawi, E.O.; Jacobsen, N.; Coleman, C.; et al. European Respiratory Society statement on thoracic ultrasound. Eur. Respir. J. 2021, 57, 2001519. [Google Scholar] [CrossRef]
- Soldati, G.; Copetti, R. Ecografia Toracica, 2nd ed.; Edizioni Medico Scientifiche: Pavia, Italy, 2012. [Google Scholar]
- Lichtenstein, D.A.; Meziere, G.; Lascols, N.; Biderman, P.; Courret, J.P.; Gepner, A.; Goldstein, I.; Tenoudji-Cohen, M. Ultrasound diagnosis of occult pneumothorax. Crit. Care Med. 2005, 33, 1231–1238. [Google Scholar] [CrossRef][Green Version]
- Cox, M.; Soudack, M.; Podberesky, D.J.; Epelman, M. Pediatric chest ultrasound: A practical approach. Pediatr. Radiol. 2017, 47, 1058–1068. [Google Scholar] [CrossRef]
- Riccabona, M. Ultrasound of the chest in children (mediastinum excluded). Eur. Radiol. 2008, 18, 390–399. [Google Scholar] [CrossRef]
- Volpicelli, G.; Mussa, A.; Garofalo, G.; Cardinale, L.; Casoli, G.; Perotto, F.; Fava, C.; Frascisco, M. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am. J. Emerg. Med. 2006, 24, 689–696. [Google Scholar] [CrossRef]
- Berce, V.; Tomazin, M.; Gorenjak, M.; Berce, T.; Lovrenčič, B. The Usefulness of Lung Ultrasound for the Aetiological Diagnosis of Community-Acquired Pneumonia in Children. Sci. Rep. Nat. Res. 2019, 9, 17957. [Google Scholar] [CrossRef][Green Version]
- Miyashita, N. Atypical pneumonia: Pathophysiology, diagnosis, and treatment. Respir. Investig. 2022, 60, 56–67. [Google Scholar] [CrossRef]
- Dropulic, L.K.; Lederman, H.M. Overview of Infections in the Immunocompromised Host. Microbiol. Spectr. 2016, 4, 1–50. [Google Scholar] [CrossRef]
- Harding, E. WHO global progress report on tuberculosis elimination. Lancet Respir. Med. 2020, 8, 19. [Google Scholar] [CrossRef]
- Meli, M.; La Spina, M.; Lo Nigro, L.; Trobia, G.L.; Russo, G.; Di Cataldo, A. Pleuro-pulmonary ultrasound in the diagnosis and follow-up of lung infections in children with cancer: A pilot study. J. Ultrasound 2022, 25, 865–875. [Google Scholar] [CrossRef]
- Stewart, L.A.; Clarke, M.; Rovers, M. Preferred reporting items for systematic review and meta-analyses of individual participant data: The PRISMA-IPD statement. JAMA 2015, 313, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Gargani, L.; Volpicelli, G. How I do it: Lung ultrasound. Cardiovasc. Ultrasound 2014, 12, 25. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mento, F.; Khan, U.; Faita, F.; Smargiassi, A.; Inchingolo, R.; Perrone, T.; Demi, L. State of the Art in Lung Ultrasound, Shifting from Qualitative to Quantitative Analyses. Ultrasound Med. Biol. 2022, 48, 2398–2416. [Google Scholar] [CrossRef] [PubMed]
- Stassen, J.; Bax, J.J. How to do lung ultrasound. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Havelock, T.; Teoh, R.; Laws, D.; Gleeson, F.; BTS Pleural Disease Guideline Group 2010. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010, 65, ii61–ii76. [Google Scholar] [CrossRef][Green Version]
- Hallifax, R.J.; Corcoran, J.P.; Ahmed, A.; Nagendran, M.; Rostom, H.; Hassan, N.; Maruthappu, M.; Psallidas, I.; Manuel, A.; Gleeson, F.V.; et al. Physician-based ultrasound-guided biopsy for diagnosing pleural disease. Chest 2014, 146, 1001–1006. [Google Scholar] [CrossRef]
- Laursen, C.B.; Sloth, E.; Lassen, A.T.; Christensen, R.D.; Lambrechtsen, J.; Madsen, P.H.; Henriksen, D.P.; Davidsen, J.R.; Rasmussen, F. Point-of-care ultrasonography in patients admitted with respiratory symptoms: A single-blind, randomised controlled trial. The Lancet. Respir. Med. 2014, 2, 638–646. [Google Scholar] [CrossRef]
- Agostinis, P.; Copetti, R.; Lapini, L.; Badona Monteiro, G.; N’Deque, A.; Baritussio, A. Chest ultrasound findings in pulmonary tuberculosis. Trop. Dr. 2017, 47, 320–328. [Google Scholar] [CrossRef]
- Hunter, L.; Bélard, S.; Janssen, S.; van Hoving, D.J.; Heller, T. Miliary tuberculosis: Sonographic pattern in chest ultrasound. Infection 2016, 44, 243–246. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Q.L.; Guo, R.J.; Lv, X.Z.; Yang, X. Quantitative evaluation and significance of ultrasound in bronchoalveolar lavage for lung consolidation in children with severe mycoplasma pneumonia. Transl. Pediatr. 2021, 10, 2325–2334. [Google Scholar] [CrossRef]
- Tripaldi, C.; Polito, M.; Iacoviello, O.; Basile, V.; De Bellis, T.; Fortunato, M.; Laforgia, F.; Scalini, E.; Silletti, M.; Lofù, I. Ultrasound Studies on Mycoplasma Bronchopneumonia. J. Curr. Med. Res. Opin. 2021, 5, 1301–1315. [Google Scholar] [CrossRef]
- Buonsenso, D.; Musolino, A.; Ferro, V.; De Rose, C.; Morello, R.; Ventola, C.; Liotti, F.M.; De Sanctis, R.; Chiaretti, A.; Biasucci, D.G.; et al. Role of lung ultrasound for the etiological diagnosis of acute lower respiratory tract infection (ALRTI) in children: A prospective study. J. Ultrasound 2022, 25, 185–197. [Google Scholar] [CrossRef]
- Liu, G.; Wang, G.; Yang, Z.; Liu, G.; Ma, H.; Lv, Y.; Ma, F.; Zhu, W. A Lung Ultrasound-Based Nomogram for the Prediction of Refractory Mycoplasma pneumoniae Pneumonia in Hospitalized Children. Infect. Drug. Resist. 2022, 31, 6343–6355. [Google Scholar] [CrossRef]
- Trinavarat, P.; Chatchatri, P.; Chandtranuwatana, P. Color Doppler sonography of pulmonary aspergillosis in infants with chronic granulomatous disease. Asian Biomed. 2012, 6, 129–133. [Google Scholar]
- Alamdaran, S.A.; Heidarzadeh, H.; Zavvar, N.; Badlee, Z.; Jaberi, M.; Ghasemi, A. Presentation of Sonographic Features of Pulmonary Invasive Fungal Disease in Six Children with Leukemia. Int. J. Pediatr. 2021, 9, 13203–13211. [Google Scholar] [CrossRef]
- Liu, J.; Ma, H.R.; Fu, W. Lung Ultrasound to Diagnose Pneumonia in Neonates with Fungal Infection. Diagnostics 2022, 12, 1776. [Google Scholar] [CrossRef]
- Tikkakoski, P.; Lohela, M.; Paivansalo, T.; Kerola, T. Pleuro-pulmonary aspergillosis: US and US-guided biopsy as an aid to diagnosis. Acta Radiol. 1995, 36, 122–126. [Google Scholar] [CrossRef]
- Grabala, J.; Grabala, M.; Onichimowski, D.; Grabala, P. Possibilities of using ultrasound for diagnosis of invasive pulmonary mucormycosis—A case study. Pol. Ann. Med. 2017, 24, 224–227. [Google Scholar] [CrossRef]
- Greco, R.; Lazzari, L.; Xue, E.; Assanelli, A.; Marktel, S.; Giglio, F.; Clerici, D.; Lupo Stanghellini, M.T.; Corti, C.; Bernardi, M.; et al. Lung ultrasound to evaluate invasive fungal diseases after allogeneic hematopoietic stem cell transplantation. Infect. Chemother. 2019, 51, 386–392. [Google Scholar] [CrossRef]
- Ruby, L.C.; Kadavigere, R.; Sheshadri, S.; Saravu, K.; Bélard, S. Pulmonary aspergilloma on transthoracic ultrasound. Infection 2021, 49, 1337–1340. [Google Scholar] [CrossRef]
- Heuvelings, C.C.; Bélard, S.; Andronikou, S.; Jamieson-Luff, N.; Grobusch, M.P.; Zar, H.J. Chest ultrasound findings in children with suspected pulmonary tuberculosis. Pediatr. Pulmonol. 2019, 54, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Montuori, M.; Casella, F.; Casazza, G.; Franzetti, F.; Pini, P.; Invernizzi, C.; Torzillo, D.; Rizzardini, G.; Galli, M.; Cogliati, C. Lung ultrasonography in pulmonary tuberculosis: A pilot study on diagnostic accuracy in a high-risk population. Eur. J. Intern. Med. 2019, 66, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Fentress, M.; Ugarte-Gil, C.; Cervantes, M.; Rivas, D.; Moore, D.; Caliguiri, P.; Bergman, K.; Noazin, S.; Padovani, A.; Gilman, R.H. Lung Ultrasound Findings Compared with Chest X-Ray Findings in Known Pulmonary Tuberculosis Patients: A Cross-Sectional Study in Lima, Peru. Am. J. Trop. Med. Hyg. 2020, 103, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Cocco, G.; Boccatonda, A.; Rossi, I.; D’Ardes, D.; Corvino, A.; Delli Pizzi, A.; Ucciferri, C.; Falasca, K.; Vecchiet, J. Early detection of pleuro-pulmonary tuberculosis by bedside lung ultrasound: A case report and review of literature. Clin. Case Rep. 2022, 10, e05739. [Google Scholar] [CrossRef]
- Parlamento, S.; Copetti, R.; Di Bartolomeo, S. Evaluation of lung ultrasound for the diagnosis of pneumonia in the ED. Am. J. Emerg. Med. 2009, 27, 379–384. [Google Scholar] [CrossRef]
- Reali, F.; Sferrazza Papa, G.F.; Carlucci, P.; Fracasso, P.; Di Marco, F.; Mandelli, M.; Soldi, S.; Riva, E.; Centanni, S. Can lung ultrasound replace chest radiography for the diagnosis of pneumonia in hospitalized children? Respiration 2014, 88, 112–115. [Google Scholar] [CrossRef]
- Reissig, A.; Copetti, R.; Mathis, G.; Mempel, C.; Schuler, A.; Zechner, P.; Aliberti, S.; Neumann, R.; Kroegel, C.; Hoyer, H. Lung ultrasound in the diagnosis and follow-up of community-acquired pneumonia: A prospective, multicenter, diagnostic accuracy study. Chest 2012, 142, 965–972. [Google Scholar] [CrossRef][Green Version]
- Blaivas, M. Lung ultrasound in evaluation of pneumonia. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2012, 31, 823–826. [Google Scholar] [CrossRef][Green Version]
- Caiulo, V.A.; Gargani, L.; Caiulo, S.; Fisicaro, A.; Moramarco, F.; Latini, G.; Picano, E.; Mele, G. Lung ultrasound characteristics of community-acquired pneumonia in hospitalized children. Pediatr. Pulmonol. 2013, 48, 280–287. [Google Scholar] [CrossRef]
- Copetti, R.; Cattarossi, L. Ultrasound diagnosis of pneumonia in children. Radiol. Med. 2008, 113, 190–198. [Google Scholar] [CrossRef]
- Corradi, F.; Brusasco, C.; Garlaschi, A.; Paparo, F.; Ball, L.; Santori, G.; Pelosi, P.; Altomonte, F.; Vezzani, A.; Brusasco, V. Quantitative analysis of lung ultrasonography for the detection of community-acquired pneumonia: A pilot study. BioMed Res. Int. 2015, 2015, 868707. [Google Scholar] [CrossRef][Green Version]
- Cortellaro, F.; Colombo, S.; Coen, D.; Duca, P.G. Lung ultrasound is an accurate diagnostic tool for the diagnosis of pneumonia in the emergency department. Emerg. Med. J. 2012, 29, 19–23. [Google Scholar] [CrossRef][Green Version]
- Iuri, D.; De Candia, A.; Bazzocchi, M. Evaluation of the lung in children with suspected pneumonia: Usefulness of ultrasonography. Radiol. Med. 2009, 114, 321–330. [Google Scholar] [CrossRef]
- Nazerian, P.; Volpicelli, G.; Vanni, S.; Gigli, C.; Betti, L.; Bartolucci, M.; Zanobetti, M.; Ermini, F.R.; Iannello, C.; Grifoni, S. Accuracy of lung ultrasound for the diagnosis of consolidations when compared to chest computed tomography. Am. J. Emerg. Med. 2015, 33, 620–625. [Google Scholar] [CrossRef][Green Version]
- Bhalla, D.; Naranje, P.; Jana, M.; Bhalla, A.S. Pediatric lung ultrasonography: Current perspectives. Pediatr. Radiol. 2022, 52, 2038–2050. [Google Scholar] [CrossRef]
- Principi, N.; Esposito, A.; Giannitto, C.; Esposito, S. Lung ultrasonography to diagnose community-acquired pneumonia in children. BMC Pulm. Med. 2017, 17, 212. [Google Scholar] [CrossRef][Green Version]
- Ambroggio, L.; Sucharew, H.; Rattan, M.S.; O’Hara, S.M.; Babcock, D.S.; Clohessy, C.; Steinhoff, M.C.; Macaluso, M.; Shah, S.S.; Coley, B.D. Lung Ultrasonography: A Viable Alternative to Chest Radiography in Children with Suspected Pneumonia? J. Pediatr. 2016, 176, 93–98.e7. [Google Scholar] [CrossRef]
- Joshi, P.; Vasishta, A.; Gupta, M. Ultrasound of the pediatric chest. Br. J. Radiol. 2019, 92, 20190058. [Google Scholar] [CrossRef]
- Goh, Y.; Kapur, J. Sonography of the Pediatric Chest. J. Ultrasound Med. 2016, 35, 1067–1080. [Google Scholar] [CrossRef][Green Version]
- Rea, G.; Sperandeo, M.; Di Serafino, M.; Vallone, G.; Tomà, P. Neonatal and pediatric thoracic ultrasonography. J. Ultrasound 2019, 22, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Bigio, J.; Kohli, M.; Klinton, J.S.; MacLean, E.; Gore, G.; Small, P.M.; Ruhwald, M.; Weber, S.F.; Jha, S.; Pai, M. Diagnostic accuracy of point-of-care ultrasound for pulmonary tuberculosis: A systematic review. PLoS ONE 2021, 16, e0251236. [Google Scholar] [CrossRef] [PubMed]
- Bloise, S.; La Regina, D.P.; Pepino, D.; Iovine, E.; Laudisa, M.; Di Mattia, G.; Nicolai, A.; Nenna, R.; Petrarca, L.; Mancino, E.; et al. Lung ultrasound compared to chest X-ray for the diagnosis of CAP in children. Pediatr. Int. 2021, 63, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Lichosik, M.; Rustecka, A.; Placzyńska, M.; Wawrzyniak, A.; Kalicki, B. Transthoracic lung ultrasound in children with signs of acute lower respiratory infection. Pediatr. Med. Rodz. 2020, 16, 87–92. [Google Scholar] [CrossRef]
- Stadler, J.A.M.; Andronikou, S.; Zar, H.J. Lung ultrasound for the diagnosis of community-acquired pneumonia in children. In Pediatr. Radiol. 2017, 47, 1412–1419. [Google Scholar] [CrossRef][Green Version]
- Sansone, F.; Attanasi, M.; Di Filippo, P.; Sferrazza Papa, G.F.; Di Pillo, S.; Chiarelli, F. Usefulness of Lung Ultrasound in Paediatric Respiratory Diseases. Diagnostics 2021, 11, 1783. [Google Scholar] [CrossRef]
- Man, M.A.; Dantes, E.; Domokos Hancu, B.; Bondor, C.I.; Ruscovan, A.; Parau, A.; Motoc, N.S.; Marc, M. Correlation between Transthoracic Lung Ultrasound Score and HRCT Features in Patients with Interstitial Lung Diseases. J. Clin. Med. 2019, 8, 1199. [Google Scholar] [CrossRef][Green Version]
- Pereda, M.A.; Chavez, M.A.; Hooper-Miele, C.C.; Gilman, R.H.; Steinhoff, M.C.; Ellington, L.E.; Gross, M.; Price, C.; Tielsch, J.M.; Checkley, W. Lung ultrasound for the diagnosis of pneumonia in children: A meta-analysis. Pediatrics 2015, 135, 714–722. [Google Scholar] [CrossRef][Green Version]
- Reynolds, J.H.; McDonald, G.; Alton, H.; Gordon, S.B. Pneumonia in the immunocompetent patient. Br. J. Radiol. 2010, 83, 998–1009. [Google Scholar] [CrossRef][Green Version]
- Lerchbaumer, M.H.; Lauryn, J.H.; Bachmann, U.; Enghard, P.; Fischer, T.; Grune, J.; Hegemann, N.; Khadzhynov, D.; Kruse, J.M.; Lehner, L.J.; et al. Point-of-care lung ultrasound in COVID-19 patients: Inter- and intra-observer agreement in a prospective observational study. Sci. Rep. 2021, 11, 10678. [Google Scholar] [CrossRef]
- Li, Z.; Lu, G.; Meng, G. Pathogenic Fungal Infection in the Lung. Front. Immunol. 2019, 3, 1524. [Google Scholar] [CrossRef][Green Version]
- Buonsenso, D.; Brancato, F.; Valentini, P.; Curatola, A.; Supino, M.; Musolino, A.M. The use of lung ultrasound to monitor the antibiotic response of community-acquired pneumonia in children: A preliminary hypothesis. J. Ultrasound Med. 2020, 39, 817–826. [Google Scholar] [CrossRef]
- Iorio, G.; Capasso, M.; Prisco, S.; De Luca, G.; Mancusi, C.; Laganà, B.; Piscopo, M.A.; Comune, V. Lung Ultrasound Findings Undetectable by Chest Radiography in Children with Community-Acquired Pneumonia. Ultrasound Med. Biol. 2018, 44, 1687–1693. [Google Scholar] [CrossRef]
- Panigrahi, M.K.; Manju, R.; Kumar, S.V.; Toi, P.C. Pulmonary mucormycosis presenting as nonresolving pneumonia in a patient with diabetes mellitus. Respir. Care 2014, 59, e201–e205. [Google Scholar] [CrossRef][Green Version]
- Tedder, M.; Spratt, J.A.; Anstadt, M.P.; Hegde, S.S.; Tedder, S.D.; Lowe, J.E. Pulmonary mucormycosis: Results of medical and surgical therapy. Ann. Thorac. Surg. 1994, 57, 1044–1050. [Google Scholar] [CrossRef]
- Chamilos, G.; Marom, E.M.; Lewis, R.E.; Lionakis, M.S.; Kontoyiannis, D.P. Predictors of pulmonary zygomycosis versus invasive pulmonary Aspergillosis in patients with cancer. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2005, 4, 60–66. [Google Scholar] [CrossRef][Green Version]
- Kousha, M.; Tadi, R.; Soubani, A.O. Pulmonary aspergillosis: A clinical review. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2011, 20, 156–174. [Google Scholar] [CrossRef][Green Version]
- Gao, Y.; Soubani, A. Advances in the diagnosis and management of pulmonary aspergillosis. Adv. Respir. Med. 2019, 87, 231–243. [Google Scholar] [CrossRef][Green Version]
- El-Baba, F.; Gao, Y.; Soubani, A.O. Pulmonary Aspergillosis: What the Generalist Needs to Know. Am. J. Med. 2020, 133, 668–674. [Google Scholar] [CrossRef]
- Kanj, A.; Abdallah, N.; Soubani, A.O. The spectrum of pulmonary aspergillosis. Respir. Med. 2018, 141, 121–131. [Google Scholar] [CrossRef]
- Garg, M.; Prabhakar, N.; Gulati, A.; Agarwal, R.; Dhooria, S. Spectrum of imaging findings in pulmonary infections. Part 2: Fungal, mycobacterial, and parasitic. Pol. J. Radiol. 2019, 22, 214–223. [Google Scholar] [CrossRef]
- Katragkou, A.; Fisher, B.T.; Groll, A.H.; Roilides, E.; Walsh, T.J. Diagnostic Imaging and Invasive Fungal Diseases in Children. J. Pediatr. Infect. Dis. Soc. 2017, 6, S22–S31. [Google Scholar] [CrossRef] [PubMed]
- Aquino, S.L.; Kee, S.T.; Warnock, M.L.; Gamsu, G. Pulmonary Aspergillosis: Imaging findings with pathologic correlation. Am. J. Roentgenol. 1994, 163, 811–815. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pesle, G.D.; Monod, O. Bronchiectasis due to asperigilloma. Dis. Chest 1954, 25, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Pinto, E.; Lopes, S.; Principe, F.; Costa, J.; Macedo, G. Pulmonary Aspergillosis diagnosed by endoscopic ultrasound fine-needle aspiration. Endosc. Ultrasound 2016, 5, 58–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Panse, J.; von Schwanewede, K.; Jost, E.; Dreher, M.; Müller, T. Pulmonary infections in patients with and without hematological malignancies: Diagnostic yield and safety of flexible bronchoscopy—A retrospective analysis. J. Thorac. Dis. 2020, 12, 4860–4867. [Google Scholar] [CrossRef]
- Panse, P.; Smith, M.; Cummings, K.; Jensen, E.; Gotway, M.; Jokerst, C. The many faces of pulmonary aspergillosis: Imaging findings with pathologic correlation. Radiol. Infect. Dis. 2016, 3, 192–200. [Google Scholar] [CrossRef]
- Ruhnke, M.; Behre, G.; Buchheidt, D.; Christopeit, M.; Hamprecht, A.; Heinz, W.; Heussel, C.P.; Horger, M.; Kurzai, O.; Karthaus, M.; et al. Diagnosis of invasive fungal diseases in haematology and oncology: 2018 update of the recommendations of the infectious diseases working party of the German society for hematology and medical oncology (AGIHO). Mycoses 2018, 61, 796–813. [Google Scholar] [CrossRef][Green Version]
- Davda, S.; Kowa, X.Y.; Aziz, Z.; Ellis, S.; Cheasty, E.; Cappocci, S.; Balan, A. The development of pulmonary aspergillosis and its histologic, clinical, and radiologic manifestations. Clin. Radiol. 2018, 73, 913–921. [Google Scholar] [CrossRef]
- Sotgiu, G.; Sulis, G.; Matteelli, A. Tuberculosis—A World Health Organization Perspective. Microbiol. Spectr. 2017, 5, 211–228. [Google Scholar] [CrossRef]
- Pai, M.; Behr, M.A.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C.C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H.; et al. Tuberculosis. Nat. Rev. Dis. Prim. 2016, 2, 16076. [Google Scholar] [CrossRef]
- Chen, X.; Hu, T.Y. Strategies for advanced personalized tuberculosis diagnosis: Current technologies and clinical approaches. Precis. Clin. Med. 2021, 4, 35–44. [Google Scholar] [CrossRef]
- Rea, G.; Sperandeo, M.; Lieto, R.; Bocchino, M.; Quarato, C.M.I.; Feragalli, B.; Valente, T.; Scioscia, G.; Giuffreda, E.; Foschino Barbaro, M.P.; et al. Chest Imaging in the Diagnosis and Management of Pulmonary Tuberculosis: The Complementary Role of Thoracic Ultrasound. Front. Med. 2021, 8, 753821. [Google Scholar] [CrossRef]
- Di Gennaro, F.; Pisani, L.; Veronese, N.; Pizzol, D.; Lippolis, V.; Saracino, A.; Monno, L.; Huson, M.A.M.; Copetti, R.; Putoto, G.; et al. Potential Diagnostic Properties of Chest Ultrasound in Thoracic Tuberculosis-A Systematic Review. Int. J. Environ. Res. Public Health 2018, 15, 2235. [Google Scholar] [CrossRef][Green Version]
- Morello, R.; De Rose, C.; Ferrari, V.; Valentini, P.; Musolino, A.M.; Biasucci, D.G.; Vetrugno, L.; Buonsenso, D. Utility and Limits of Lung Ultrasound in Childhood Pulmonary Tuberculosis: Lessons from a Case Series and Literature Review. J. Clin. Med. 2022, 11, 5714. [Google Scholar] [CrossRef]
- Giannelli, F.; Cozzi, D.; Cavigli, E.; Campolmi, I.; Rinaldi, F.; Giachè, S.; Rogasi, P.G.; Miele, V.; Bartolucci, M. Lung ultrasound (LUS) in pulmonary tuberculosis: Correlation with chest CT and X-ray findings. J. Ultrasound 2022, 25, 625–634. [Google Scholar] [CrossRef]
- Akhan, O.; Demirkazik, F.B.; Ozmen, M.N.; Balkanci, F.; Ozkara, S.; Cöplü, L.; Emri, A.; Besim, A. Tuberculous pleural effusions: Ultrasonic diagnosis. J. Clin. Ultrasound 1992, 20, 461–465. [Google Scholar] [CrossRef]
- Sharma, S.K.; Mohan, A.; Sharma, A.; Mitra, D.K. Miliary tuberculosis: New insights into an old disease. Lancet Infect. Dis. 2005, 5, 415–430. [Google Scholar] [CrossRef]
- Bosch-Marcet, J.; Serres-Créixams, X.; Zuasnabar-Cotro, A.; Codina-Puig, X.; Català-Puigbó, M.; Simon-Riazuelo, J.L. Comparison of ultrasound with plain radiography and CT for the detection of mediastinal lymphadenopathy in children with tuberculosis. Pediatr. Radiol. 2004, 34, 895–900. [Google Scholar] [CrossRef]
- Bosch-Marcet, J.; Serres-Créixams, X.; Borrás-Pérez, V.; Coll-Sibina, M.T.; Guitet-Juliá, M.; Coll-Rosell, E. Value of sonography for follow-up of mediastinal lymphadenopathy in children with tuberculosis. J. Clin. Ultrasound 2007, 35, 118–124. [Google Scholar] [CrossRef]










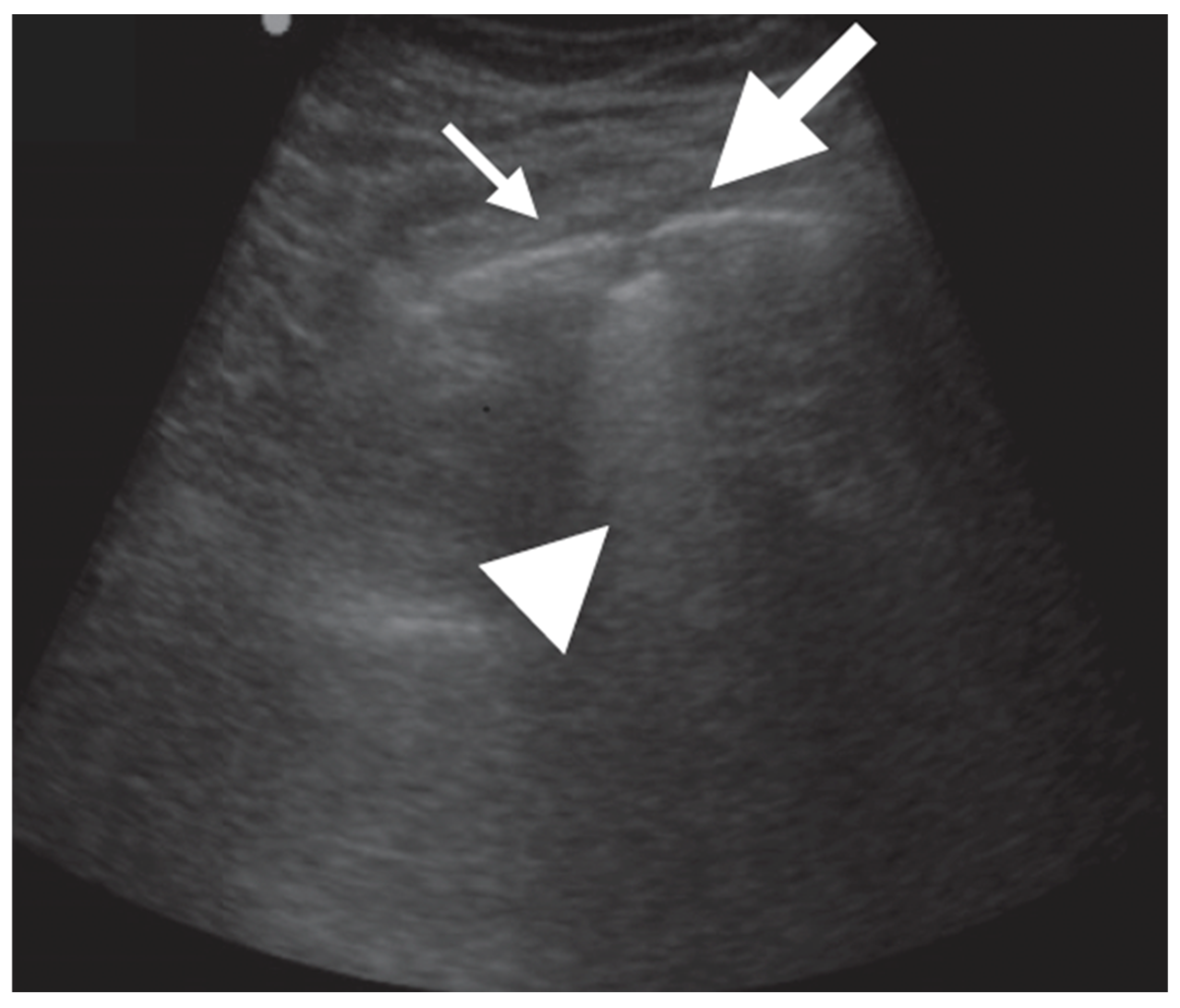
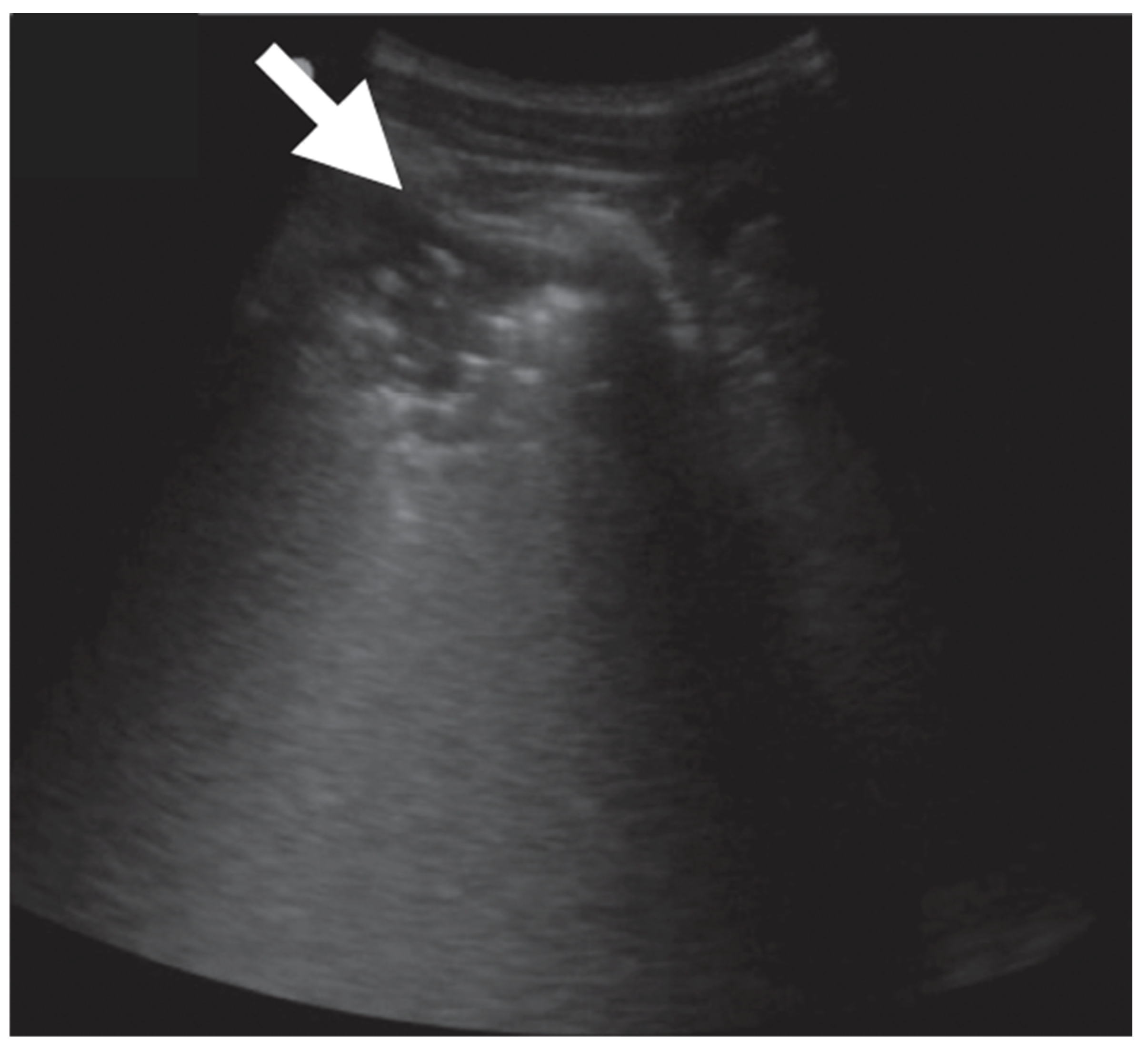

| Definition | |
|---|---|
| Consolidation | Area in which lung tissue is de-aerated with density similar to parenchymal tissues [23] |
| Atelectasis | Type of consolidation shown as hyperechogenic tissue structure visualized as solid parenchyma with static air bronchogram [11,24] |
| Cavitation | Solid, hypoechoic, heterogeneous lesions with sharp lobulated margins [11,25] |
| Pleural effusion | Hypo- or anechogenic structure, delineated by the chest wall and the diaphragm [11,26] |
| B-lines | Vertical reverberation artefacts from the pleural line to the edge of the scree; laserlike, vertical hyperechogenic artefacts synchronized with pleural line [11,23,27] |
| Pleural irregularities | Reduction or interruption of pleural line [11,28] |
| Sub-pleural nodes/granularities | Hyperechogenic subcentimetric granularities or consolidation under the pleural line [29,30] |
| N. of Patients | Age | Pleural Effusion | Consolidation | B-Lines | Atelectasias | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total | >1–1.5 cm | Total | Scattered | Confluent | |||||
| Li, 2021 [31] | 30 | Mean 9 yrs | 16 | 30 (100%) | NS | 30 | 30 | 30 | 18 |
| (53%) | (100%) | (100%) | (100%) | (60%) | |||||
| Tripaldi, 2021 [32] | 40 | Mean 4 yrs | 6 | 34 | 31 | 13 | NS | NS | NS |
| (15%) | (85%) | (77%) | (32%) | ||||||
| Buonsenso, 2022 [33] | 43 | Mean 7 yrs | 7 | 38 | 19 | 28 | 28 | 0 | 26 |
| (16%) | (88%) | (44%) | (65%) | (65%) | (60%) | ||||
| Liu G., 2022 [34] | 161 | Median 4 yrs | 48 | 136 | NS | 161 | 3 | 154 | NS |
| (29%) | (84%) | (100%) | (1%) | (95%) | |||||
| Total | 274 | 77 | 238 (86%) | 50 | 232 | 61 | 184 | 44 | |
| (28%) | (60%) | (84%) | (26%) | (79%) | (60%) | ||||
| N. of Patients | Age | Microorganism | Consolidation | Atelectasis | Cavitation | Hyper– Echoic Nodule with Hypo– Echoic Rim | Hypo– Echoic Nodule with Hyper– Echoic Rim | Pleural Effusion | B–lines | |
|---|---|---|---|---|---|---|---|---|---|---|
| Children | ||||||||||
| Trinavarat 2012 [35] | 1 | 6 weeks | Aspergillus | 1 | 1 | |||||
| (100%) | (100%) | |||||||||
| Alamdara, 2021 [36] | 6 | 5-11 yrs | 1 Mucurmicosis, 5 Aspegillus | 5 | 2 | 2 | 4 | |||
| (83%) | (33%) | (33%) | (66%) | |||||||
| Liu J., 2022 [37] | 7 | Premature newborns | 5 C. albicans, 1 C. parapsilosis, 1 Aspergillus | 7 | 2 | 2 | 7 | |||
| (100%) | (33%) | (33%) | (100%) | |||||||
| Total | 14 | 13 | 2 | 3 | 2 | 4 | 2 (14%) | 7 | ||
| (93%) | (14%) | (21%) | (14%) | (28%) | (50%) | |||||
| Adults | ||||||||||
| Tikkakoski, 1995 [38] | 4 | 49-79 yrs | 4 Aspergillus (1 A.niger, 2 A.fumigatus) | 4 | 4 | 1 (25%) | ||||
| (100%) | (100%) | |||||||||
| Grabala, 2017 [39] | 1 | 41 yrs | 1 Mucurmicosis | 1 | 1 (100%) | |||||
| (100%) | ||||||||||
| Greco, 2019 [40] | 10 | Mean 44 yrs | Not specified | 10 | 4 | 4 (40%) | 8 | |||
| (100%) | (40%) | (80%) | ||||||||
| Ruby, 2021 [41] | 1 | 45 yr | 1 Aspergillus | 1 | 1 | 1 | ||||
| (100%) | (100%) | (100%) | ||||||||
| Total | 16 | 16 | 4 | 5 | 1 | 5 (31%) | 9 | |||
| (100%) | (25%) | (31%) | (6%) | (56%) | ||||||
| N. of Patients | Mean Age | Pleural Effusion | Pleural Irregularities | Consolidation | Cavitation | Subpleural Nodes | Subpleural Granularities | B-Lines | |
|---|---|---|---|---|---|---|---|---|---|
| Children | |||||||||
| Heuvelings, 2019 [42] | 40 | 2 yrs | 12 (30%) | 31 | 22 | 0 | 0 | 0 | 13 |
| (78%) | (55%) | (33%) | |||||||
| Adults | |||||||||
| Hunter, 2016 [30] * | 10 | 33 yrs median | 0 | 0 | 0 | 0 | 0 | 10 | 10 |
| (100%) | (100%) | ||||||||
| Agostinis, 2017 [29] | 60 | 32 yrs | 11 | NS | 28 | 3 | 58 | 4 | NS |
| (18%) | (46%) | (5%) | (98%) | (6%) | |||||
| Montuori, 2019 [43] | 51 | 34 yrs | 10 | 37 | 40 | 9 | 37 | 0 | 0 |
| (20%) | (73%) | (77%) | (30%) | (73%) | |||||
| Fentress, 2020 [44] | 51 | 34 yrs | 4 | NS | 41 | 3 | 41 | 0 | 20 |
| (7%) | (80%) | (6%) | (80%) | (39%) | |||||
| Cocco, 2022 [45] | 1 | 36 yrs | 1 (100%) | 0 | 1 | 1 | 0 | 0 | 0 |
| (100%) | (100%) | ||||||||
| Total (adults) | 173 | 26 | 37 | 110 | 16 | 136 | 14 | 30 | |
| (15%) | (59%) | (63%) | (9%) | (78%) | (8%) | (26%) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meli, M.; Spicuzza, L.; Comella, M.; La Spina, M.; Trobia, G.L.; Parisi, G.F.; Di Cataldo, A.; Russo, G. The Role of Ultrasound in the Diagnosis of Pulmonary Infection Caused by Intracellular, Fungal Pathogens and Mycobacteria: A Systematic Review. Diagnostics 2023, 13, 1612. https://doi.org/10.3390/diagnostics13091612
Meli M, Spicuzza L, Comella M, La Spina M, Trobia GL, Parisi GF, Di Cataldo A, Russo G. The Role of Ultrasound in the Diagnosis of Pulmonary Infection Caused by Intracellular, Fungal Pathogens and Mycobacteria: A Systematic Review. Diagnostics. 2023; 13(9):1612. https://doi.org/10.3390/diagnostics13091612
Chicago/Turabian StyleMeli, Mariaclaudia, Lucia Spicuzza, Mattia Comella, Milena La Spina, Gian Luca Trobia, Giuseppe Fabio Parisi, Andrea Di Cataldo, and Giovanna Russo. 2023. "The Role of Ultrasound in the Diagnosis of Pulmonary Infection Caused by Intracellular, Fungal Pathogens and Mycobacteria: A Systematic Review" Diagnostics 13, no. 9: 1612. https://doi.org/10.3390/diagnostics13091612
APA StyleMeli, M., Spicuzza, L., Comella, M., La Spina, M., Trobia, G. L., Parisi, G. F., Di Cataldo, A., & Russo, G. (2023). The Role of Ultrasound in the Diagnosis of Pulmonary Infection Caused by Intracellular, Fungal Pathogens and Mycobacteria: A Systematic Review. Diagnostics, 13(9), 1612. https://doi.org/10.3390/diagnostics13091612






