General Overview and Diagnostic (Imaging) Techniques for Neurogenic Thoracic Outlet Syndrome
Abstract
1. Introduction
2. History of Neurogenic Thoracic Outlet Syndrome
3. Neurogenic Thoracic Outlet Syndrome (NTOS)
4. Gilliatt-Sumner Hand
Diagnosing GSH
5. NTOS without Signs of GSH
- Local findings include symptoms consistent with inflammation at the scalene triangle. Symptoms of referred pain can be in areas near the inflammation, for example, in the chest wall, axilla, upper back, shoulder, trapezius region, neck, or head. This pain can be triggered by palpation of the affected areas;
- Peripheral findings include symptoms consistent with nerve compression. This can include numbness, pain, paresthesia’s, vasomotor changes, and weakness. This can be tested using provocative tests that narrow the scalene triangle, an elevated arm stress test (EAST), by stretching the brachial plexus, or by an upper limb tension test (ULTT);
- The absence of diagnoses of other diseases that could explain these symptoms. For example, cervical radiculopathy, shoulder disease, carpal tunnel syndrome, and complex regional pain syndrome;
- A positive anterior scalene and/or pectoralis minor test injection.
6. Diagnosing NTOS
6.1. Initial Physiotherapy
6.1.1. History
6.1.2. Physical Examination
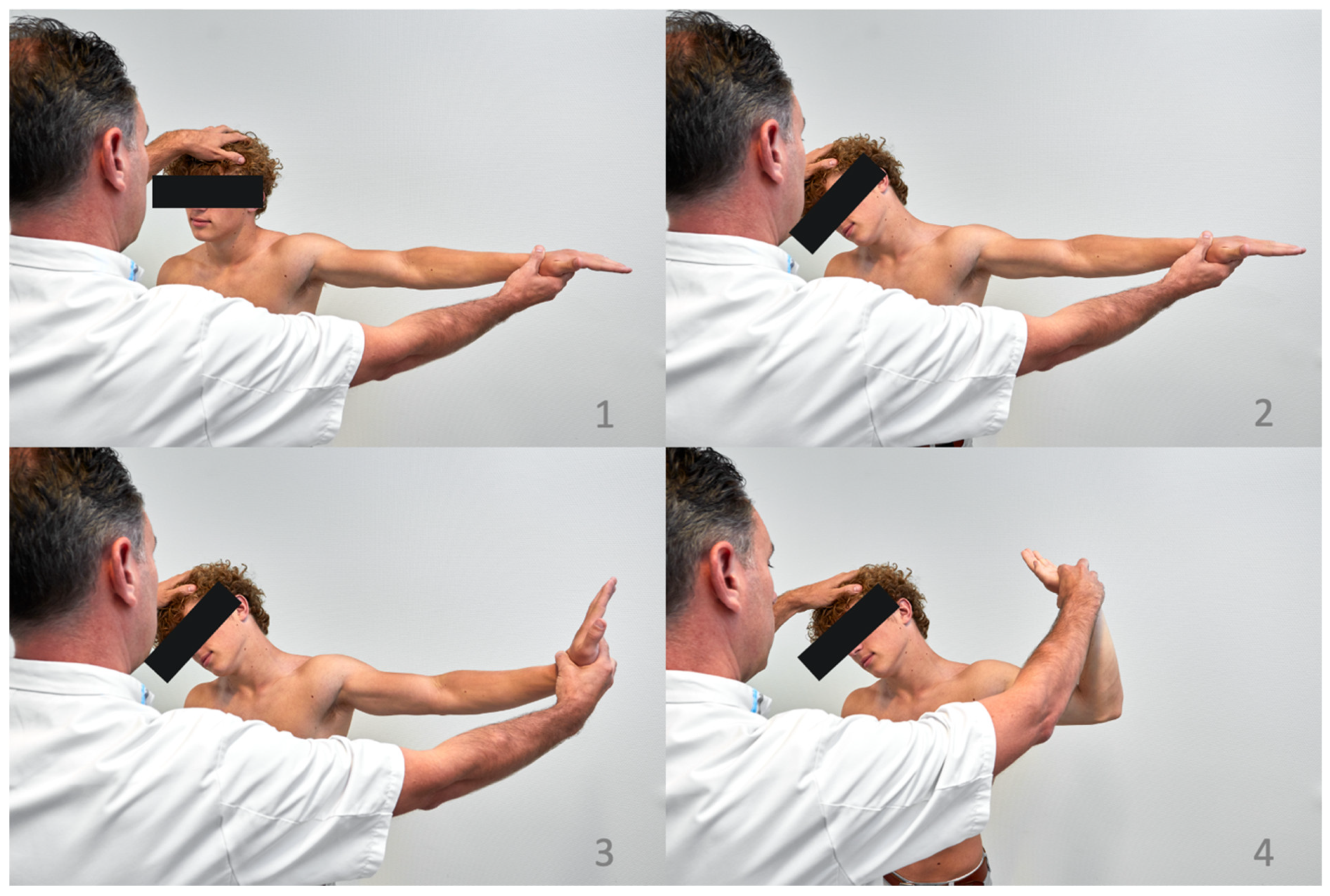
6.2. Provocation Tests
6.2.1. Morley’s Compression Test
6.2.2. Tinel Sign
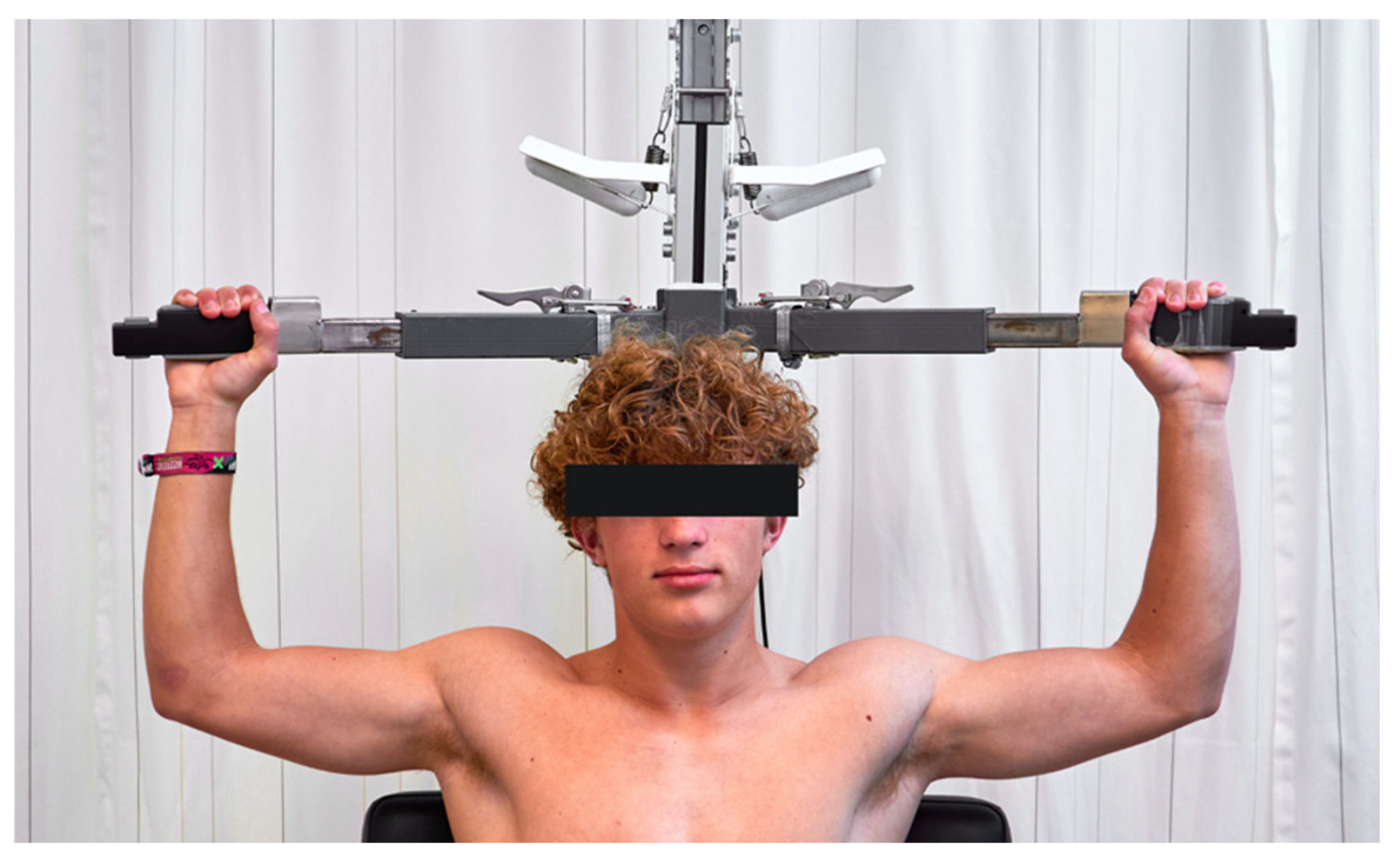
6.2.3. EAST
6.2.4. ULTT
6.3. Additional Imaging Techniques
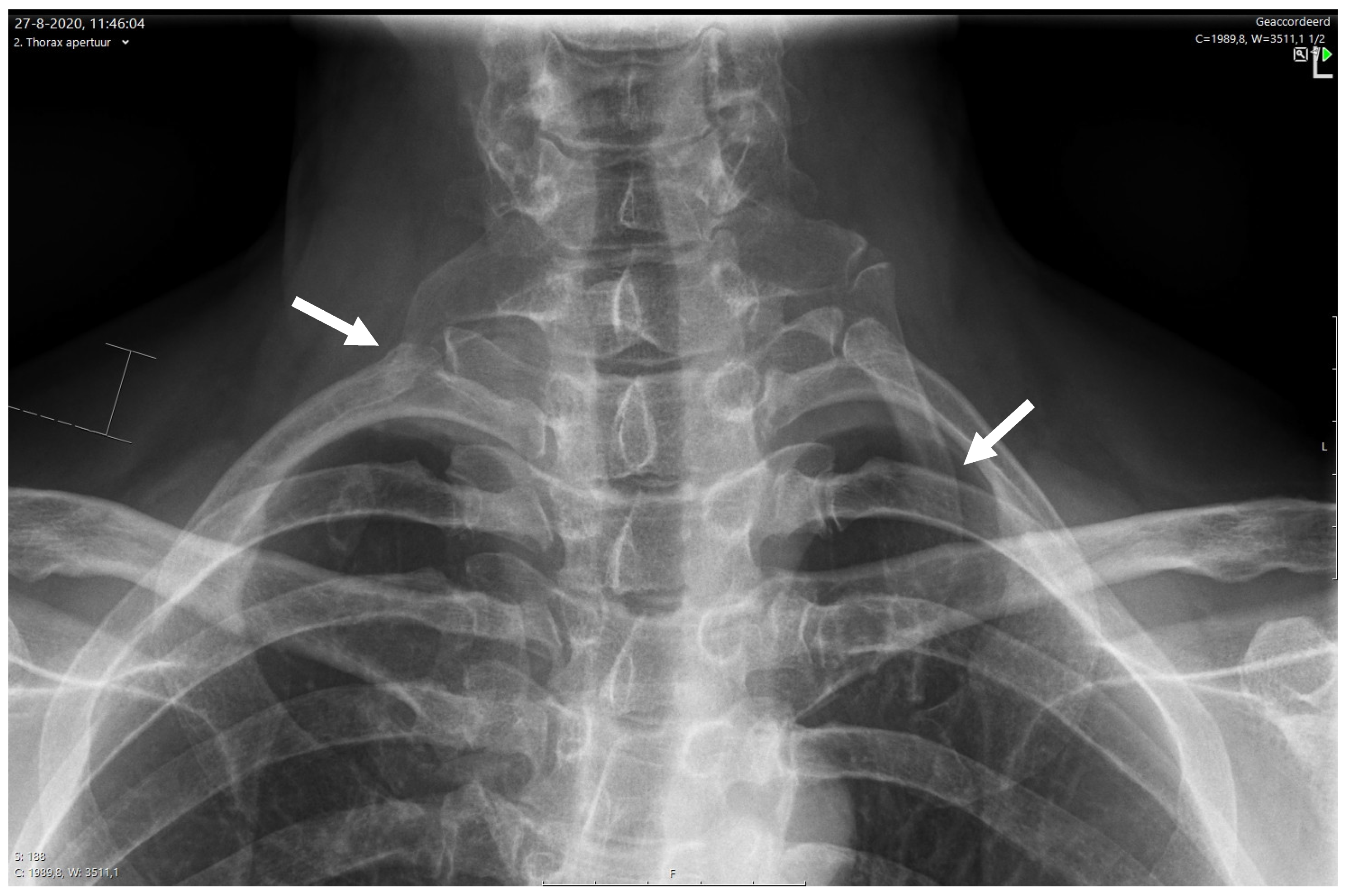
6.3.1. X-ray for NTOS
6.3.2. Magnetic Resonance Imaging for NTOS
6.3.3. Electrodiagnostic Study for NTOS
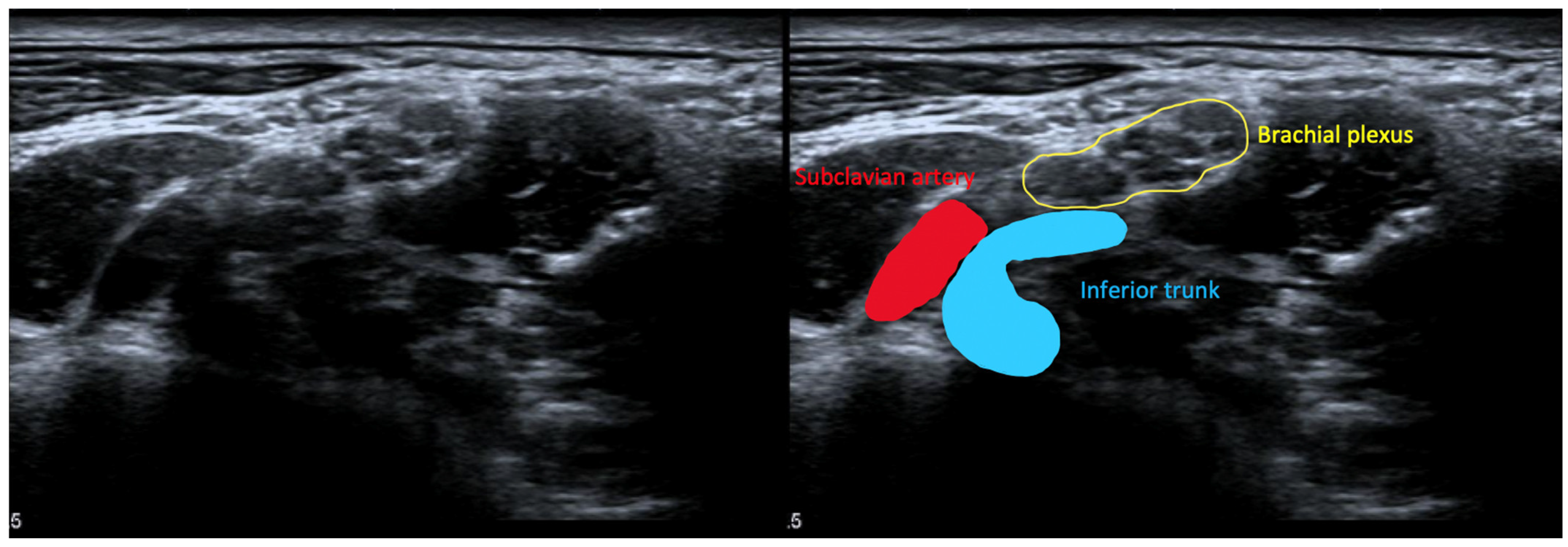
6.3.4. High-Resolution Ultrasound for NTOS
6.3.5. Duplex for NTOS
6.3.6. Scalene Muscle Block (SMB)
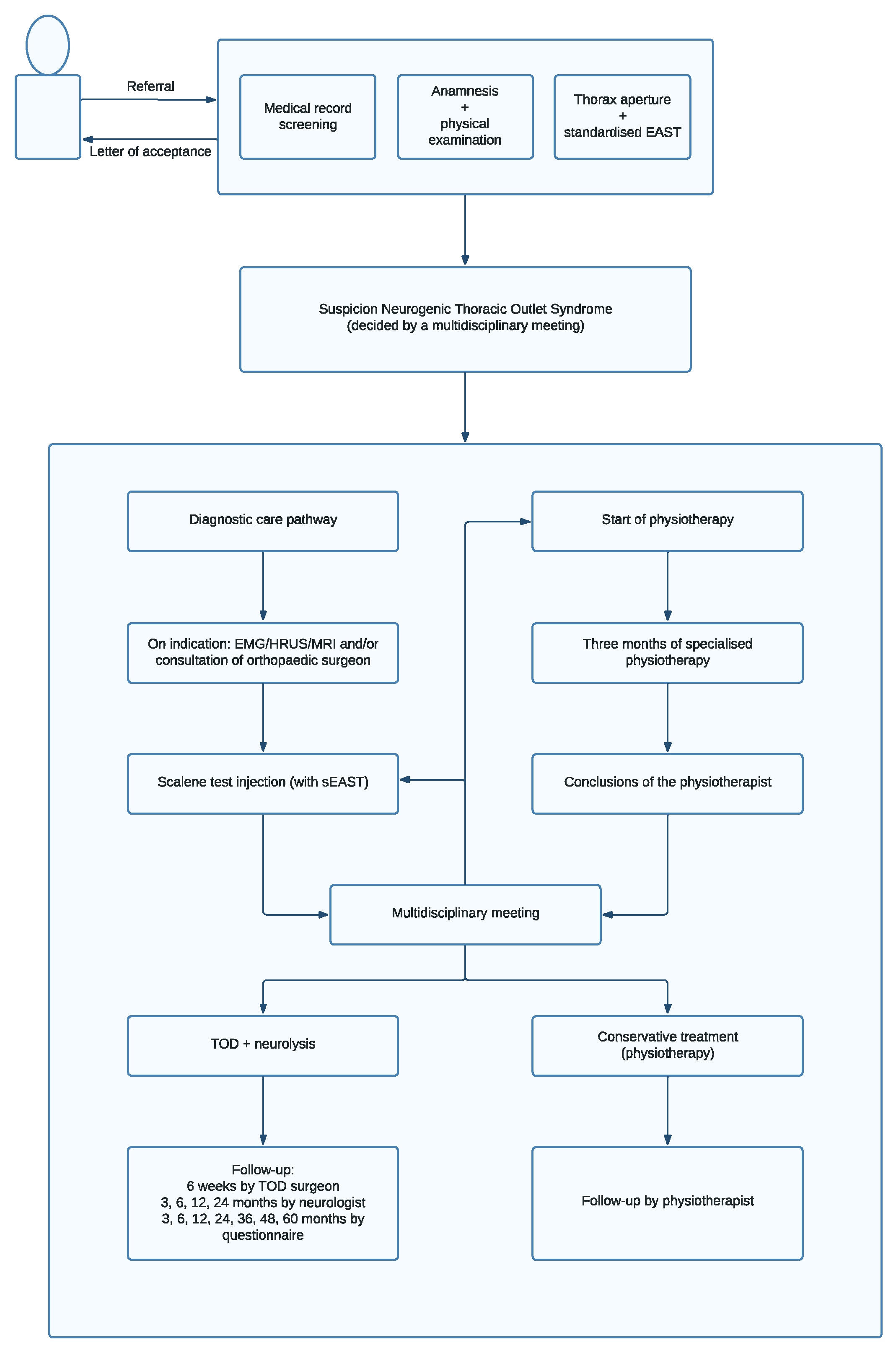
7. Multidisciplinary Meeting
Thoracic Outlet Decompression Surgery (TOD)
8. Postoperative Rehabilitation Program
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Illig, K.A.; Donahue, D.; Duncan, A.; Freischlag, J.; Gelabert, H.; Johanson, K. Reporting standards of the Society for Vascular Surgery for thoracic outlet syndrome: Executive summary. J. Vasc. Surg. 2016, 64, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Makhoul, R.G.; Machleder, H.I. Developmental anomalies at the thoracic outlet: An analysis of 200 consecutive cases. J. Vasc. Surg. 1992, 16, 534–542; discussion 542–545. [Google Scholar] [CrossRef] [PubMed]
- Sanders, R.J.; Donahue, D.M. Pathology and Pathophysiology of NTOS. In Thoracic Outlet Syndrome; Illig, K.A., Thompson, R.W., Freischlag, J.A., Donahue, D.M., Jordan, S.E., Lum, Y.W., Gelabert, H.A., Eds.; Springer International Publishing: Cham, Switwerland, 2021; pp. 53–60. [Google Scholar]
- Burt, B.M. Thoracic outlet syndrome for thoracic surgeons. J. Thorac. Cardiovasc. Surg. 2018, 156, 1318–1323.e1311. [Google Scholar] [CrossRef] [PubMed]
- Sanders, R.J.; Hammond, S.L.; Rao, N.M. Diagnosis of thoracic outlet syndrome. J. Vasc. Surg. 2007, 46, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Machleder, H.I. A Brief History of the Thoracic Outlet Compression Syndromes. In Thoracic Outlet Syndrome; Illig, K.A., Thompson, R.W., Freischlag, J.A., Donahue, D.M., Jordan, S.E., Lum, Y.W., Gelabert, H.A., Eds.; Springer International Publishing: Cham, Switwerland, 2021; pp. 7–16. [Google Scholar]
- Stopford, J.S.B.; Telford, E.D. Compression of the lower trunk of the brachial plexus by a first dorsal rib. With a note on the surgical treatment. Br. J. Surg. 2005, 7, 168–177. [Google Scholar] [CrossRef]
- Gilliatt, R.W.; Le Quesne, P.M.; Logue, V.; Sumner, A.J. Wasting of the hand associated with a cervical rib or band. J. Neurol. Neurosurg. Psychiatry 1970, 33, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Dale, W.A. Thoracic outlet compression syndrome. Critique in 1982. Arch. Surg. 1982, 117, 1437–1445. [Google Scholar] [CrossRef]
- Horowitz, S.H. Brachial plexus injuries with causalgia resulting from transaxillary rib resection. Arch. Surg. 1985, 120, 1189–1191. [Google Scholar] [CrossRef]
- Povlsen, B.; Hansson, T.; Povlsen, S.D. Treatment for thoracic outlet syndrome. Cochrane Database Syst. Rev. 2014, 11, CD007218. [Google Scholar] [CrossRef]
- Roos, D.B. Congenital anomalies associated with thoracic outlet syndrome. Anatomy, symptoms, diagnosis, and treatment. Am. J. Surg. 1976, 132, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Ohman, J.W.; Thompson, R.W. Thoracic Outlet Syndrome in the Overhead Athlete: Diagnosis and Treatment Recommendations. Curr. Rev. Musculoskelet. Med. 2020, 13, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Gilliatt, R.W. Thoracic outlet syndrome. Br. Med. J. 1983, 287, 764. [Google Scholar] [CrossRef] [PubMed]
- Mul, K.; Pesser, N.; Vervaart, K.; Teijink, J.; van Nuenen, B.; van Alfen, N. Variability in electrodiagnostic findings associated with neurogenic thoracic outlet syndrome. Muscle Nerve 2022, 65, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Dollinger, P.; Böhm, J.; Arányi, Z. Combined nerve and vascular ultrasound in thoracic outlet syndrome: A sensitive method in identifying the site of neurovascular compression. PLoS ONE 2022, 17, e0268842. [Google Scholar] [CrossRef] [PubMed]
- Goeteyn, J.; Pesser, N.; van Sambeek, M.; Vervaart, K.; van Nuenen, B.F.L.; Teijink, J.A.W. Thoracic outlet decompression surgery for Gilliatt-Sumner hand as a presentation of neurogenic thoracic outlet syndrome. J. Vasc. Surg. 2022, 75, 1985–1992. [Google Scholar] [CrossRef]
- Arányi, Z.; Csillik, A.; Böhm, J.; Schelle, T. Ultrasonographic Identification of Fibromuscular Bands Associated with Neurogenic Thoracic Outlet Syndrome: The “Wedge-Sickle” Sign. Ultrasound Med. Biol. 2016, 42, 2357–2366. [Google Scholar] [CrossRef]
- Illig, K.A.; Donahue, D.; Duncan, A.; Freischlag, J.; Gelabert, H.; Johansen, K.; Jordan, S.; Sanders, R.; Thompson, R. Reporting standards of the Society for Vascular Surgery for thoracic outlet syndrome. J. Vasc. Surg. 2016, 64, e23–e35. [Google Scholar] [CrossRef]
- Thompson, R.W. Diagnosis of Neurogenic Thoracic Outlet Syndrome: 2016 Consensus Guidelines and Other Strategies. In Thoracic Outlet Syndrome; Illig, K.A., Thompson, R.W., Freischlag, J.A., Donahue, D.M., Jordan, S.E., Lum, Y.W., Gelabert, H.A., Eds.; Springer International Publishing: Cham, Switwerland, 2021; pp. 67–97. [Google Scholar]
- Pesser, N.; Goeteyn, J.; van der Sanden, L.; Houterman, S.; van Alfen, N.; van Sambeek, M.; van Nuenen, B.F.L.; Teijink, J.A.W. Feasibility and Outcomes of a Multidisciplinary Care Pathway for Neurogenic Thoracic Outlet Syndrome: A Prospective Observational Cohort Study. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 1017–1024. [Google Scholar] [CrossRef]
- Pesser, N.; de Bruijn, B.I.; Goeteyn, J.; Houterman, S.; van Sambeek, M.R.H.M.; Thompson, R.W.; Teijink, J.A.W.; van Nuenen, B.F.L. Reliability and validity of the elevated arm stress test in the diagnosis of neurogenic thoracic outlet syndrome. J. Vasc. Surg. 2022, 76, 814–820. [Google Scholar] [CrossRef]
- Pesser, N.; de Bruijn, B.I.; Goeteyn, J.; Verhofstad, N.; Houterman, S.; van Sambeek, M.; Thompson, R.W.; van Nuenen, B.F.L.; Teijink, J.A.W. Reliability and validity of the standardized elevated arm stress test in the diagnosis of neurogenic thoracic outlet syndrome. J. Vasc. Surg. 2022, 76, 821–829.e821. [Google Scholar] [CrossRef]
- Collins, E.; Orpin, M. Physical Therapy Management of Neurogenic Thoracic Outlet Syndrome. Thorac. Surg. Clin. 2021, 31, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.T. Therapist management of thoracic outlet syndrome. J. Hand. Ther. 1994, 7, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Balderman, J.; Abuirqeba, A.A.; Eichaker, L.; Pate, C.; Early, J.A.; Bottros, M.M.; Jayarajan, S.N.; Thompson, R.W. Physical therapy management, surgical treatment, and patient-reported outcomes measures in a prospective observational cohort of patients with neurogenic thoracic outlet syndrome. J. Vasc. Surg. 2019, 70, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Povlsen, S.; Povlsen, B. Diagnosing Thoracic Outlet Syndrome: Current Approaches and Future Directions. Diagnostics 2018, 8, 21. [Google Scholar] [CrossRef]
- Kuhn, J.E.; Lebus, V.G.; Bible, J.E. Thoracic outlet syndrome. J. Am. Acad. Orthop. Surg. 2015, 23, 222–232. [Google Scholar] [CrossRef]
- Goeteyn, J.; Pesser, N.; van Sambeek, M.; Thompson, R.W.; van Nuenen, B.F.L.; Teijink, J.A.W. Duplex Ultrasound Studies Are Neither Necessary or Sufficient for the Diagnosis of Neurogenic Thoracic Outlet Syndrome. Ann. Vasc. Surg. 2022, 81, 232–239. [Google Scholar] [CrossRef]
- Balderman, J.; Holzem, K.; Field, B.J.; Bottros, M.M.; Abuirqeba, A.A.; Vemuri, C.; Thompson, R.W. Associations between clinical diagnostic criteria and pretreatment patient-reported outcomes measures in a prospective observational cohort of patients with neurogenic thoracic outlet syndrome. J. Vasc. Surg. 2017, 66, 533–544.e532. [Google Scholar] [CrossRef]
- Ferrante, M.A.; Ferrante, N.D. The thoracic outlet syndromes: Part 2. The arterial, venous, neurovascular, and disputed thoracic outlet syndromes. Muscle Nerve 2017, 56, 663–673. [Google Scholar] [CrossRef]
- Goeteyn, J.; Pesser, N.; van Nuenen, B.; van Sambeek, M.; Teijink, J. Familial predisposition of thoracic outlet syndrome: Does a familial syndrome exist? Report of cases and review of literature. Acta Chir. Belg. 2021, 121, 211–214. [Google Scholar] [CrossRef]
- Nakatsuchi, Y.; Saitoh, S.; Hosaka, M.; Uchiyama, S. Long thoracic nerve paralysis associated with thoracic outlet syndrome. J. Shoulder Elb. Surg. 1994, 3, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Nord, K.M.; Kapoor, P.; Fisher, J.; Thomas, G.; Sundaram, A.; Scott, K.; Kothari, M.J. False positive rate of thoracic outlet syndrome diagnostic maneuvers. Electromyogr. Clin. Neurophysiol. 2008, 48, 67–74. [Google Scholar] [PubMed]
- Plewa, M.C.; Delinger, M. The false-positive rate of thoracic outlet syndrome shoulder maneuvers in healthy subjects. Acad. Emerg. Med. 1998, 5, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Morley’s compression test. [CrossRef]
- Ho, T.; Braza, M.E. Hoffmann Tinel Sign. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2020. [Google Scholar]
- Roos, D.B.; Owens, J.C. Thoracic outlet syndrome. Arch. Surg. 1966, 93, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Dessureault-Dober, I.; Bronchti, G.; Bussières, A. Diagnostic Accuracy of Clinical Tests for Neurogenic and Vascular Thoracic Outlet Syndrome: A Systematic Review. J. Manip. Physiol. Ther. 2018, 41, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Gillard, J.; Pérez-Cousin, M.; Hachulla, E.; Remy, J.; Hurtevent, J.F.; Vinckier, L.; Thévenon, A.; Duquesnoy, B. Diagnosing thoracic outlet syndrome: Contribution of provocative tests, ultrasonography, electrophysiology, and helical computed tomography in 48 patients. Jt. Bone Spine 2001, 68, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Fouasson-Chailloux, A.; Daley, P.; Menu, P.; Louguet, B.; Gadbled, G.; Bouju, Y.; Abraham, P.; Dauty, M. Hand Strength Deficit in Patients with Neurogenic Thoracic Outlet Syndrome. Diagnostics 2021, 11, 874. [Google Scholar] [CrossRef] [PubMed]
- Raptis, C.A.; Sridhar, S.; Thompson, R.W.; Fowler, K.J.; Bhalla, S. Imaging of the Patient with Thoracic Outlet Syndrome. Radiographics 2016, 36, 984–1000. [Google Scholar] [CrossRef]
- Szaro, P.; McGrath, A.; Ciszek, B.; Geijer, M. Magnetic resonance imaging of the brachial plexus. Part 1: Anatomical considerations, magnetic resonance techniques, and non-traumatic lesions. Eur. J. Radiol. Open 2022, 9, 100392. [Google Scholar] [CrossRef] [PubMed]
- Khalilzadeh, O.; Glover, M.; Torriani, M.; Gupta, R. Imaging Assessment of Thoracic Outlet Syndrome. Thorac. Surg. Clin. 2021, 31, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Daley, P.; Pomares, G.; Gross, R.; Menu, P.; Dauty, M.; Fouasson-Chailloux, A. Use of Electroneuromyography in the Diagnosis of Neurogenic Thoracic Outlet Syndrome: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 5206. [Google Scholar] [CrossRef] [PubMed]
- Pesser, N.; Teijink, J.A.W.; Vervaart, K.; Goeteyn, J.; Gons, R.A.R.; van Sambeek, M.R.H.M.; van Nuenen, B.F.L. Value of Ultrasound in the Diagnosis of Neurogenic Thoracic Outlet Syndrome. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 852–853. [Google Scholar] [CrossRef] [PubMed]
- Fouasson-Chailloux, A.; Menu, P.; Daley, P.; Gautier, G.; Gadbled, G.; Abraham, P.; Dauty, M. Subclavian Vessel Compression Assessed by Duplex Scanning in Patients with Neurogenic Thoracic Outlet Syndrome and No Vascular Signs. Diagnostics 2021, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Lum, Y.W.; Brooke, B.S.; Likes, K.; Modi, M.; Grunebach, H.; Christo, P.J.; Freischlag, J.A. Impact of anterior scalene lidocaine blocks on predicting surgical success in older patients with neurogenic thoracic outlet syndrome. J. Vasc. Surg. 2012, 55, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Goeteyn, J.; Van Der Sanden, L.; Pesser, N.; Houterman, S.; van Sambeek, M.; van Nuenen, B.F.L.; Teijink, J.A.W. Redo surgery for neurogenic thoracic outlet syndrome is useful. J. Vasc. Surg. 2022, 76, 531–537.e531. [Google Scholar] [CrossRef]
- Gelabert, H.A.; Jabori, S.; Barleben, A.; Kiang, S.; O’Connell, J.; Jimenez, J.C.; Derubertis, B.; Rigberg, D. Regrown first rib in patients with recurrent thoracic outlet syndrome. Ann. Vasc. Surg. 2014, 28, 933–938. [Google Scholar] [CrossRef]
- Povlsen, B.; Belzberg, A.; Hansson, T.; Dorsi, M. Treatment for thoracic outlet syndrome. Cochrane Database Syst. Rev. 2010, 1, CD007218. [Google Scholar] [CrossRef]
- Caputo, F.J.; Wittenberg, A.M.; Vemuri, C.; Driskill, M.R.; Earley, J.A.; Rastogi, R.; Emery, V.B.; Thompson, R.W. Supraclavicular decompression for neurogenic thoracic outlet syndrome in adolescent and adult populations. J. Vasc. Surg. 2013, 57, 149–157. [Google Scholar] [CrossRef]
- Gelabert, H.; Rigberg, D.A.; Jiminez, J.C.; Jabori, S.; Farley, S.; O’Connell, J.B. Transaxillary decompression of thoracic outlet syndrome patients presenting with cervical ribs. J. Vasc. Surg. 2018, 68, 1143–1149. [Google Scholar] [CrossRef]
- Phillips, W.W.; Donahue, D.M. Reoperation for Persistent or Recurrent Neurogenic Thoracic Outlet Syndrome. Thorac. Surg. Clin. 2021, 31, 89–96. [Google Scholar] [CrossRef]
| Neurological | Musculoskeletal System | Vascular/Lymphogenic | Other |
|---|---|---|---|
| Brachial plexus lesion: - Traumatic - Malignancy - Latrogen | AC joint arthritis | Arm vein thrombosis caused by malignancy or catheter | Complex regional pain syndrome |
| Carpal tunnel syndrome | Biceps tendinopathy | ATOS | Pancoast tumor |
| Cervical radiculopathy | Cervical arthritis | Lymphoedema | Psychogenic pain |
| Multiple sclerosis | Costochondritis | Raynaud’s disease | Rheumatoid arthritis |
| Neuralgic amyotrophy (Parsonage–Turner) | Fibromyalgia | VTOS | Scleroderma |
| Ulnar neuropathy | Rotator cuff tendinopathy | ||
| Scapular dyskinesia | |||
| Subacromial syndrome |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teijink, S.B.J.; Pesser, N.; Goeteyn, J.; Barnhoorn, R.J.; van Sambeek, M.R.H.M.; van Nuenen, B.F.L.; Gelabert, H.A.; Teijink, J.A.W. General Overview and Diagnostic (Imaging) Techniques for Neurogenic Thoracic Outlet Syndrome. Diagnostics 2023, 13, 1625. https://doi.org/10.3390/diagnostics13091625
Teijink SBJ, Pesser N, Goeteyn J, Barnhoorn RJ, van Sambeek MRHM, van Nuenen BFL, Gelabert HA, Teijink JAW. General Overview and Diagnostic (Imaging) Techniques for Neurogenic Thoracic Outlet Syndrome. Diagnostics. 2023; 13(9):1625. https://doi.org/10.3390/diagnostics13091625
Chicago/Turabian StyleTeijink, Stijn B. J., Niels Pesser, Jens Goeteyn, Renée J. Barnhoorn, Marc R. H. M. van Sambeek, Bart F. L. van Nuenen, Hugh A. Gelabert, and Joep A. W. Teijink. 2023. "General Overview and Diagnostic (Imaging) Techniques for Neurogenic Thoracic Outlet Syndrome" Diagnostics 13, no. 9: 1625. https://doi.org/10.3390/diagnostics13091625
APA StyleTeijink, S. B. J., Pesser, N., Goeteyn, J., Barnhoorn, R. J., van Sambeek, M. R. H. M., van Nuenen, B. F. L., Gelabert, H. A., & Teijink, J. A. W. (2023). General Overview and Diagnostic (Imaging) Techniques for Neurogenic Thoracic Outlet Syndrome. Diagnostics, 13(9), 1625. https://doi.org/10.3390/diagnostics13091625






