Application of a Novel One-Side Cell Quartz Crystal Microbalance Immunosensor in the Determination of Alpha-Fetoprotein from Human Serum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
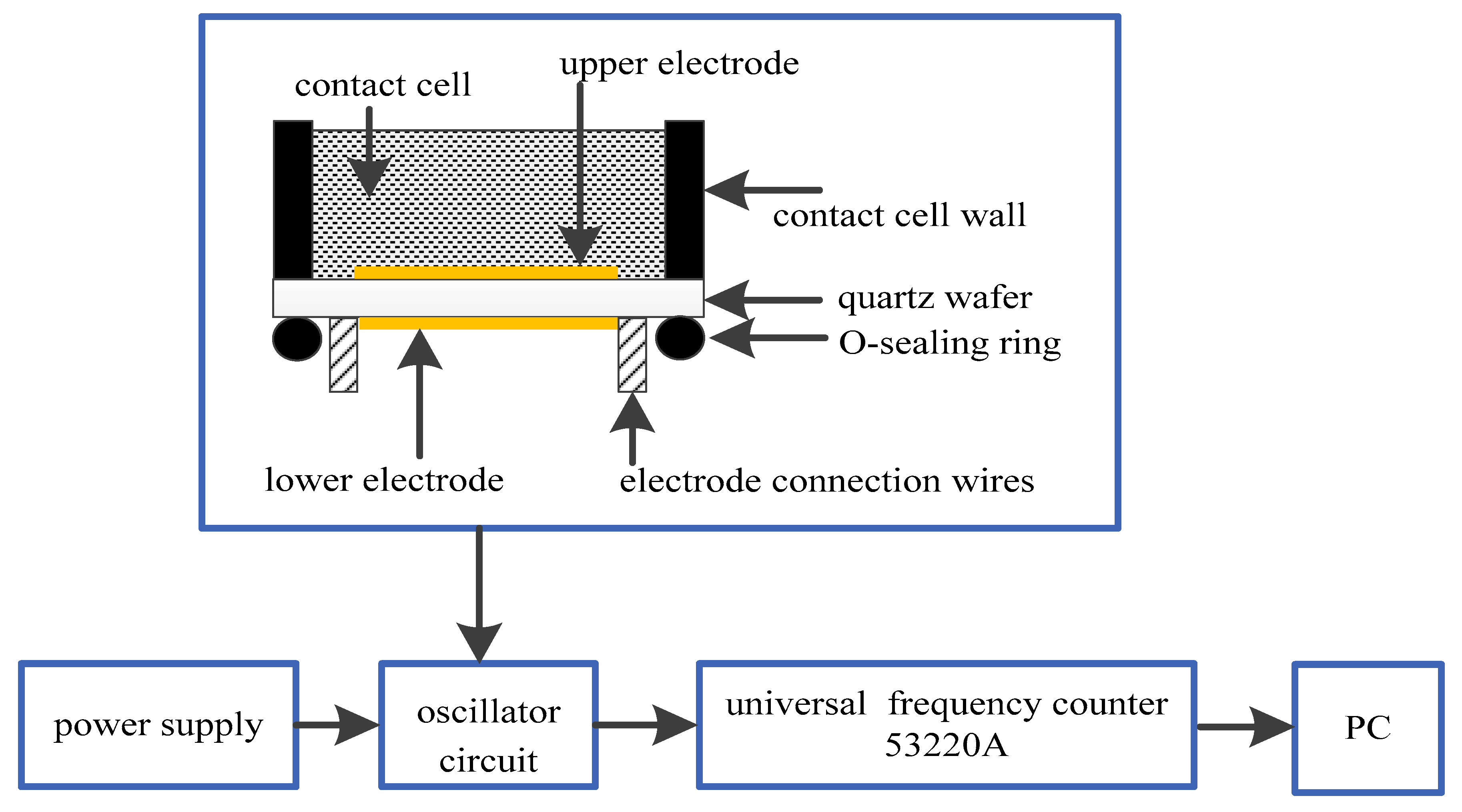
2.2. Equipment and Apparatus
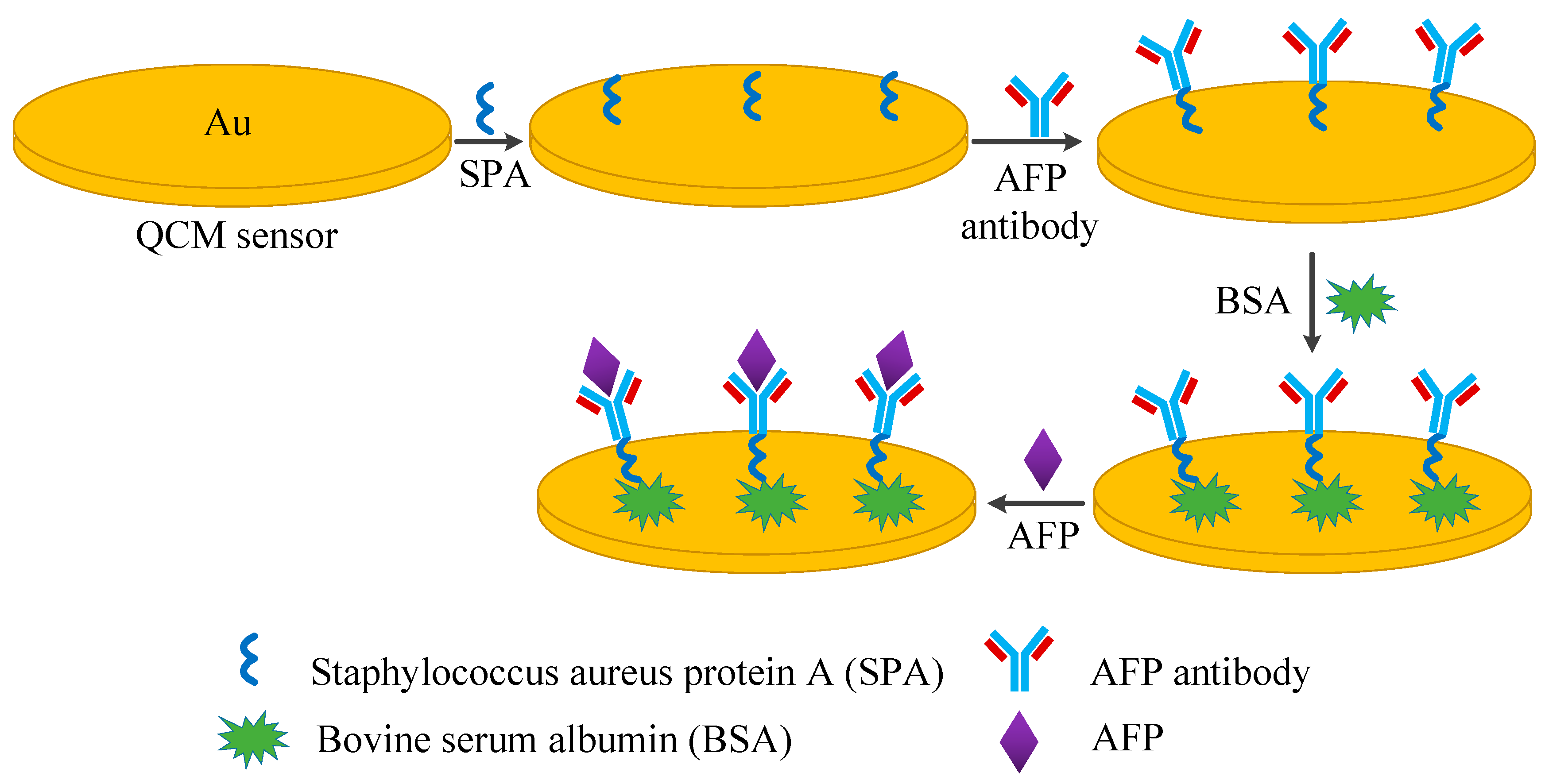
2.3. Antibody Immobilization Procedure
2.3.1. Quartz Crystal Preparation and Pre-Treatment
2.3.2. Antibody Immobilization
2.4. Detection Procedure
3. Results and Discussion
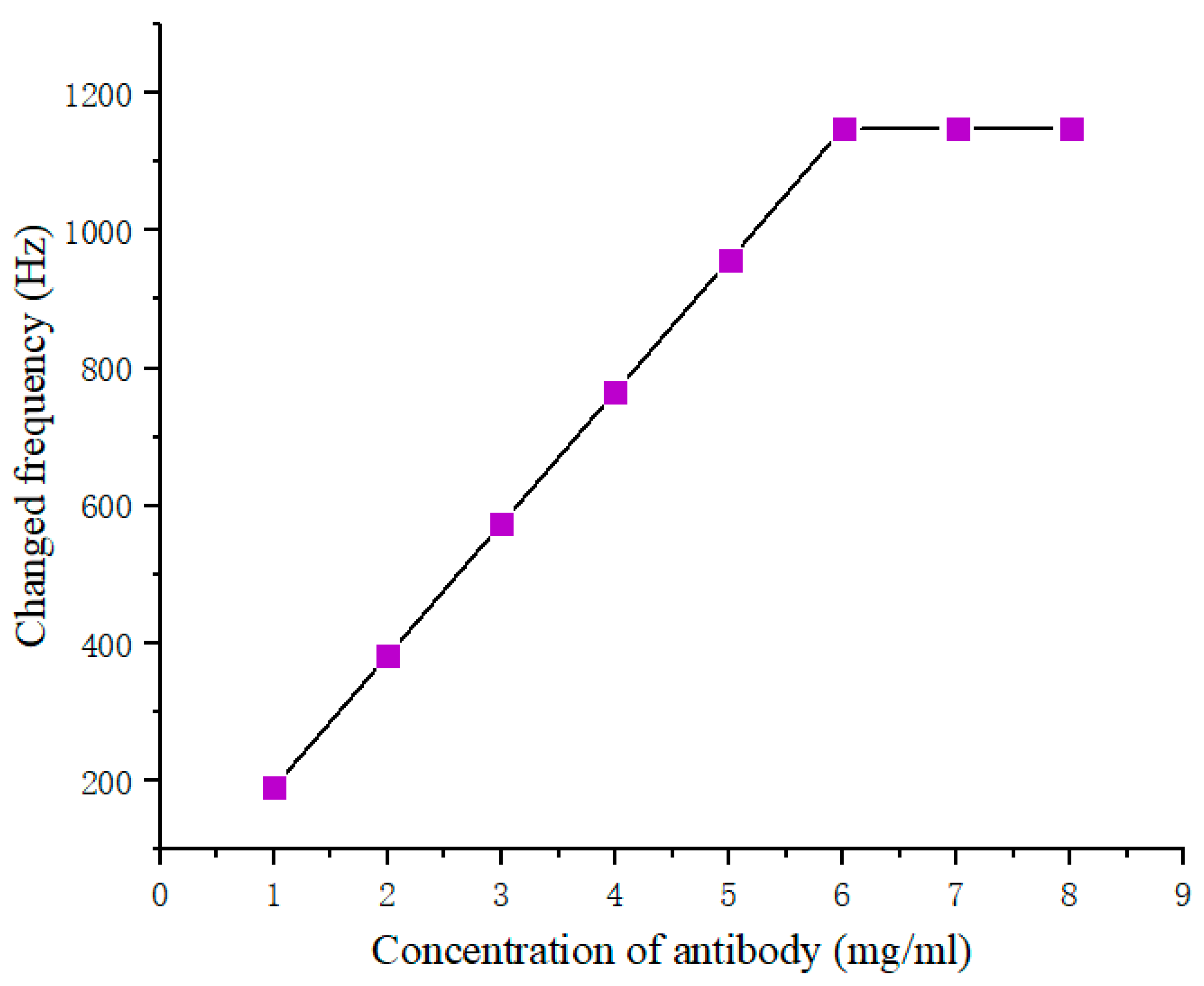
3.1. Concentration Selection for AFP Antibody
3.2. Reproducibility of Antibody Immobilization
3.3. Optimal Detection Time of QCM Immunosensor Device
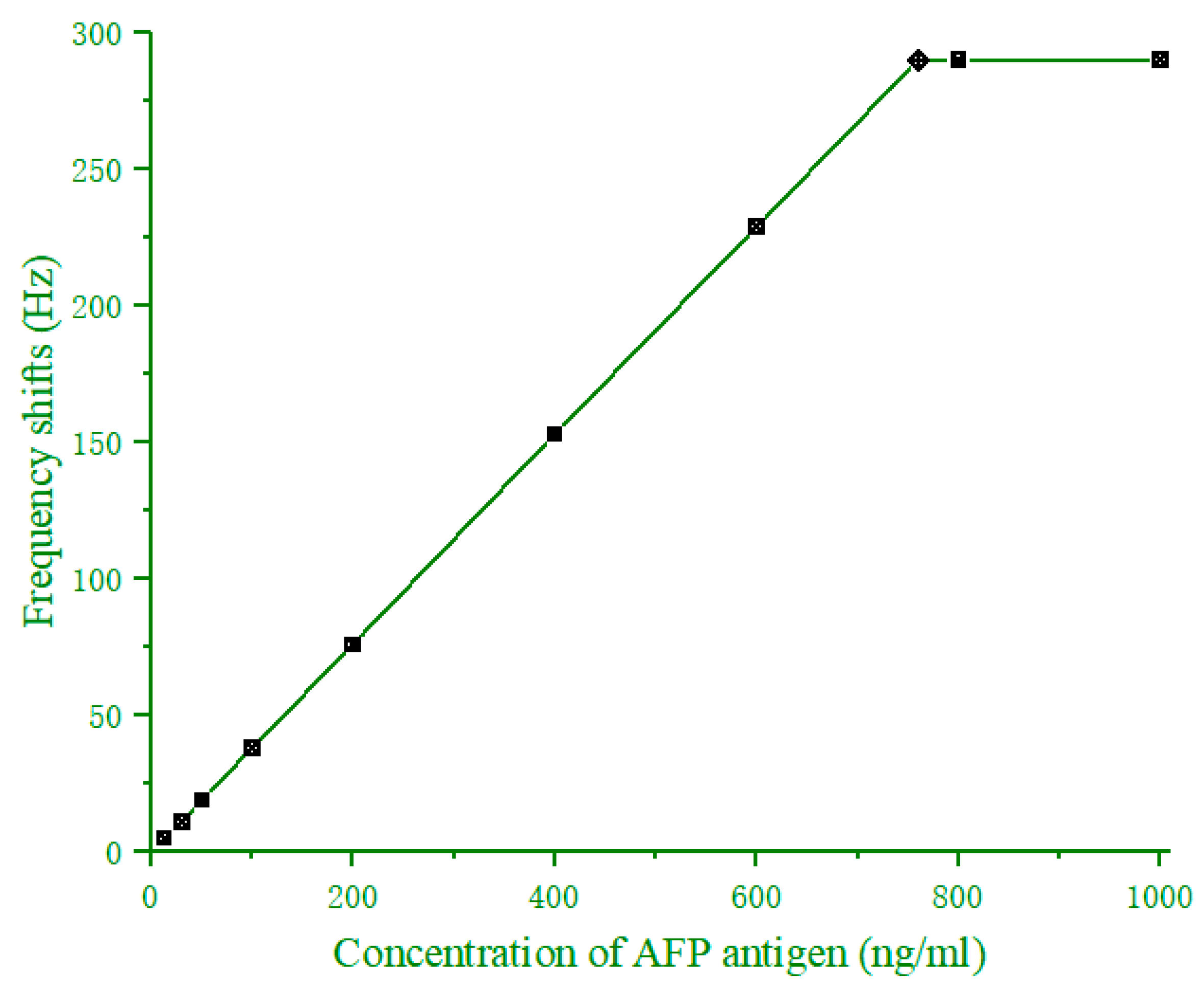
3.4. Standard Curve for Determination of AFP
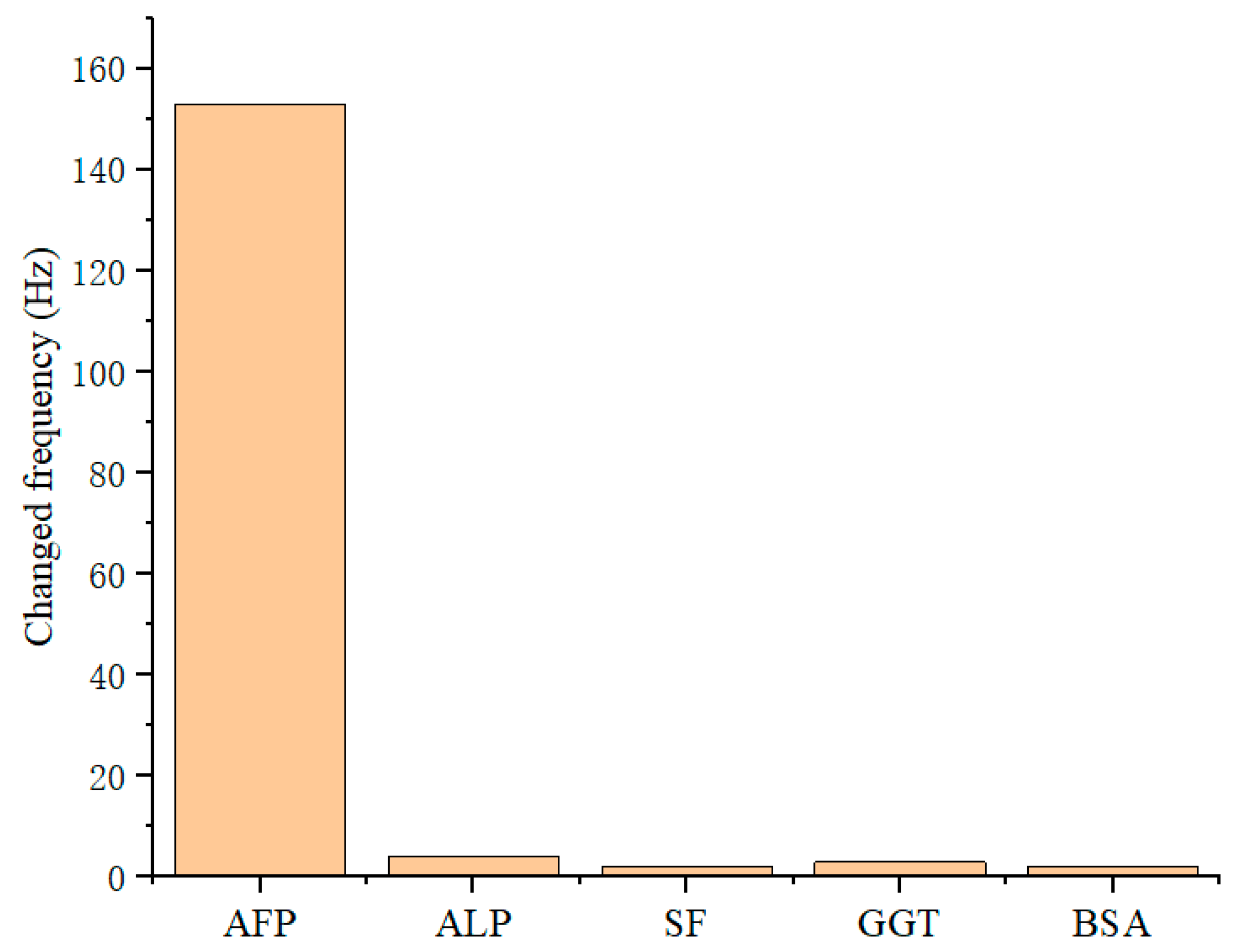
3.5. Selectivity of QCM Sensor
3.6. Detection of AFP from Real Serum Samples
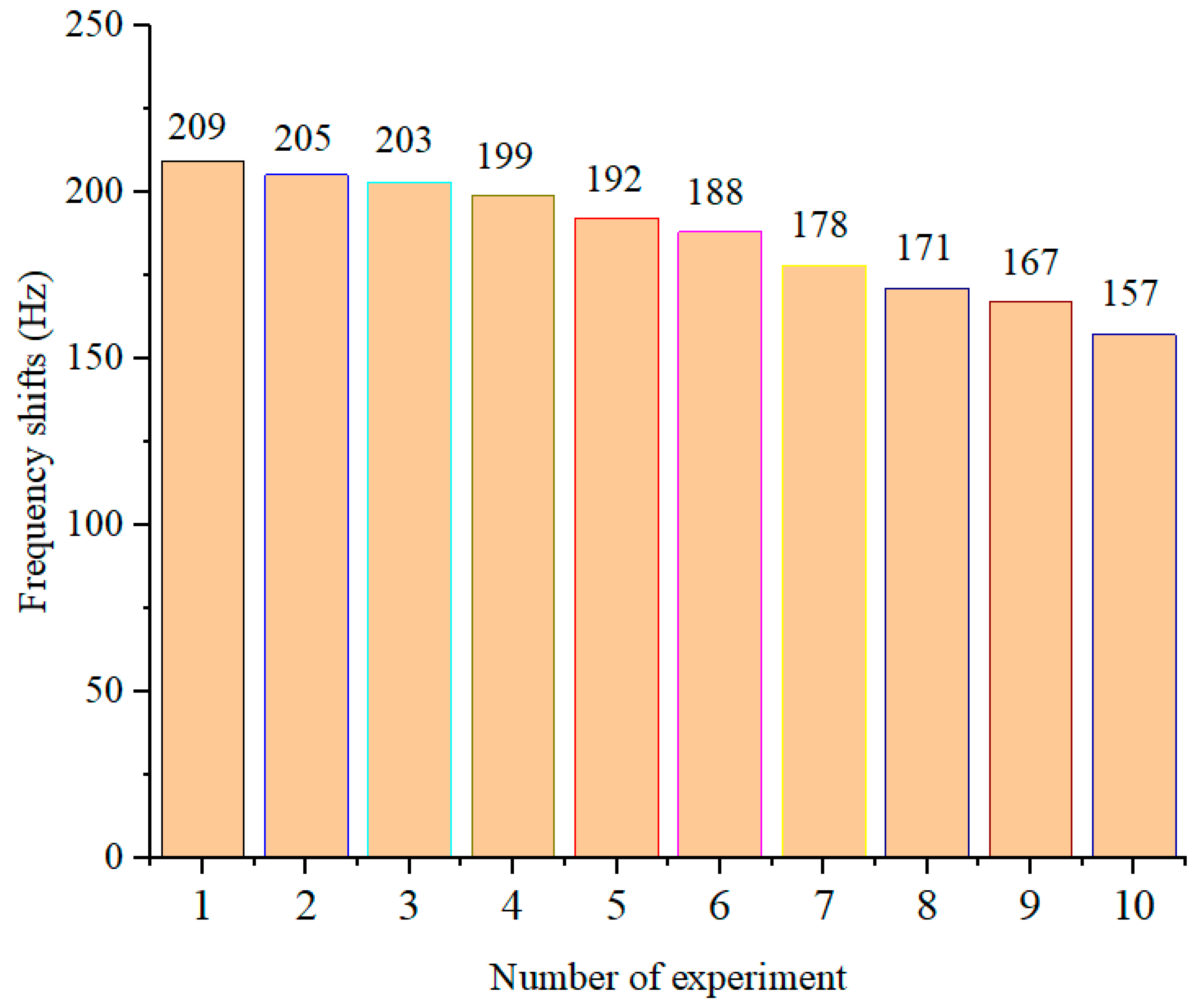
3.7. Reproducibility of QCM Immunosensors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, I.; Soerjomataram, A.; Jemal, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Gao, S.; Xu, J.; Zheng, M.; Huang, Y.; Yu, Y.; Lin, J. A novel serum protein purification technique combined with surface-enhanced raman spectroscopy for liver cancer detection. Spectrosc. Lett. 2021, 54, 113–121. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, S.; Wei, C.; Xing, Z.; Zhang, S.; Zhang, X. Metal stable isotope tagging: Renaissance of radioimmunoassay for multiplex and absolute quantification of biomolecules. Acc. Chem. Res. 2016, 49, 775–783. [Google Scholar] [CrossRef]
- Waldmann, T.A.; Mclntire, K.R. The use of a radioimmunoassay for alpha-fetoprotein in the diagnosis of malignancy. Cancer 1974, 34, 1510–1515. [Google Scholar] [CrossRef]
- Liu, J.W.; Zhao, J.J.; Li, S.T.; Zhang, L.L.; Huang, Y.; Zhao, S.L. A novel microchip electrophoresis-based chemiluminescence immunoassay for the detection of alpha-fetoprotein in human serum. Talanta 2017, 165, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, Y.; Wang, X.; Jiao, J.; Li, Y.; Gu, W.; Liang, X. A pH Indicator-linked Immunosorbent assay following direct amplification strategy for colorimetric detection of protein biomarkers. Biosens. Bioelectron. 2017, 90, 1–5. [Google Scholar]
- Shao, F.; Jiao, L.; Miao, L.; Wei, Q.; Li, H. Automatic time-resolved fluorescence immunoassay of serum alpha fetoprotein-l3 variant via LCA magnetic cationic polymeric liposomes improves the diagnostic accuracy of liver cancer. Int. J. Nanomed. 2020, 15, 4933–4941. [Google Scholar]
- Chen, J.Y.; Penn, L.S.; Xi, J. Quartz crystal microbalance: Sensing cell-substrate adhesion and beyond. Biosens. Bioelectron. 2018, 99, 593–602. [Google Scholar] [CrossRef]
- Liu, S.M.; Liu, X.Y.; Pan, Q.W.; Dai, Z.H.; Pan, M.F.; Wang, S. A portable, label-free, reproducible quartz crystal microbalance immunochip for the detection of zearalenone in food samples. Biosensors 2021, 11, 53. [Google Scholar] [CrossRef]
- Lim, H.J.; Saha, T.; Tey, B.T.; Tan, W.S.; Ooi, C.W. Quartz crystal microbalance based biosensors as rapid diagnostic devices for infectious diseases. Biosens. Bioelectron. 2020, 168, 112513. [Google Scholar] [CrossRef]
- Dultsev, F.N.; Tronin, A.V. Rapid sensing of hepatitis B virus using QCM in the thickness shear mode. Sens. Actuators B Chem. 2015, 216, 1–5. [Google Scholar] [CrossRef]
- Pandey, L.M. Design of engineered surfaces for prospective detection of SARS-CoV-2 using quartz crystal microbalance based techniques. Expert Rev. Proteomics 2020, 17, 425–432. [Google Scholar] [CrossRef]
- Yang, L.; Huang, X.H.; Sun, L.; Xu, L. A piezoelectric immunosensor for the rapid detection of p16INK4a expression in liquid-based cervical cytology specimens. Sens. Actuators B Chem. 2016, 224, 863–867. [Google Scholar] [CrossRef]
- Suthar, J.; Parsons, E.S.; Hoogenboom, B.W.; Williams, G.R.; Guldin, S. Acoustic immunosensing of exosomes using a quartz crystal microbalance with dissipation monitoring. Anal. Chem. 2020, 92, 4082–4093. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrey, G.Z. The use of quartz oscillators for weighing layers and for micro-weighing. Z. Phys. A 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Torad, N.L.; Zhang, S.H.; Amer, W.A.; Ayad, M.M.; Kim, M.J.; Kim, J.H.; Ding, B.; Zhang, X.G.; Kimura, T.; Yamauchi, Y. Advanced nanoporous material–based QCM devices: A new horizon of interfacial mass sensing technology. Adv. Mater. Interfaces 2019, 6, 1900849. [Google Scholar] [CrossRef]
- Bunde, R.L.; Jarvi, E.J.; Rosentreter, J.J. Review piezoelectric quartz crystal biosensors. Talanta 1998, 46, 1223–1236. [Google Scholar] [CrossRef]
- Ajmani, P.S. Immunohematology and Blood Banking, Blood Group and Immunology, 1st ed.; Springer: Singapore, 2020; pp. 7–23. [Google Scholar]
- Zhao, S.H.; Zhao, Y. Crystal Oscillator, 1st ed.; Science Press: Beijing, China, 2008; pp. 88–89. [Google Scholar]
- Liu, K.; Zhang, C. Volatile organic compounds gas sensor based on quartz crystal microbalance for fruit freshness detection: A review. Food Chem. 2021, 334, 127615. [Google Scholar] [CrossRef]
- Yang, M.; Thompson, M. Multiple chemical information from the thickness shear mode acoustic wave sensor in the liquid phase. Anal. Chem. 1993, 65, 1158–1168. [Google Scholar] [CrossRef]
- Kurosawa, S.; Tawara, E.; Kamo, N.; Kobatake, Y. Oscillating frequency of piezoelectric quartz crystal in solutions. Anal. Chim. Acta 1990, 230, 41–49. [Google Scholar] [CrossRef]
- Dong, Y.G.; Feng, G.P. Effects of surface physical sorption on characteristic of coated quartz crystal humidity sensors. Sens. Actuators B Chem. 1995, 24, 62–64. [Google Scholar] [CrossRef]
- Hillier, A.C.; Ward, M.D. Scanning electrochemical mass sensitivity mapping of the quartz crystal microbalance in liquid media. Anal. Chem. 1992, 64, 2539–2554. [Google Scholar] [CrossRef]


| Crystal Number | AFP Antibody Immobilization
(Hz) | a Antigen Recognition
(Hz) |
|---|---|---|
| 1 | 1147 | 150 |
| 2 | 1148 | 156 |
| 3 | 1142 | 151 |
| 4 | 1146 | 152 |
| 5 | 1149 | 155 |
| 6 | 1144 | 158 |
| 7 | 1147 | 153 |
| 8 | 1143 | 154 |
| 9 | 1146 | 152 |
| 10 | 1148 | 159 |
| Average | 1146 | 154 |
| SD | 2.2 | 2.82 |
| CV(%) | 0.19 | 1.8 |
| Serum Samples | 1 a | 2 b | 3 c | 4 c | 5 c | 6 c | 7 c | 8 c |
|---|---|---|---|---|---|---|---|---|
| QCM number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| QCM (ng.mL−1) | 15.6 | 18.2 | 423.4 | 289.5 | 355.8 | 199.3 | 501.1 | 545.3 |
| RIA (ng.mL−1) | 15.2 | 17.7 | 436.1 | 280.3 | 342.4 | 190.9 | 517.1 | 520.5 |
| Relative deviation (%) | 2.6 | 2.8 | −2.9 | 3.3 | 3.9 | 4.4 | −3.1 | 4.8 |
| correlation coefficient (r) | 0.9978 | |||||||
| Method | Analytic Time | Radio Contamination | Labeled Antibody | Cost | Analysis Process | References |
|---|---|---|---|---|---|---|
| QCM | Half hour | No | No | Low | Simple | This study |
| RIA | Hours | Yes | Yes | High | Cumbersome | [4] |
| QCM Numbers | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Frequency changes (Hz) | 209 | 207 | 203 | 211 | 205 | 206 | 210 | 208 | 204 | 209 |
| Average (Hz) | 207 | |||||||||
| SD (Hz) | 2.5 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Shi, H.; Mu, B. Application of a Novel One-Side Cell Quartz Crystal Microbalance Immunosensor in the Determination of Alpha-Fetoprotein from Human Serum. Diagnostics 2023, 13, 1630. https://doi.org/10.3390/diagnostics13091630
Chen Y, Shi H, Mu B. Application of a Novel One-Side Cell Quartz Crystal Microbalance Immunosensor in the Determination of Alpha-Fetoprotein from Human Serum. Diagnostics. 2023; 13(9):1630. https://doi.org/10.3390/diagnostics13091630
Chicago/Turabian StyleChen, Yan, Huashan Shi, and Bo Mu. 2023. "Application of a Novel One-Side Cell Quartz Crystal Microbalance Immunosensor in the Determination of Alpha-Fetoprotein from Human Serum" Diagnostics 13, no. 9: 1630. https://doi.org/10.3390/diagnostics13091630




