Application of Hybridization Chain Reaction/CRISPR-Cas12a for the Detection of SARS-CoV-2 Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HCR Probes Testing
2.3. Sensitivity Testing of HCR Reaction
2.4. CRISPR-Cas12a Detection
2.5. Clinical Evaluation of HCR Reaction
3. Results
3.1. HCR Probes Testing
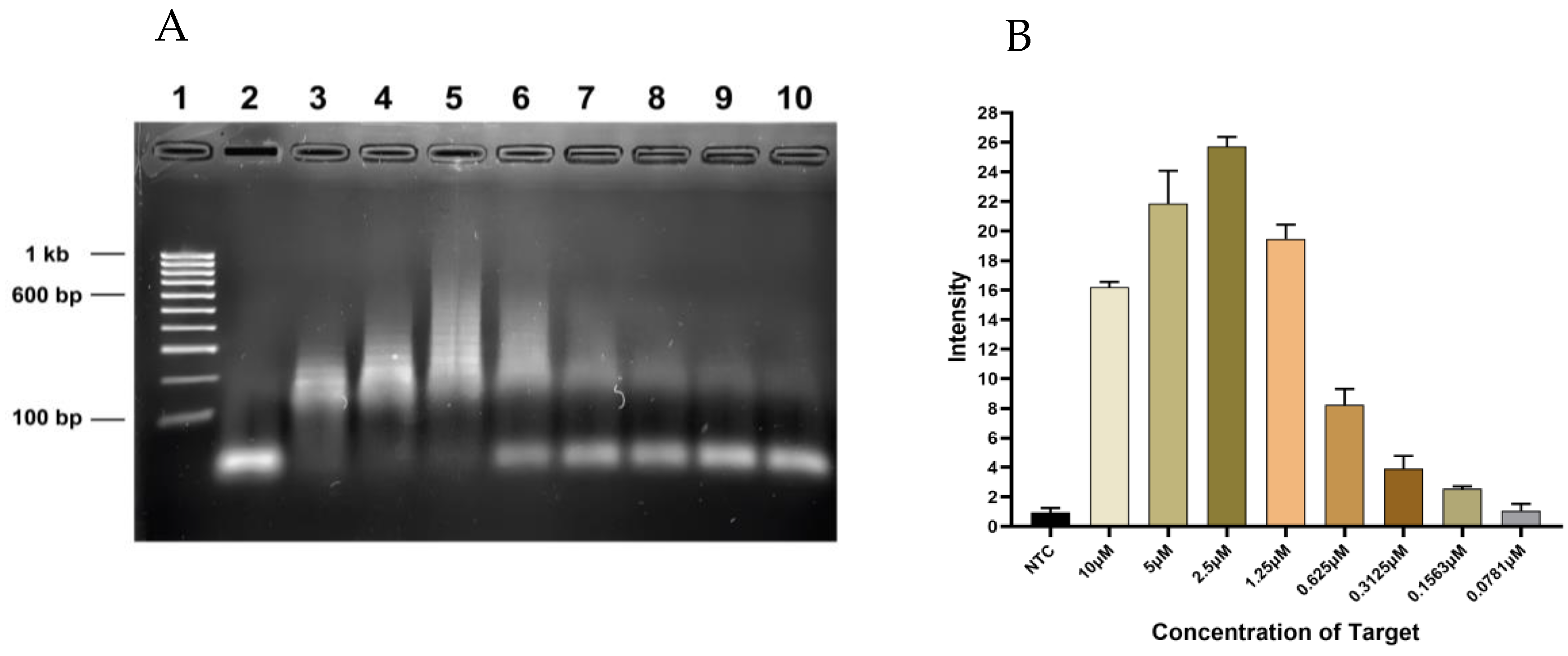
3.2. Sensitivity Testing of HCR Reaction
3.3. CRISPR-Cas12a Detection
3.4. Clinical Evaluation of HCR Reaction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Jain, U. Effect of COVID-19 on the Organs. Cureus 2020, 12, e9540. [Google Scholar] [CrossRef]
- Sodagar, A.; Javed, R.; Tahir, H.; Razak, S.I.A.; Shakir, M.; Naeem, M.; Yusof, A.H.A.; Sagadevan, S.; Hazafa, A.; Uddin, J.; et al. Pathological Features and Neuroinflammatory Mechanisms of SARS-CoV-2 in the Brain and Potential Therapeutic Approaches. Biomolecules 2022, 12, 971. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. COVID-19 Weekly Epidemiological Update; World Health Organization: Geneva, Switzerland, 2022; pp. 1–33. Available online: https://www.who.int/publications/m/item/covid-19-weekly-epidemiological-update (accessed on 12 March 2023).
- Mathieu, E.; Ritchie, H.; Ortiz-Ospina, E.; Roser, M.; Hasell, J.; Appel, C.; Giattino, C.; Rodés-Guirao, L. A global database of COVID-19 vaccinations. Nat. Hum. Behav. 2021, 5, 947–953. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Martini, F.; Maritati, M.; Caselli, E.; Gallenga, C.E.; Guarino, M.; De Giorgio, R.; Mazziotta, C.; Tramarin, M.L.; Badiale, G.; et al. Advanced Molecular and Immunological Diagnostic Methods to Detect SARS-CoV-2 Infection. Microorganisms 2022, 10, 1193. [Google Scholar] [CrossRef]
- Song, Q.; Sun, X.; Dai, Z.; Gao, Y.; Gong, X.; Zhou, B.; Wu, J.; Wen, W. Point-of-care testing detection methods for COVID-19. Lab Chip 2021, 21, 1634–1660. [Google Scholar] [CrossRef] [PubMed]
- Yüce, M.; Filiztekin, E.; Özkaya, K.G. COVID-19 diagnosis—A review of current methods. Biosens. Bioelectron. 2021, 172, 112752. [Google Scholar] [CrossRef]
- Chaibun, T.; Puenpa, J.; Ngamdee, T.; Boonapatcharoen, N.; Athamanolap, P.; O’Mullane, A.P.; Vongpunsawad, S.; Poovorawan, Y.; Lee, S.Y.; Lertanantawong, B. Rapid electrochemical detection of coronavirus SARS-CoV-2. Nat. Commun. 2021, 12, 802. [Google Scholar] [CrossRef]
- Kobia, F.; Gitaka, J. Open Peer Review COVID-19: Are Africa’s Diagnostic Challenges Blunting Response Effectiveness? [Version 1; Peer Review: 2 Approved]. 2020, pp. 1–11. Available online: https://doi.org/10.12688/aasopenres.13061.1 (accessed on 12 March 2023).
- Vatankhah, M.; Azizi, A.; Sanajouyan Langeroudi, A.; Ataei Azimi, S.; Khorsand, I.; Kerachian, M.A.; Motaei, J. CRISPR-based biosensing systems: A way to rapidly diagnose COVID-19. Crit. Rev. Clin. Lab. Sci. 2021, 58, 225–241. [Google Scholar] [CrossRef]
- Shademan, B.; Nourazarian, A.; Hajazimian, S.; Isazadeh, A.; Biray Avci, C.; Oskouee, M.A. CRISPR Technology in Gene-Editing-Based Detection and Treatment of SARS-CoV-2. Front. Mol. Biosci. 2022, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Malik, Y.S.; Ganesh, B.; Rahangdale, S.; Saurabh, S.; Natesan, S.; Srivastava, A.; Sharun, K.; Yatoo, M.I.; Tiwari, R.; et al. CRISPR-Cas System: An Approach With Potentials for COVID-19 Diagnosis and Therapeutics. Front. Cell. Infect. Microbiol. 2020, 10, 576875. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.G.; Ang, G.Y.; Yu, C.Y.; Yean, C.Y. Harnessing crispr-cas to combat covid-19: From diagnostics to therapeutics. Life 2021, 11, 1210. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Dai, W.; Gong, J.; Li, G.; Liu, N.; Wu, W.; Pan, J.; Chen, C.; Jiao, Y.; Deng, H.; et al. Rapid detection of SARS-CoV-2 with CRISPRCas12a. PLoS Biol. 2020, 18, e3000978. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Wang, J.; Liu, G. CRISPR/Cas Systems towards Next-Generation Biosensing. Trends Biotechnol. 2019, 37, 730–743. [Google Scholar] [CrossRef]
- Kaminski, M.M.; Abudayyeh, O.O.; Gootenberg, J.S.; Zhang, F.; Collins, J.J. CRISPR-based diagnostics. Nat. Biomed. Eng. 2021, 5, 643–656. [Google Scholar] [CrossRef]
- Gadwal, A.; Roy, D.; Khokhar, M.; Modi, A.; Sharma, P.; Purohit, P. CRISPR/Cas-New Molecular Scissors in Diagnostics and Therapeutics of COVID-19. Indian J. Clin. Biochem. 2021, 36, 459–467. [Google Scholar] [CrossRef]
- Kellner, M.J.; Koob, J.G.; Gootenberg, J.S.; Abudayyeh, O.O.; Zhang, F. SHERLOCK: Nucleic acid detection with CRISPR nucleases. Nat. Protoc. 2019, 14, 2986–3012. [Google Scholar] [CrossRef]
- Broughton, J.P.; Deng, X.; Yu, G.; Fasching, C.L.; Servellita, V.; Singh, J.; Miao, X.; Streithorst, J.A.; Granados, A.; Sotomayor-Gonzalez, A.; et al. CRISPR–Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020, 38, 870–874. [Google Scholar] [CrossRef]
- Huang, Z.; Tian, D.; Liu, Y.; Lin, Z.; Lyon, C.J.; Lai, W.; Fusco, D.; Drouin, A.; Yin, X.; Hu, T.; et al. Ultra-sensitive and high-throughput CRISPR-p owered COVID-19 diagnosis. Biosens. Bioelectron. 2020, 164, 112316. [Google Scholar] [CrossRef]
- Zhao, R.; Yu, C.; Lu, B.; Li, B. Coupling nucleic acid circuitry with the CRISPR-Cas12a system for universal and signal-on detection. RSC Adv. 2022, 12, 10374–10378. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, Z. Hybridization Chain Reaction for Direct mRNA Detection without Nucleic Acid Purification. RNA Detect. Methods Protoc. 2018, 1649, 455–471. [Google Scholar] [CrossRef]
- Dirks, R.M.; Pierce, N.A. Triggered amplification by hybridization chain reaction. Proc. Natl. Acad. Sci. USA 2004, 101, 15275–15278. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Balcioglu, M.; Robertson, N.M.; Hizir, M.S.; Yumak, S.; Yigit, M.V. Low picomolar, instrument-free visual detection of mercury and silver ions using low-cost programmable nanoprobes. Chem. Sci. 2017, 8, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Lu, Z.; Huang, Q.; Li, H.; Wang, Y.; Lai, Y.; He, Y.; Deng, M.; Liu, W. An ultrasensitive hybridization chain reaction-amplified CRISPR-Cas12a aptasensor for extracellular vesicle surface protein quantification. Theranostics 2020, 10, 10262–10273. [Google Scholar] [CrossRef] [PubMed]
- Miti, A.; Zuccheri, G. Hybridization Chain Reaction Design and Biosensor Implementation. In DNA Nanotechnology; Humana Press: New York, NY, USA, 2018; pp. 115–135. [Google Scholar] [CrossRef]
- Wu, T.H.; Chang, C.C.; Yang, C.H.; Lin, W.Y.; Ee, T.J.; Lin, C.W. Hybridization chain reactions targeting the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Int. J. Mol. Sci. 2020, 21, 3216. [Google Scholar] [CrossRef]
- Kachwala, M.J.; Smith, C.W.; Nandu, N.; Yigit, M.V. Reprogrammable Gel Electrophoresis Detection Assay Using CRISPR-Cas12a and Hybridization Chain Reaction. Anal. Chem. 2021, 93, 1934–1938. [Google Scholar] [CrossRef]
- Zhou, H.; Bu, S.; Xu, Y.; Xue, L.; Li, Z.; Hao, Z.; Wan, J.; Tang, F. CRISPR/Cas13a combined with hybridization chain reaction for visual detection of influenza A (H1N1) virus. Anal. Bioanal. Chem. 2022, 8437–8445. [Google Scholar] [CrossRef]
- Liu, X.; Bu, S.; Feng, J.; Wei, H.; Wang, Z.; Li, X.; Zhou, H.; He, X.; Wan, J. Electrochemical biosensor for detecting pathogenic bacteria based on a hybridization chain reaction and CRISPR-Cas12a. Anal. Bioanal. Chem. 2022, 414, 1073–1080. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Zhou, X. A CRISPR-based and post-amplification coupled SARS-CoV-2 detection with a portable evanescent wave biosensor. Biosens. Bioelectron. 2021, 190, 113418. [Google Scholar] [CrossRef]
- Schulte, S.J.; Huang, J.; Pierce, N.A. Hybridization Chain Reaction Lateral Flow Assays for Amplified Instrument-Free At-Home SARS-CoV-2 Testing. ACS Infect. Dis. 2023, 9, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, J.N.; Steenberg, C.D.; Bois, J.S.; Wolfe, B.R.; Pierce, M.B.; Khan, A.R.; Dirks, R.M.; Pierce, N.A. NUPACK: Analysis and Design of Nucleic Acid Systems. J. Comput. Chem. 2011, 32, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Ngamsom, B.; Iles, A.; Kamita, M.; Kimani, R.; Rodriguez-Mateos, P.; Mungai, M.; Dyer, C.E.; Walter, C.; Gitaka, J.; Pamme, N. An integrated lab-on-a-chip device for RNA extraction, amplification and CRISPR-Cas12a-assisted detection for COVID-19 screening in resource-limited settings. medRxiv 2022. [Google Scholar] [CrossRef]
- CDC. CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel; CDC: Atlanta, GA, USA, 2020; pp. 1–38. [Google Scholar]
- Schwarzkopf, M.; Liu, M.C.; Schulte, S.J.; Ives, R.; Husain, N.; Choi, H.M.T.; Pierce, N.A. Hybridization chain reaction enables a unified approach to multiplexed, quantitative, high-resolution immunohistochemistry and in situ hybridization. Development 2021, 148, dev199847. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.M.T.; Beck, V.A.; Pierce, N.A. Next-generation in situ hybridization chain reaction: Higher gain, lower cost, greater durability. ACS Nano 2014, 8, 4284–4294. [Google Scholar] [CrossRef]
- Zhang, Q. Application of Hybridizaftion Chain Reaction (HCR) in Electrochemical Analysis. Int. J. Electrochem. Sci. 2022, 17, 220227. [Google Scholar] [CrossRef]
- Breitbach, A. Lateral Flow Readout for CRISPR/Cas-Based Detection Strategies; Milenia Biotec GmbH: Gießen, Germany, 2020. [Google Scholar]
- Khorramdelazad, H.; Hossein, M.; Najafi, A. Immunopathological similarities between COVID-19 and influenza: Investigating the consequences of Co-infection Hossein. Microb. Pathog. 2021, 152, 2–13. [Google Scholar] [CrossRef]
- Liang, Y.; Lin, H.; Zou, L.; Zhao, J.; Li, B.; Wang, H.; Lu, J.; Sun, J.; Yang, X.; Deng, X.; et al. CRISPR-Cas12a-Based Detection for the Major SARS-CoV-2 Variants of Concern. Microbiol. Spectr. 2021, 9, e01017-21. [Google Scholar] [CrossRef]
- Jiang, Y.; Hu, M.; Liu, A.A.; Lin, Y.; Liu, L.; Yu, B.; Zhou, X.; Pang, D.W. Detection of SARS-CoV-2 by CRISPR/Cas12a-Enhanced Colorimetry. ACS Sensors 2021, 6, 1086–1093. [Google Scholar] [CrossRef]




| Name | Sequence 5′-3′ |
|---|---|
| Target | GAACGCTGAAGCGCTGGGGGCAAA |
| H1 Probe | TTTGCCCCCAGCGCTTCAGCGTTCAATGCGGAACGCTGAAGCGCTGGG |
| H2 Probe | GAACGCTGAAGCGCTGGGGGCAAACCCAGCGCTTCAGCGTTCCGCATT |
| Grna | UAAUUUCUACUAAGUGUAGAUCCCCCAGCGCUUCAGCGUUC |
| Reporter | /56-FAM/TTATTATT/3Bio/ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagoe, K.O.; Kyama, M.C.; Maina, N.; Kamita, M.; Njokah, M.; Thiong’o, K.; Kanoi, B.N.; Wandera, E.A.; Ndegwa, D.; Kinyua, D.M.; et al. Application of Hybridization Chain Reaction/CRISPR-Cas12a for the Detection of SARS-CoV-2 Infection. Diagnostics 2023, 13, 1644. https://doi.org/10.3390/diagnostics13091644
Sagoe KO, Kyama MC, Maina N, Kamita M, Njokah M, Thiong’o K, Kanoi BN, Wandera EA, Ndegwa D, Kinyua DM, et al. Application of Hybridization Chain Reaction/CRISPR-Cas12a for the Detection of SARS-CoV-2 Infection. Diagnostics. 2023; 13(9):1644. https://doi.org/10.3390/diagnostics13091644
Chicago/Turabian StyleSagoe, Kate Obaayaa, Mutinda Cleophas Kyama, Naomi Maina, Moses Kamita, Muturi Njokah, Kelvin Thiong’o, Bernard N. Kanoi, Ernest Apondi Wandera, Davies Ndegwa, Dickson Mwenda Kinyua, and et al. 2023. "Application of Hybridization Chain Reaction/CRISPR-Cas12a for the Detection of SARS-CoV-2 Infection" Diagnostics 13, no. 9: 1644. https://doi.org/10.3390/diagnostics13091644
APA StyleSagoe, K. O., Kyama, M. C., Maina, N., Kamita, M., Njokah, M., Thiong’o, K., Kanoi, B. N., Wandera, E. A., Ndegwa, D., Kinyua, D. M., & Gitaka, J. (2023). Application of Hybridization Chain Reaction/CRISPR-Cas12a for the Detection of SARS-CoV-2 Infection. Diagnostics, 13(9), 1644. https://doi.org/10.3390/diagnostics13091644






