Exploring the Role of Intraoperative Positive Culture of Allograft Bone in Subsequent Postoperative Infections among Donors and Recipients in Bone Bank Processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
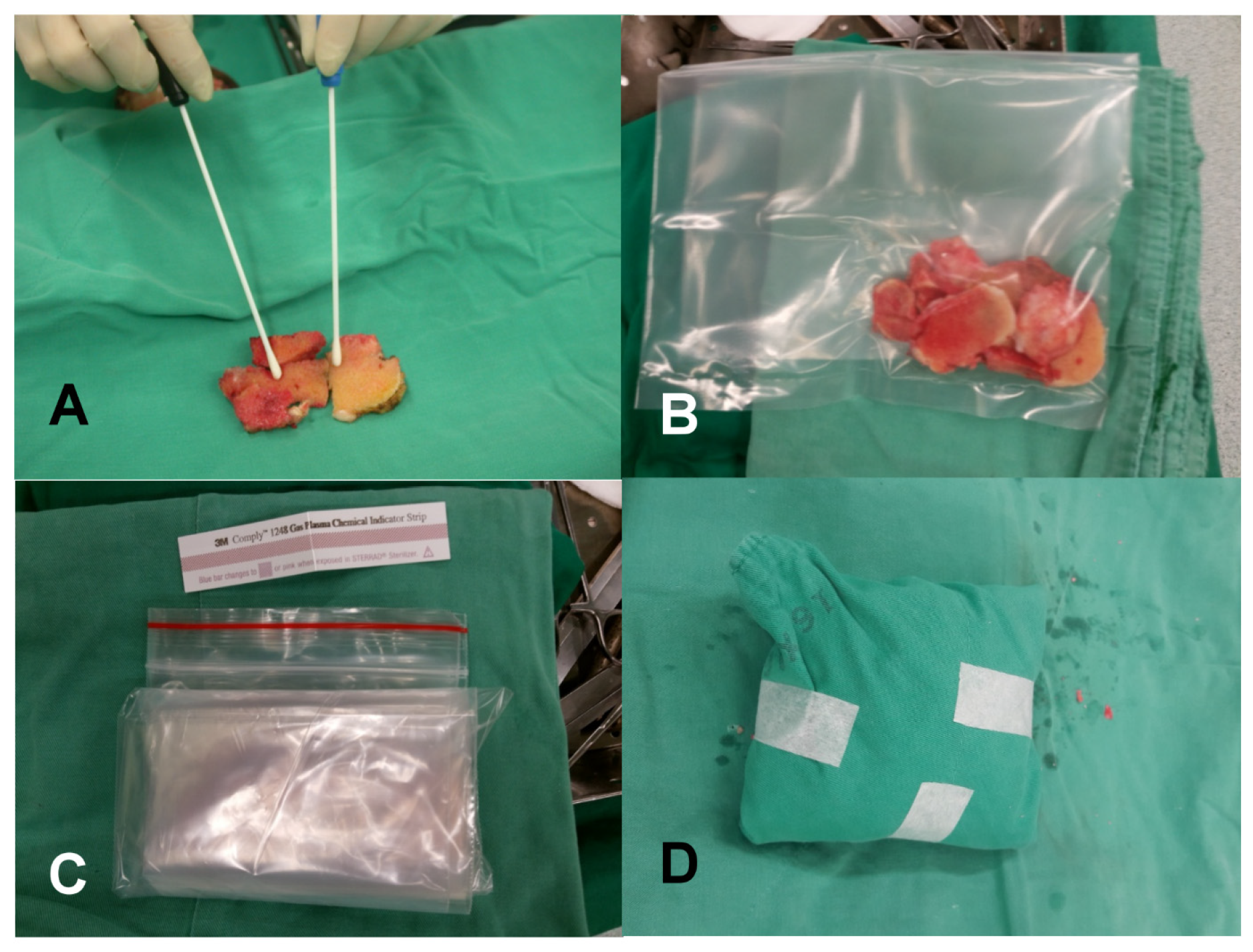
2.2. Allograft Retrieval and Bone Banking Process
2.3. Allograft Implantation
2.4. Microbiology Laboratory Procedures
2.5. Clinical Assessment
2.6. Statistical Analysis
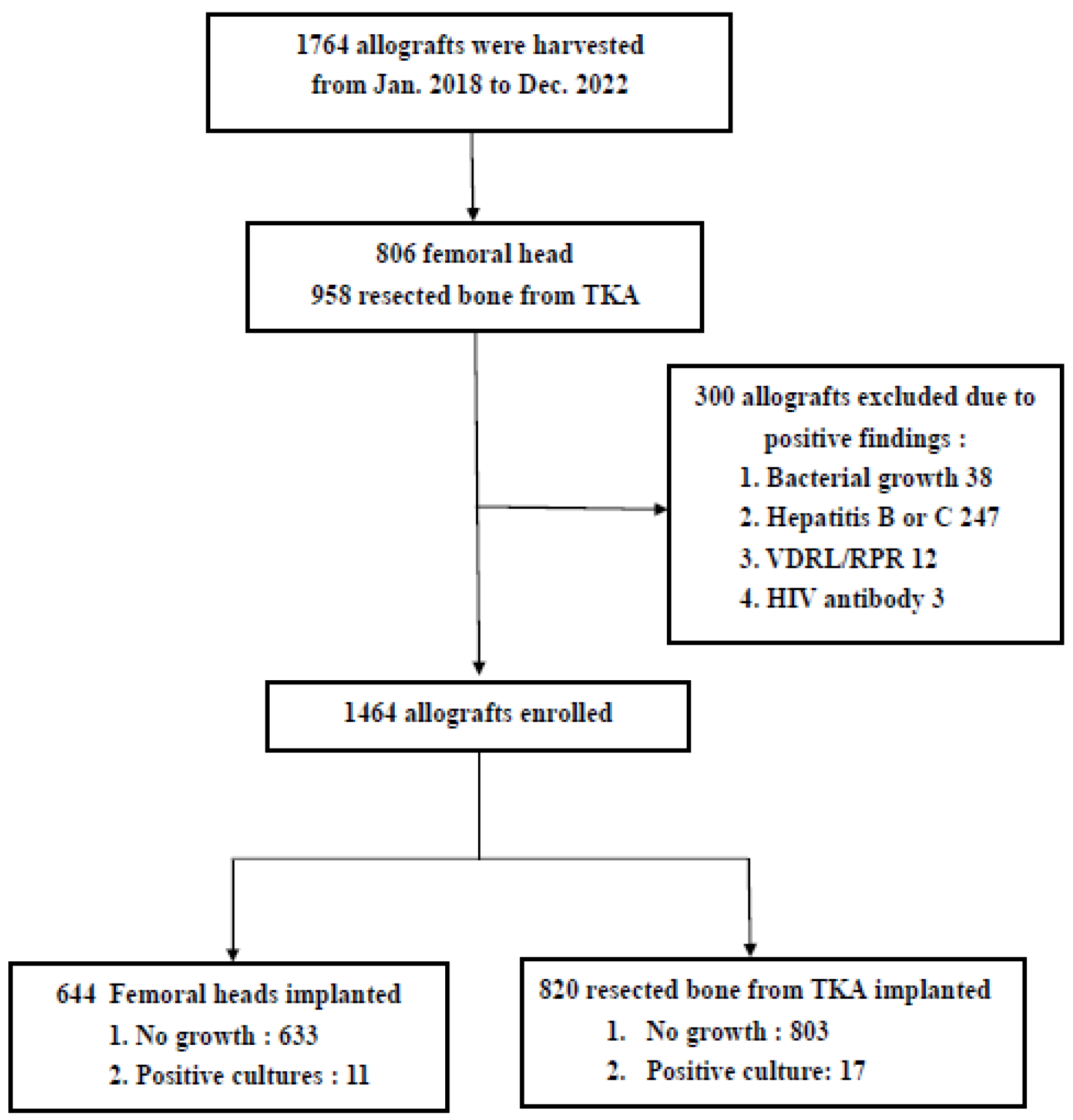
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sims, L.; Kulyk, P.; Woo, A. Intraoperative culture positive allograft bone and subsequent postoperative infections: A retrospective review. Can. J. Surg. 2017, 60, 94–100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, J.W.; Chao, L.H.; Su, L.H.; Wang, J.W.; Wang, C.J. Experience with a bone bank operation and allograft bone infection in recipients at a medical centre in southern Taiwan. J. Hosp. Infect. 2002, 50, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Zamborsky, R.; Svec, A.; Bohac, M.; Kilian, M.; Kokavec, M. Infection in bone allograft transplants. Exp. Clin. Transplant. 2016, 14, 484–490. [Google Scholar] [PubMed]
- Hovanyecz, P.; Lorenti, A.; Lucero, J.M.; Gorla, A.; Castiglioni, A.E. Living donor bone banking: Processing and discarding—From procurement to therapeutic use. Cell Tissue Bank. 2015, 16, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.H.; Liu, J.Y.; Huang, C.C.; Lin, F.l.; Yang, R.S.; Hou, C.H. Quality control processes in allografting: A twenty-year retrospective review of a hospital-based bone bank in Taiwan. PLoS ONE 2017, 12, e0184809. [Google Scholar] [CrossRef] [PubMed]
- Kappe, T.; Cakir, B.; Mattes, T.; Reichel, H.; Flören, M. Infections after bone allograft surgery: A prospective study by a hospital bone bank using frozen femoral heads from living donors. Cell Tissue Bank. 2010, 11, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Delloye, C.; Cornu, O.; Druez, V.; Barbier, O. Bone allografts: What they can offer and what they cannot. J. Bone Jt. Surg. Br. 2007, 89, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Verma, A.; Jain, A.; Goyal, T.; Kandwal, P.; Arora, S.S. Infection and utilization rates of bone allografts in a hospital-based musculoskeletal tissue bank in north India. J. Clin. Orthop. Trauma 2021, 23, 101635. [Google Scholar] [CrossRef]
- Deijkers, R.L.M.; Bloem, R.M.; Petit, P.L.C.; Brand, R.; Veh Meyer, S.B.W.; Veen, M.R. Contamination of bone allografts: Analysis of incidence and predisposing factors. J. Bone Jt. Surg. Br. 1997, 79, 161–166. [Google Scholar] [CrossRef]
- Sommerville, S.M.M.; Johnson, N.; Bryce, S.L.; Journeaux, S.F.; Morgan, D.A.F. Contamination of banked femoral head allograft: Incidence, bacteriology and donor follow up. Aust. N. Z. J. Surg. 2000, 70, 480–484. [Google Scholar] [CrossRef]
- Wu, C.; Hsieh, P.; Jiang, J.F.; Shih, H.; Chen, C.; Hu, C. A positive bacterial culture from allograft bone at implantation does not correlate with subsequent surgical site infection. Bone Jt. J. 2015, 97, 427–431. [Google Scholar] [CrossRef] [PubMed]
- van de Pol, G.J.; Sturm, P.D.; van Loon, C.J.; Verhagen, C.; Schreurs, B.W. Microbiological cultures of allografts of the femoral head just before transplantation. J. Bone Jt. Surg. Br. 2007, 89, 1225–1228. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barnhart, B.; Allan, D.G.; Milbrandt, J.C.; Khardori, N.; Hall, A.; Barenfanger, J. Intra-operative culturing of donor allograft bone: A lack of clinical utility. U Pa Orthop. J. 2009, 19. Available online: https://www.upoj.org/wp-content/uploads/v19/v19_10.pdf (accessed on 27 November 2023).
- Stepanovic, Z.L.; Ristic, B.M. The effectiveness of bone banking in Central Serbia: Audit of the first seven years. Cell Tissue Bank. 2014, 15, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Nather, A. Musculoskeletal tissue banking in Singapore: 15 years of experience (1988–2003). J. Orthop. Surg. 2004, 12, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, I.; Al-Rabiah, A.M.; Alhussainan, T.S.; Alrumaih, H.A.; Fallatah, A.B.; Alsakran, S.A.; Al-Mohrej, O.A. Principles of bone and tissue banking in Saudi Arabia: 10-year experience report. Cell Tissue Bank. 2021, 22, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Baseri, N.; Meysamie, A.; Campanile, F.; Hamidieh, A.A.; Jafarian, A. Bacterial contamination of bone allografts in the tissue banks: A systematic review and meta-analysis. J. Hosp. Infect. 2022, 123, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Warnock, J.M.; Rowan, C.H.; Davidson, H.; Millar, C.; McAlinden, M.G. Improving efficiency of a regional stand alone bone bank. Cell Tissue Bank. 2016, 17, 85–90. [Google Scholar] [CrossRef] [PubMed]
- James, L.A.; Ibrahim, T.; Esler, C.N. Microbiological culture results for the femoral head. Are they important to the donor? J. Bone Jt. Surg. Br. 2004, 86, 797–800. [Google Scholar] [CrossRef]
- Vehmeyer, S.B.W.; Slooff, A.R.M.; Bloem, R.M.; Petit, P.L.C. Bacterial contamination of femoral head allografts from living donors. Acta Orthop. Scand. 2002, 73, 165–169. [Google Scholar] [CrossRef]
- James, L.A.; Gower, A. The clinical significance of femoral head culture results in donors after hip arthroplasty—A preliminary report. J. Arthroplast. 2002, 17, 355–358. [Google Scholar] [CrossRef]
- Ibrahim, T.; Aswad, M.G.; Dias, J.J.; Brown, A.R.; Esler, C.N. Long-term outcome of total hip replacement in patients with or without femoral head contamination. J. Orthop. Surg. 2011, 19, 174–176. [Google Scholar] [CrossRef]
- Phuong, D.T.K.; Park, K.S.; Hwang, S.Y.; Lee, D.H.; Yoon, T.R. Microbiological culture findings of the femoral heads as a prognostic factor in the total hip replacement surgery. Clin. Orthop. Surg. 2013, 5, 105–109. [Google Scholar] [CrossRef]
- Justesen, T.; Olsen, J.; Hesselvig, A.B.; Mørup-Petersen, A.; Odgaard, A. Does intraoperative contamination during primary knee arthroplasty affect patient-reported outcomes for patients who are uninfected 1 year after surgery? A prospective cohort study of 714 patients. Acta Orthop. 2020, 91, 750–755. [Google Scholar] [CrossRef]
- Jonsson, E.O.; Johannesdottir, H.; Robertsson, O.; Mogensen, B. Bacterial contamination of the wound during primary total hip and knee replacement: Median 13 years of follow-up of 90 replacements. Acta Orthop. 2014, 85, 159–164. [Google Scholar] [CrossRef]
- Naves, G.G.; Silva, A.F.; Antebi, U.; Cristovam, P.C.; Honda, E.K.; Guimarães, R.P. Analysis of potential contamination factors in musculoskeletal tissues. Cell Tissue Bank. 2018, 19, 659–666. [Google Scholar] [CrossRef]
- Pinto, F.M.; de Souza, R.Q.; da Silva, C.B.; Mimica, L.M.; Graziano, K.U. Analysis of the microbial load in instruments used in orthopedic surgeries. Am. J. Infect. Control 2010, 38, 229–233. [Google Scholar] [CrossRef] [PubMed]
| Variable | Number of Allografts | Prosthetic Joint Infection |
|---|---|---|
| Staphylococcus epidermidis | 9 | 0 |
| Staphylococcus haemolyticus | 4 | 0 |
| Staphylococcus capitis | 3 | 0 |
| Staphylococcus caprae | 2 | 0 |
| Staphylococcus hominis | 1 | 0 |
| CoNS | 4 | 0 |
| MSSA | 3 | 0 |
| MRSA | 2 | 0 |
| Klebsiella pneumonia and Micrococcus | 1 | 1 |
| Clostrium perfringens | 1 | 0 |
| Peptococcus | 1 | 0 |
| Peptoniphilus harei | 1 | 0 |
| Propionibacterium acnes | 1 | 0 |
| Enterococcus faecium | 1 | 0 |
| Bacillus cereus | 2 | 0 |
| Gram-positive bacilli | 2 | 0 |
| 38 | 1 |
| Number | Sex | Age | Allograft Type | Underlying Chronic Disease | Operative Procedure | Microorganism | Intravenous Antibiotics | IVA Duration | Oral Antibiotics Duration | Results | Preoperative Condtion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 78 | Femoral head | Heart disease, Gout | Revision THA | Staphylococcus haemolyticus | Cefazolin | 7 | 7 | N | N |
| 2 | M | 81 | Femoral head | HT | Lumbar spine surgery | Staphylococcus haemolyticus | Cefazolin | 14 | 0 | N | N |
| 3 | M | 58 | Femoral head | Alcoholism | Revision THA | Gram-positive bacilli | Cefazolin | 1 | 0 | N | PJI-Staph. epidermidis |
| 4 | F | 67 | Femoral head | Heart disease | TKA | Gram-negative bacilli | Cefazolin | 1 | 0 | N | N |
| 5 | F | 70 | Femoral head | HT | Humeral fracture | Staphylococcus epidermidis | Cefazolin | 7 | 14 | N | N |
| 6 | M | 62 | Femoral head | HT | High tibial osteotomy | Propionibacterium spp. | Cefazolin | 1 | 0 | N | N |
| 7 | M | 55 | Femoral head | DM, HT, ESRD, CAD | Ankle fusion | Enterococcus faecium, MM | Cefazolin | 7 | 14 | N | N |
| 8 | F | 64 | Femoral head | DM, HT, ESRD | Femoral fracture | Staphylococcus epidermidis | Cefazolin | 3 | 0 | N | N |
| 9 | M | 77 | Femoral head | HT, HB, COPD | Thoracic spine surgery | Staphylococcus epidermidis | Cefazolin | 7 | 14 | N | N |
| 10 | M | 55 | Femoral head | DM, ESRD | Revision THA | MRSA | Teicoplanin | 7 | 60 | N | PJI-MRSA |
| 11 | F | 71 | Femoral head | DM, HT | Revision TKA | Staphylococcus saprophytica | Cefazolin | 7 | 0 | N | N |
| 12 | F | 57 | TKA bone chips | DM, HT | Tibial fracture | Staphylococcus epidermidis | Cefazolin | 2 | 0 | N | N |
| 13 | M | 78 | TKA bone chips | Heart disease, Gout | Revision THA | Staphylococcus haemolyticus | Cefazolin | 7 | 7 | N | N |
| 14 | F | 63 | TKA bone chips | DM, HT, Gout | Lumbar spine surgery | Stenotrophomonas maltophilia | Cefazolin | 14 | 0 | N | N |
| 15 | F | 78 | TKA bone chips | DM, HT | Lumbar spine surgery | Enterococcus faecium | Cefazolin | 7 | 14 | N | N |
| 16 | F | 59 | TKA bone chips | DM, HT | Lumbar spine surgery | Gram-positive bacilli | Cefazolin | 3 | 0 | N | N |
| 17 | F | 67 | TKA bone chips | DM, HT | Patellar fracture | Staphylococcus haemolyticus | Cefazolin | 1 | 0 | N | N |
| 18 | M | 58 | TKA bone chips | HT | THA | Staphylococcus epidermidis | Ceftazidime | 14 | 0 | N | Pneumonia- Pseudomonas |
| 19 | F | 76 | TKA bone chips | HT | Radial fracture | Staphylococcus epidermidis | Cefazolin | 1 | 0 | N | N |
| 20 | M | 66 | TKA bone chips | HB, Heart disease | Lumbar spine surgery | Staphylococcus capitis | Cefazolin | 3 | 0 | N | N |
| 21 | F | 65 | TKA bone chips | HT | Calcaneus fracture | Staphylococcus epidermidis | Cefazolin | 7 | 0 | N | N |
| 22 | M | 63 | TKA bone chips | HCC, LC | Revision THA | CoNS | Cefazolin | 1 | 14 | N | N |
| 23 | M | 68 | TKA bone chips | HB, HC | Thoracic spine surgery | Staphylococcus epidermidis | Cefazolin | 7 | 7 | N | N |
| 24 | F | 50 | TKA bone chips | HB | Tibial fx | Bacillus cereus | Cefuroxime | 14 | 0 | N | N |
| 25 | M | 68 | TKA bone chips | DM, Cancer | Humeral fracture | Enterococcus faecium | Cefazolin | 2 | 7 | Y | N |
| 26 | F | 81 | TKA bone chips | HT | Radial fracture | Aerococcus viridans | Cefazolin | 1 | 0 | N | N |
| 27 | M | 56 | TKA bone chips | HT, ESRD, HC | Lumbar spine surgery | Bacillus flexus | Flomocef | 7 | 0 | N | N |
| 28 | F | 61 | TKA bone chips | HB | Lumbar spine surgery | Micrococcus luteus | Flomocef | 7 | 7 | N | N |
| Intraoperative Positive Culture Group (N = 28) | Intraoperative Negative Culture Group (N = 1436) | p Value | |
|---|---|---|---|
| No infections | 27 | 1424 | 0.223 |
| Infection | 1 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-H.; Chen, H.-Y.; Huang, T.-Y.; Chen, J.-L.; Kuo, L.-T.; Huang, K.-C. Exploring the Role of Intraoperative Positive Culture of Allograft Bone in Subsequent Postoperative Infections among Donors and Recipients in Bone Bank Processing. Diagnostics 2024, 14, 15. https://doi.org/10.3390/diagnostics14010015
Tsai Y-H, Chen H-Y, Huang T-Y, Chen J-L, Kuo L-T, Huang K-C. Exploring the Role of Intraoperative Positive Culture of Allograft Bone in Subsequent Postoperative Infections among Donors and Recipients in Bone Bank Processing. Diagnostics. 2024; 14(1):15. https://doi.org/10.3390/diagnostics14010015
Chicago/Turabian StyleTsai, Yao-Hung, Hung-Yen Chen, Tsung-Yu Huang, Jiun-Liang Chen, Liang-Tseng Kuo, and Kuo-Chin Huang. 2024. "Exploring the Role of Intraoperative Positive Culture of Allograft Bone in Subsequent Postoperative Infections among Donors and Recipients in Bone Bank Processing" Diagnostics 14, no. 1: 15. https://doi.org/10.3390/diagnostics14010015
APA StyleTsai, Y.-H., Chen, H.-Y., Huang, T.-Y., Chen, J.-L., Kuo, L.-T., & Huang, K.-C. (2024). Exploring the Role of Intraoperative Positive Culture of Allograft Bone in Subsequent Postoperative Infections among Donors and Recipients in Bone Bank Processing. Diagnostics, 14(1), 15. https://doi.org/10.3390/diagnostics14010015







