Three-Dimensional Analysis of the Cranial Base Structure in Patients with Facial Asymmetry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Subjects and Materials
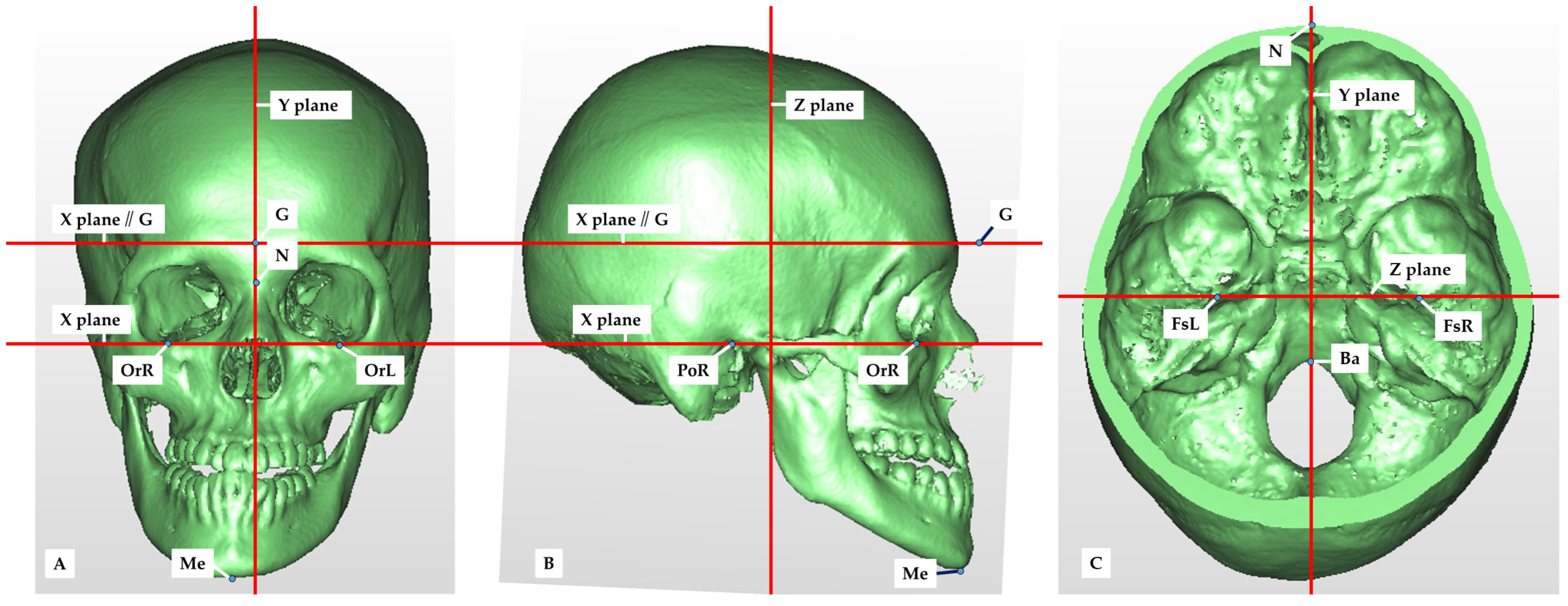
2.2. Setting of Measurement Points, Reference Planes and Measurement Items in CT Images
2.3. Measurement and Evaluation
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Cranial Deformity in Patients with Facial Asymmetry
4.2. Relationship between Cranial Deformity and Mandibular Deviation in Patients with Facial Asymmetry with Plagiocephaly
4.3. Research Methods
4.4. Clinical Application
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haraguchi, S.; Takada, K.; Yasuda, Y. Facial asymmetry in subjects with skeletal Class III deformity. Angle Orthod. 2002, 72, 28–35. [Google Scholar] [PubMed]
- Ono, S.; Tachiki, C.; Morikawa, T.; Aihara, Y.; Matsunaga, S.; Sugahara, K.; Watanabe, A.; Kawamata, T.; Nishii, Y. Morphological Evaluation of Cranium Facial Asymmetry in Class III Malocclusion Patients. Appl. Sci. 2023, 13, 6533. [Google Scholar] [CrossRef]
- Yunoki, H.; Nakajima, M.; Hayashi, H. Clinicostatistical study on orthognathic surgery for 11 years at Second Department of Oral and Maxillofacial Surgery, Osaka Dental University. Jpn. J. Jaw Deform. 1999, 9, 51–56. [Google Scholar] [CrossRef]
- Sung, J.K.; Kee, J.L.; Sang, H.L. Morphologic relationship between the cranial base and the mandible in patients with facial asymmetry. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 330–340. [Google Scholar]
- Kwon, T.G.; Park, H.S.; Ryoo, H.M. A comparison of craniofacial morphology in patients with and without facial asymmetry. Int. J. Oral Maxillofac. Surg. 2006, 35, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Captier, G.; Leboucq, N.; Bigorre, M. Plagiocephaly: Morphometry of skull base asymmetry. Surg. Radiol. Anat. 2003, 25, 226–233. [Google Scholar] [CrossRef]
- Melsen, B. The cranial base: The postnatal development of the cranial base studied histologically on human autopsy material. Am. J. Orthod. 1974, 66, 689–691. [Google Scholar]
- Maria, G.P.; Elena, C.; Francesca, T. Reverse-sequencing chewing patterns evaluation in anterior versus posterior unilateral crossbite patients. Eur. J. Orthod. 2012, 34, 536–541. [Google Scholar]
- Zhang, Y.L.; Song, J.L.; Xu, X.C. Morphologic analysis of the temporomandibular joint between patients with facial asymmetry and asymptomatic subjects by 2D and 3D evaluation: A preliminary study. Medicine 2016, 95, e3052. [Google Scholar] [CrossRef]
- Yi, L.; Fan, L.; Ying, Z.; Xin, Y. Morphological Characteristics of the Cranial Base in Sagittal Malocclusion. J. Hard Tissue Biol. 2013, 22, 249–254. [Google Scholar]
- Gaia, S.; Santiago, B.A.; Chiara, N.; Santiago, B.A.; Emily, S.; Chwa, B.A.; Chad, A.; Purnell, M.D. Positional Plagiocephaly and Craniosynostosis. Pediatr. Ann. 2023, 52, e10–e17. [Google Scholar]
- Rachel, Y.M.; Rebecca, F.C.; Ivan, H. Sleep-Related Infant Deaths: Updated 2022 Recommendations for Reducing Infant Deaths in the Sleep Environment. Pediatrics 2022, 150, e2022057990. [Google Scholar]
- DeGrazia, M.; Ahtam, B.; Rogers-Vizena, C.R.; Proctor, M.; Porter, C.; Vyas, R.; Laurentys, C.T.; Bergling, E.; McLaughlin, K.; Grant, P.E. Brain Characteristics Noted Prior to and Following Cranial Orthotic Treatment. Child Neurol. Open. 2020, 7, 2329048X20949769. [Google Scholar] [CrossRef]
- Aihara, Y. Skull deformity: A 6-month-old child with a malformed head. Jpn. Pediatr. Surg. 2017, 49, 8–10. [Google Scholar]
- Pirttiniemi, P.M. Associations of mandibular and facial asymmetries-A review. Am. J. Orthod. Dentofac. Orthop. 1994, 106, 191–200. [Google Scholar] [CrossRef]
- Baek, S.H.; Cho, I.S.; Chang, Y.I.; Kim, M.J. Skeletodental factors affecting chin point deviation in female patients with class III malocclusion and facial asymmetry: A three-dimensional analysis using computed tomography. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 104, 628–639. [Google Scholar] [CrossRef]
- Wang, R.H.; Ho, C.T.; Lin, H.H.; Lo, L.J. Three-dimensional cephalometry for orthognathic planning: Normative data and analyses. J. Formos. Med. Assoc. 2020, 119, 191–203. [Google Scholar] [CrossRef]
- Ho, C.T.; Denadai, R.; Lo, L.J.; Lin, H.H. Average 3D Skeletofacial Model as a Template for Maxillomandibular Repositioning During Virtual Orthognathic Surgical Planning. Plast. Reconstr. Surg. 2023; ahead of print. [Google Scholar] [CrossRef]
- Steiner, C.C. Cephalometrics for you and me. Am. J. Orthod. 1953, 39, 729–755. [Google Scholar] [CrossRef]
- Jacobson, A. The “Wits” appraisal of jaw disharmony. Am. J. Orthod. 1975, 67, 125–138. [Google Scholar] [CrossRef]
- Jacobson, A. Application of the “Wits” appraisal. Am. J. Orthod. 1976, 70, 179–189. [Google Scholar] [CrossRef]
- Loveday, B.P.; de Chalain, T.B. Active counterpositioning or orthotic device to treat positional plagiocephaly? J. Craniofac. Surg. 2001, 12, 308–313. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Sasaki, A.; Kawajiri, A.; Fujimoto, K.; Uesato, T.; Toyota, A.; Suda, N. Three-dimensional analysis of cranial vault and position of mandibular fossa -part 2. Analysis of asymmetric mandibular protrusion cases. Jpn. J. Jaw Deform. 2020, 30, 267–280. [Google Scholar] [CrossRef]
- López, B.D.F.; Ruiz, B.J. Asymmetry of glenoid fossa as differential diagnosis for hemimandibular elongation. Rev. Mex. Ortodon. 2017, 5, 217–226. [Google Scholar]
- Poikela, A.; Kantomaa, T.; Pirttiniemi, P. Craniofacial growth after a period of unilateral masticatory function in young rabbits. Eur. J. Oral Sci. 1997, 105, 331–337. [Google Scholar] [CrossRef]
- Kreiborg, S.; Moller, E.; Bjork, A. Skeletal and functional craniofacial adaptations in plagiocephaly. J. Craniofac. Genet. Dev. Biol. Suppl. 1985, 1, 199–210. [Google Scholar]
- Ishizaki, K.; Suzuki, K.; Mito, T.; Tanaka, E.M.; Sato, S. Morphologic, functional, and occlusal characterization of mandibular lateral displacement malocclusion. Am. J. Orthod. 2010, 137, 454.e1–454.e9. [Google Scholar] [CrossRef]
- St John, D.; Mulliken, J.B.; Kaban, L.B. Anthropometric analysis of mandibular asymmetry in infants with deformational posterior plagiocephaly. J. Oral. Maxillofac. Surg. 2002, 60, 873–877. [Google Scholar] [CrossRef]
- Enlow, D.H. Facial Growth, 3rd ed.; W. B. Saunders: Philadelphia, PA, USA, 1990; pp. 58–163. [Google Scholar]
- Choi, H.W.; Kim, B.; Kim, J.Y. Three-dimensional computed tomography evaluation of craniofacial characteristics according to lateral deviation of chin. Maxillofac. Plast. Reconstr. Surg. 2019, 41, 57. [Google Scholar] [CrossRef]
- Sgouros, S.; Goldin, J.H.; Hockley, A.D.; Wake, M.J.; Natarajan, K. Intracranial volume change in childhood. J. Neurosurg. 1999, 91, 610–616. [Google Scholar] [CrossRef]
- Melnick, A.K. A cephalometric study of mandibular asymmetry in a longitudinally followed sample of growing children. Am. J. Orthod. Dentofac. Orthop. 1992, 101, 355–366. [Google Scholar] [CrossRef]
- Ferros, I.; Mora, M.J.; Obeso, I.F.; Jimenez, P.; Martinez, I.A. Relationship between the cranial base and the mandible in artificially deformed skulls. Orthod. Craniofac. Res. 2016, 19, 222–233. [Google Scholar] [CrossRef]
- Kim, Y.H.; Sato, K.; Mitani, H. Asymmetry of the sphenoid bone and its suitability as a reference for analyzing craniofacial asymmetry. Dentofac. Orthop. 2003, 124, 656–662. [Google Scholar] [CrossRef]
- Schlicher, W.; Nielsen, I.; Huang, J.C.; Maki, K.; Hatcher, D.C.; Miller, A.J. Consistency and precision of landmark identification in three-dimensional cone beam computed tomography scans. Eur. J. Orthod. 2012, 34, 263–275. [Google Scholar] [CrossRef]
- Kawamura, T.; Fukui, T.; Nishiyama, H. Three-dimensional analysis of the temporal bone and mandibular morphology in mandibular prognathism with facial asymmetry. Oral Sci. Int. 2021, 19, 44–51. [Google Scholar] [CrossRef]
- Severt, T.R.; Proffit, W.R. The prevalence of facial asymmetry in the dentofacial deformities population at the University of North Carolina. Int. J. Adult Orthodon. Orthognath. Surg. 1997, 12, 171–176. [Google Scholar]
- Samman, N.; Tong, A.C.; Cheung, D.L. Analysis of 300 dentofacial deformities in Hong Kong. Int. J. Adult Orthodon. Orthognath. Surg. 1992, 7, 181–185. [Google Scholar]
- Haraguchi, S.; Iguchi, Y.; Takada, K. Asymmetry of the face in orthodontic patients. Angle Orthod. 2008, 78, 421–426. [Google Scholar] [CrossRef]
- Milos, D.; Pavlic, A.; Vandevska, R.V.; Zigante, M.; Matthewson, A.; Spalj, S. Craniofacial Growth in Adolescence and its Influence on the Mandibular Incisor Crowding. Acta Stomatol. Croat. 2021, 55, 37–44. [Google Scholar] [CrossRef]
- The American Association of Oral and Maxillofacial Surgeons. Criteria for Orthognathic Surgery. Available online: http://www.aaoms.org/docs/practice_resouces/clinical_resources/ortho_criteria.pdf (accessed on 1 April 2022).
- Masuoka, N.; Muramatsu, A.; Ariji, Y. Discriminative thresholds of cephalometric indexes in the subjective evaluation of facial asymmetry. Am. J. Orthod. Dentofac. Orthop. 2007, 131, 609–613. [Google Scholar] [CrossRef]
- Farronato, M.; Baselli, G.; Baldini, B.; Favia, G.; Tartaglia, G.M. 3D Cephalometric Normality Range: Auto Contractive Maps (ACM) Analysis in Selected Caucasian Skeletal Class I Age Groups. Bioengineering 2022, 9, 216. [Google Scholar] [CrossRef] [PubMed]




| Symmetry (n = 60) | Asymmetry (n = 60) | |
|---|---|---|
| Sex | ||
| Male | 20 | 16 |
| Female | 40 | 44 |
| Age (y) | ||
| Mean | 20.9 | 24.4 |
| Range | 14.9–49.9 | 14.2–49.4 |
| Measurement values | ||
| Mean ANB (°) | −2.85 | −3.34 |
| Mean Wits appraisal (mm) | −11.70 | −10.22 |
| Difference in position of Me (mm) | 1.75 | 7.85 |
| Range in position of Me (mm) | 0.14–2.59 | 4.19–16.29 |
| Abbreviation | Explanation | |
|---|---|---|
| Reference point | ||
| Glabella | G | Most prominent point between the eyebrows |
| Nasion | Na | Most anterior point of the frontal nasal suture |
| Orbitale | Or | Lowest point of the orbital bone margin |
| Porion | Po | Sublingual neural tube opening |
| Foramen spinosum | Fs | Opening of the foramen spinosum |
| Basion | Ba | Lowest point on the anterior margin of the foramen magnum occipitalis |
| Menton | Me | Lowest point of the mandibular symphysis |
| Measurement point | ||
| Anterior cranial base | ||
| Orbitale | Or | Lowest point on the orbital bony margin |
| Frontozygomatic suture | Fz | Most medial point of the zygomatico-frontal suture |
| Anterior clinoid process | Acl | Last point of the anterior process of the anterior floor |
| Middle cranial base | ||
| Posterior clinoid process | Pcl | Most lateral point of the posterior process of the floor |
| Sphenoid lesser wing | Sl | Most centrifugal point of the lesser wing of the sphenoid |
| Foramen rotundum | Fr | Foramen magnum opening |
| Foramen spinosum | Fs | Foramen spinosum opening |
| Articular process | At | Lowest point of the articular process |
| Condyle | Co | Uppermost point of the condyle |
| Mandibular fossa | Mf | Deepest point of the mandibular fossa |
| Pterygoid hamulus | Ph | Most posterior point of the pterygoid hook |
| Posterior cranial base | ||
| Styloid process | Sty | Lowermost point of the stromal process |
| Mastoid process | M | Lowest point of the mastoid process |
| Hypoglossal canal | Hyp | Sublingual neural tube opening |
| X | Y | Z | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symmetry | Asymmetry | Symmetry | Asymmetry | Symmetry | Asymmetry | ||||||||||
| Avg. | SD | Avg. | SD | p | Avg. | SD | Avg. | SD | p | Avg. | SD | Avg. | SD | p | |
| Anterior cranial base | |||||||||||||||
| Or | 0.93 | 0.71 | 0.92 | 0.72 | NS | 1.48 | 0.91 | 1.86 | 1.39 | NS | 1.48 | 1.21 | 2.23 | 2.13 | 0.02 * |
| Fz | 1.52 | 1.05 | 1.52 | 1.10 | NS | 0.69 | 0.65 | 0.87 | 0.84 | NS | 2.07 | 1.55 | 2.80 | 2.41 | NS |
| Acl | 1.00 | 1.14 | 0.90 | 0.66 | NS | 1.21 | 0.86 | 1.23 | 0.91 | NS | 1.10 | 0.93 | 1.00 | 0.84 | NS |
| Middle cranial base | |||||||||||||||
| Pcl | 1.71 | 2.28 | 1.38 | 0.96 | NS | 1.28 | 0.92 | 1.36 | 1.19 | NS | 1.01 | 1.18 | 0.92 | 0.65 | NS |
| Sl | 2.29 | 1.44 | 2.11 | 1.70 | NS | 2.60 | 2.12 | 2.65 | 2.35 | NS | 2.17 | 1.75 | 2.34 | 1.85 | NS |
| Fr | 1.40 | 1.37 | 1.29 | 1.09 | NS | 1.36 | 1.13 | 1.71 | 1.42 | NS | 1.65 | 1.11 | 1.84 | 1.81 | NS |
| Fs | 0.00 | 0.00 | 0.00 | 0.00 | NS | 2.11 | 1.49 | 2.23 | 1.96 | NS | 2.14 | 1.59 | 2.34 | 1.83 | NS |
| At | 1.35 | 0.97 | 1.20 | 1.04 | NS | 1.42 | 0.91 | 1.88 | 1.22 | 0.03 * | 3.07 | 2.19 | 2.99 | 2.45 | NS |
| Co | 0.70 | 0.70 | 0.88 | 0.71 | NS | 1.60 | 1.41 | 2.20 | 1.62 | 0.04 * | 1.62 | 1.31 | 1.89 | 1.57 | NS |
| Mf | 0.79 | 0.73 | 0.75 | 0.66 | NS | 1.30 | 1.18 | 1.97 | 1.79 | 0.02 * | 1.68 | 1.36 | 1.79 | 1.27 | NS |
| Ph | 0.89 | 0.72 | 0.97 | 0.81 | NS | 1.75 | 1.18 | 2.45 | 1.83 | 0.02 * | 1.19 | 0.91 | 1.28 | 1.09 | NS |
| Posterior cranial base | |||||||||||||||
| Sty | 2.77 | 3.11 | 3.17 | 3.37 | NS | 2.34 | 1.80 | 3.16 | 2.69 | NS | 1.67 | 1.51 | 2.61 | 2.96 | 0.04 * |
| M | 1.24 | 0.99 | 1.63 | 1.74 | NS | 1.63 | 1.61 | 2.73 | 3.36 | 0.03 * | 2.28 | 1.96 | 2.99 | 3.21 | NS |
| Hyp | 1.22 | 1.12 | 1.25 | 1.90 | NS | 1.56 | 2.19 | 1.42 | 1.32 | NS | 1.04 | 0.75 | 1.07 | 1.01 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, Y.; Tachiki, C.; Morikawa, T.; Aihara, Y.; Matsunaga, S.; Sugahara, K.; Watanabe, A.; Kawamata, T.; Nishii, Y. Three-Dimensional Analysis of the Cranial Base Structure in Patients with Facial Asymmetry. Diagnostics 2024, 14, 24. https://doi.org/10.3390/diagnostics14010024
Hayashi Y, Tachiki C, Morikawa T, Aihara Y, Matsunaga S, Sugahara K, Watanabe A, Kawamata T, Nishii Y. Three-Dimensional Analysis of the Cranial Base Structure in Patients with Facial Asymmetry. Diagnostics. 2024; 14(1):24. https://doi.org/10.3390/diagnostics14010024
Chicago/Turabian StyleHayashi, Yuki, Chie Tachiki, Taiki Morikawa, Yasuo Aihara, Satoru Matsunaga, Keisuke Sugahara, Akira Watanabe, Takakazu Kawamata, and Yasushi Nishii. 2024. "Three-Dimensional Analysis of the Cranial Base Structure in Patients with Facial Asymmetry" Diagnostics 14, no. 1: 24. https://doi.org/10.3390/diagnostics14010024
APA StyleHayashi, Y., Tachiki, C., Morikawa, T., Aihara, Y., Matsunaga, S., Sugahara, K., Watanabe, A., Kawamata, T., & Nishii, Y. (2024). Three-Dimensional Analysis of the Cranial Base Structure in Patients with Facial Asymmetry. Diagnostics, 14(1), 24. https://doi.org/10.3390/diagnostics14010024









