Eye Pain Caused by Epithelial Damage in the Central Cornea in Aqueous-Deficient Dry Eye
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. DE-Related Symptoms Evaluated Using DE-System Questionnaire Visual Analogue Scale (DSQ-VAS)
2.3. DE-Related EP Symptoms Evaluated Using Short-Form McGill Pain Questionnaire 2 (SF-MPQ-2)
2.4. Quantitative Evaluation of EP Using PainVision®
2.5. Ocular Surface Examinations
2.6. Statistical Analysis
3. Results
3.1. Patient Background and Clinical Objective Findings
3.2. Pain Subscale of DE-Related Symptoms Evaluated Using DSQ-VAS and SF-MPQ-2 in ADDE
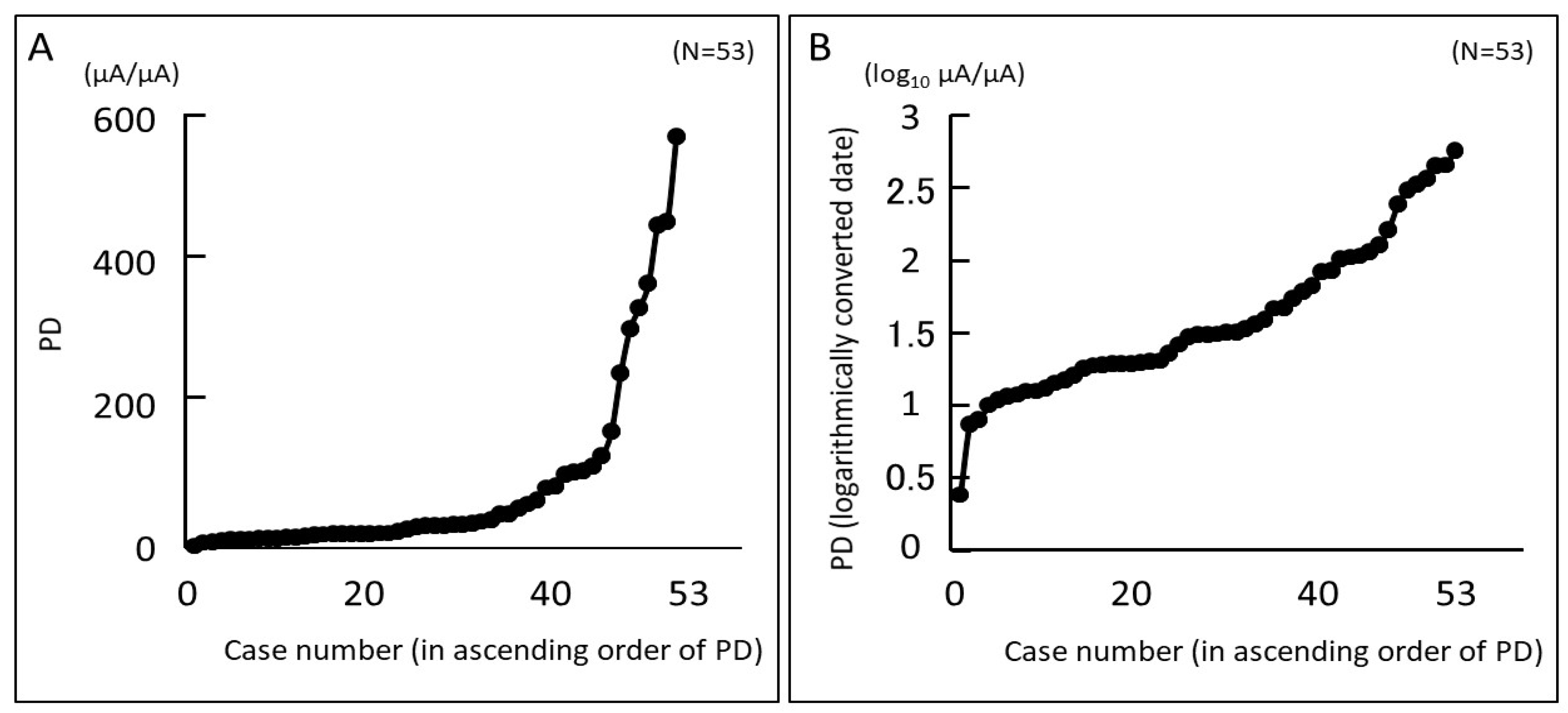
3.3. Pain Degree (PD) Evaluated Using PainVision® and Relationship between PD and Objective Findings
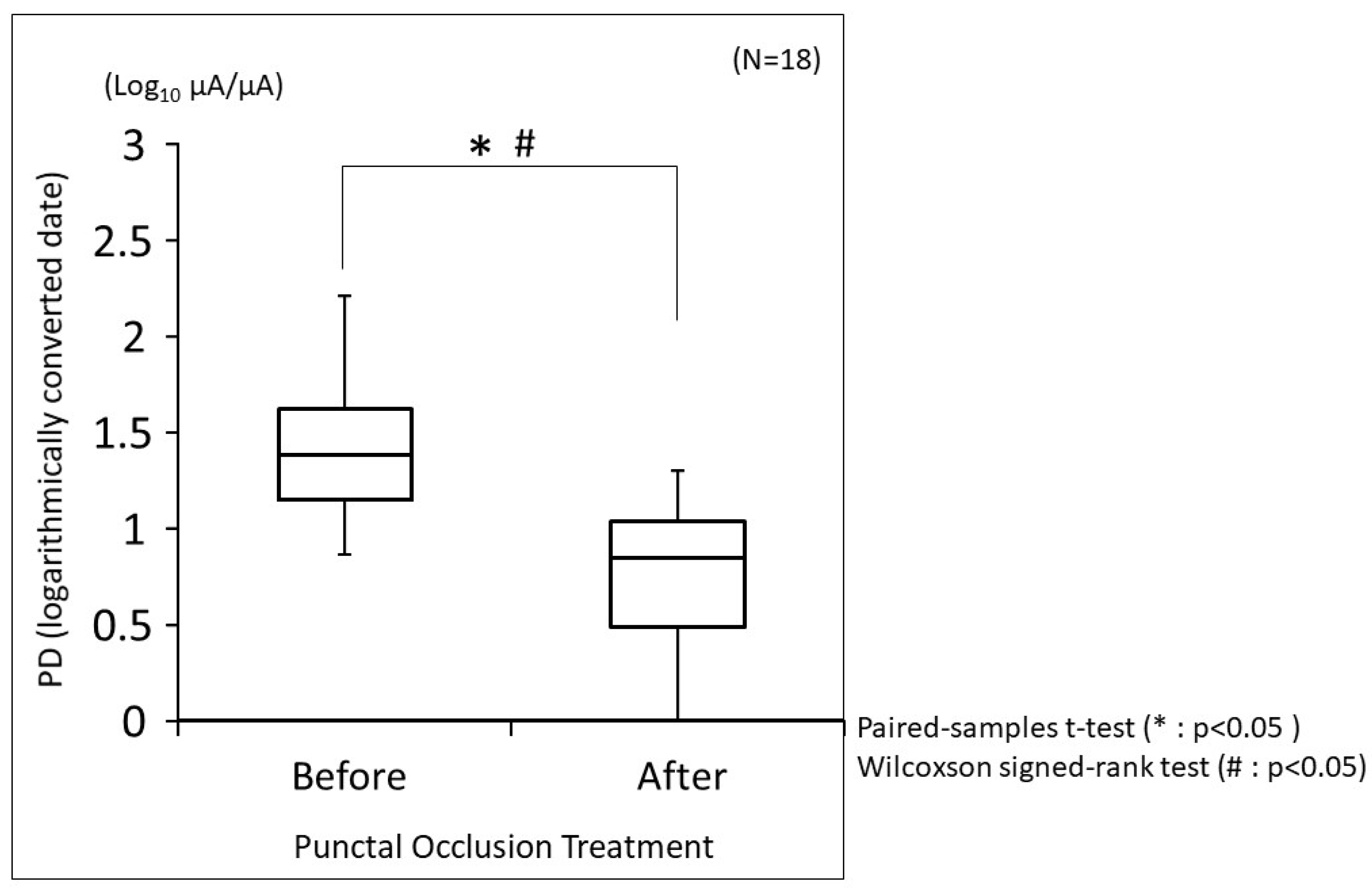
3.4. Changes in PD before and after Punctal Occlusion Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsubota, K.; Yokoi, N.; Shimazaki, J.; Watanabe, H.; Dogru, M.; Yamada, M.; Kinoshita, S.; Kim, H.M.; Tchah, H.W.; Hyon, J.Y.; et al. Asia Dry Eye Society. New perspectives on dry eye definition and diagnosis: A consensus report by the Asia Dry Eye Society. Ocul. Surf. 2017, 15, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.P.; Hammitt, K.M.; Jones, L.; et al. TFOS DEWS II Report Executive Summary. Ocul. Surf. 2017, 15, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Galor, A.; Feuer, W.; Lee, D.J.; Florez, H.; Venincasa, V.D.; Perez, V.L. Ocular surface parameters in older male veterans. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.D.; Galor, A.; Ramos-Betancourt, N.; Lisker-Cervantes, A.; Beltrán, F.; Ozorno-Zárate, J.; Sánchez-Huerta, V.; Torres-Vera, M.A.; Hernández-Quintela, E. Frequency and risk factors associated with dry eye in patients attending a tertiary care ophthalmology center in Mexico City. Clin. Ophthalmol. 2016, 10, 1335–1342. [Google Scholar] [CrossRef]
- Kheirkhah, A.; Crnej, A.; Ren, A.; Mullins, A.; Satitpitakul, V.; Hamrah, P.; Schaumberg, D.; Dana, R. Patients’ perceived treatment effectiveness in dry eye disease. Cornea 2017, 36, 893–897. [Google Scholar] [CrossRef]
- Siedlecki, A.N.; Smith, S.D.; Siedlecki, A.R.; Hayek, S.M.; Sayegh, R.R. Ocular pain response to treatment in dry eye patients. Ocul. Surf. 2020, 18, 305–311. [Google Scholar] [CrossRef]
- Sullivan, B.D.; Crews, L.A.; Messmer, E.M.; Foulks, G.N.; Nichols, K.K.; Baenninger, P.; Geerling, G.; Figueiredo, F.; Lemp, M. Correlations between commonly used objective signs and symptoms for the diagnosis of dry eye disease: Clinical implications. Acta Ophthalmol. 2012, 92, 161–166. [Google Scholar] [CrossRef]
- Galor, A.; Levitt, R.C.; Felix, E.R.; Martin, E.R.; Sarantopoulos, C.D. Neuropathic ocular pain: An important yet underevaluated feature of dry eye. Eye 2015, 29, 301–312. [Google Scholar] [CrossRef]
- Galor, A.; Covington, D.; Levitt, A.E.; McManus, K.T.; Seiden, B.; Felix, E.R.; Kalangara, J.; Feuer, W.; Patin, D.J.; Martin, E.R.; et al. Neuropathic ocular pain due to dry eye is associated with multiple comorbid chronic pain syndromes. J. Pain 2016, 17, 310–318. [Google Scholar] [CrossRef]
- Dieckmann, G.; Goyal, S.; Hamrah, P. Neuropathic corneal pain: Approaches for management. Ophthalmology 2017, 124, S34–S47. [Google Scholar] [CrossRef]
- Moein, H.R.; Akhlaq, A.; Dieckmann, G.; Abbouda, A.; Pondelis, N.; Salem, Z.; Müller, R.T.; Cruzat, A.; Cavalcanti, B.M.; Jamali, A.; et al. Visualization of micro-neuromas by using in vivo confocal microscopy: An objective biomarker for the diagnosis of neuropathic corneal pain? Ocul. Surf. 2020, 18, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Price, D.D. Psychological and neural mechanisms of the affective dimension of pain. Science 2000, 288, 1769–1772. [Google Scholar] [CrossRef] [PubMed]
- Ikeno, S.; Kawamata, M. PainVision. Masui Jpn. J. Anesthesiol. 2009, 58, 1367–1372. (In Japanese) [Google Scholar]
- Kim, J.; Lee, K.S.; Kong, S.W.; Kim, T.; Kim, M.J.; Park, S.B.; Lee, K.H. Correlations Between Electrically Quantified Pain Degree, Subjectively Assessed Visual Analogue Scale, and the McGill Pain Questionnaire A Pilot Study. Ann. Rehabil. Med. 2014, 38, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Hiraki, M.; Takemasa, I.; Uemura, M.; Haraguchi, N.; Nishimura, J.; Hata, T.; Mizushima, T.; Yamamoto, H.; Doki, Y.; Mori, M. Evaluation of invasiveness in single-site laparoscopic colectomy, using “the PainVision™ system” for quantitative analysis of pain sensation. Surg. Endosc. 2014, 28, 3216–3223. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Satoh, A.; Yamada, T.; Aisu, N.; Matsuoka, T.; Koganemaru, T.; Kajitani, R.; Munechika, T.; Matsumoto, Y.; Nagano, H.; et al. The relationship between evaluation methods for chemotherapy-induced peripheral neuropathy. Sci. Rep. 2019, 9, 20361. [Google Scholar] [CrossRef]
- Matsuoka, T.; Yoshida, Y.; Aisu, N.; Yamada, T.; Mogi, A.; Komono, A.; Sakamoto, R.; Kojima, D.; Yoshimatsu, G.; Kiyomi, F.; et al. Evaluation of vascular pain in patients with colorectal cancer receiving peripheral venous chemotherapy with or without oxaliplatin. Sci. Rep. 2019, 9, 1819. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.N.; Imai, K.; Kimura, T.; Saito, Y.; Takashima, S.; Matsuzaki, I.; Kurihara, N.; Atari, M.; Matsuo, T.; Iwai, H.; et al. Effect of lidocaine cream analgesia for chest drain tube removal after video-assisted thoracoscopic surgery for lung cancer: A randomized clinical trial. Reg. Anesth. Pain. Med. 2019, 45, 100760. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, K.; Han, S.L.; Yu, L.Z. PainVision® apparatus for assessment of efficacy of pulsed radiofrequency combined with pharmacological therapy in the treatment of postherpetic neuralgia and correlations with measurements. BioMed Res. Int. 2017, 2017, 5670219. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Yokoi, N.; Kato, H.; Sakai, R.; Komuro, A.; Sonomura, Y.; Ikeda, T.; Sotozono, C. Evaluation of eye-pain severity between dry-eye subtypes. Diagnostics 2021, 11, 166. [Google Scholar] [CrossRef]
- Yokoi, N.; Georgiev, G.A.; Kato, H.; Komuro, A.; Sonomura, Y.; Sotozono, C.; Tsubota, K.; Kinoshita, S. Classification of fluorescein breakup patterns: A novel method of differential diagnosis for dry eye. Am. J. Ophthalmol. 2017, 180, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Dogru, M.; Kawashima, M.; Nakamura, S.; Tsubota, K. Advances in the diagnosis and treatment of dry eye. Prog. Retin. Eye Res. 2020, 29, 100842. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, T.I.; Turk, D.C.; Morasco, B.J. Evaluation of the psychometric properties of the revised Short-Form McGill Pain Questionnaire. J. Pain 2012, 13, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Bron, A.; Tiffany, J.; Brown, N.; Hsuan, J.; Fowler, C. Reflective meniscometry: A non-invasive method to measure tear meniscus curvature. Br. J. Ophthalmol. 1999, 83, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Bron, A.J.; Tiffany, J.M.; Maruyama, K.; Komuro, A.; Kinoshita, S. Relationship between tear volume and tear meniscus curvature. Arch. Ophthalmol. 2004, 122, 1265–1269. [Google Scholar] [CrossRef][Green Version]
- Koh, S.; Watanabe, H.; Hosohata, J.; Hori, Y.; Hibino, S.; Nishida, K.; Maeda, N.; Tano, Y. Diagnosing dry eye using a blue-free barrier filter. Am. J. Ophthalmol. 2003, 136, 513–519. [Google Scholar] [CrossRef]
- Lemp, M.A. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995, 21, 221–232. [Google Scholar]
- Miyata, K.; Amano, S.; Sawa, M.; Nishida, T. A novel grading method for superficial punctate keratopathy magnitude and its correlation with corneal epithelial permeability. Arch. Ophthalmol. 2003, 121, 1537–1539. [Google Scholar] [CrossRef]
- van Bijsterveld, O.P. Diagnostic tests in the Sicca syndrome. Arch. Ophthalmol. 1969, 82, 10–14. [Google Scholar] [CrossRef]
- Amano, S. Meibomian gland dysfunction: Recent progress worldwide and in Japan. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES87–DES93. [Google Scholar] [CrossRef]
- Yokoi, N.; Komuro, A.; Maruyama, K.; Tsuzuki, M.; Miyajima, S.; Kinoshita, S. New surgical treatment for superior limbic keratoconjunctivitis and its association with conjunctivochalasis. Am. J. Ophthalmol. 2003, 135, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Shiraishi, A.; Sakane, Y.; Ohta, K.; Yamaguchi, M.; Ohashi, Y. Involvement of eyelid pressure in lid-wiper epitheliopathy. Curr. Eye Res. 2016, 41, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Fechner, G.T. Outline of a new principle of mathematical psychology (1851). By Gustav Theodor Fechner (translation). Psychol. Res. 1987, 49, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.B.S. Psychophysical and Clinical Investigations of Ocular Discomfort. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 2012. [Google Scholar]
- Rósza, A.J.; Beuerman, R.W. Density and organization of free nerve endings in the corneal epithelium of the rabbit. Pain 1982, 14, 105–120. [Google Scholar] [CrossRef]
- Toda, I.; Shimazaki, J.; Tsubota, K. Dry eye with only decreased tear break-up time is sometimes associated with allergic conjunctivitis. Ophthalmology 1995, 102, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Li, X.; Peng, W. Individual variation in pain sensitivity and implicit negative bias toward pain. Psychosom. Med. 2020, 82, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, A.T.; George, S.Z.; Bialosky, J.E.; Robinson, M.E. Fear of pain, pain catastrophizing, and acute pain perception: Relative prediction and timing of assessment. J. Pain 2008, 9, 806–812. [Google Scholar] [CrossRef] [PubMed]
- George, S.Z.; Hirsh, A.T. Psychologic influence on experimental pain sensitivity and clinical pain intensity for patients with shoulder pain. J. Pain 2009, 10, 293–299. [Google Scholar] [CrossRef]
- George, S.Z.; Dannecker, E.A.; Robinson, M.E. Fear of pain, not pain catastrophizing, predicts acute pain intensity, but neither factor predicts tolerance or blood pressure reactivity: An experimental investigation in pain-free individuals. Eur. J. Pain 2006, 10, 457–465. [Google Scholar] [CrossRef]
- Shetty, R.; Dua, H.S.; Tong, L.; Kundu, G.; Khamar, P.; Gorimanipalli, B.; D’Souza, S. Role of in vivo confocal microscopy in dry eye disease and eye pain. Indian J. Ophthalmol. 2023, 71, 1099–1104. [Google Scholar] [CrossRef]
| Punctal Occlusion Treatment | ||||
|---|---|---|---|---|
| Total | Before | After | p Value | |
| (n = 53) | (n = 18) | (n = 18) | ||
| Patient Demographics | ||||
| Age, yrs, mean (SD) | 64.4 (13.4) | 67.9 (10.2) | ||
| Female, n (%) | 47 (88.7%) | 17 (94.4%) | ||
| Sjögren’s syndrome, n (%) | 24 (45.3%) | 8 (44.4%) | ||
| Ocular Surface Evaluations | ||||
| Corneal staining score (0–15), $ | 8.3 (3.8) | 9.6 (3.1) | 3.5 (3.8) | <0.0001 |
| Upper | 0.64 (1.0) | 0.89 (1.3) | 0.56 (0.98) | 0.42 |
| Temporal | 1.6 (1.2) | 1.9 (1.0) | 0.44 (0.92) | 0.0002 |
| Nasal | 1.9 (1.1) | 2.3 (0.84) | 0.67 (0.97) | <0.0001 |
| Central | 2.1 (1.2) | 2.6 (0.70) | 0.39 (0.85) | <0.0001 |
| Lower | 2.1 (0.96) | 2.2 (1.0) | 0.89 (1.0) | 0.0014 |
| Conjunctival staining score (0–6), $ | 3.8 (2.1) | 4.3 (2.0) | 1.2 (1.9) | 0.0004 |
| Temporal | 1.8 (1.1) | 2.2 (1.1) | 0.56 (1.0) | 0.0002 |
| Nasal | 2.0 (1.1) | 2.1 (1.1) | 0.67 (1.0) | 0.0007 |
| SLK score (0–3), $ | 0.32 (0.75) | 0.50 (0.92) | 0.11 (0.47) | 0.09 |
| Upper LWE score (0–3), $ | 1.1 (1.0) | 1.0 (0.97) | 0.22 (0.55) | 0.0035 |
| Lower LWE score (0–3), $ | 1.3 (0.96) | 1.3 (0.96) | 0.5 (0.62) | 0.0085 |
| FBUT, seconds $ | 1.7 (1.6) | 0.87 (1.3) | 7.4 (3.1) | <0.0001 |
| TMR, mm # | 0.15 (0.06) | 0.17 (0.07) | 0.48 (0.25) | <0.0001 |
| Schirmer 1 test, mm | 4.5 (5.9) | 3.5 (5.2) | ||
| MGD, n (%) | 2 (3.8%) | 0 | 0 | |
| Anesthesia test positive, n (%) | 8 (15.0%) | 1 (5.6%) | 1 (5.6%) | |
| Pain Degree (PainVision®) | Eye Pain (VAS) | |||
|---|---|---|---|---|
| n = 57 | R | p Value | R | p Value |
| Corneal Staining Score (Total) | 0.18 | 0.19 | 0.01 | 0.92 |
| Upper | 0.17 | 0.22 | 0.01 | 0.94 |
| Temporal | 0.14 | 0.30 | 0.02 | 0.87 |
| Nasal | 0.33 | 0.017 | 0.18 | 0.20 |
| Central | 0.33 | 0.014 | 0.09 | 0.50 |
| Lower | −0.18 | 0.19 | −0.03 | 0.82 |
| Conjunctival Staining Score (Total) | 0.09 | 0.54 | −0.07 | 0.60 |
| Temporal | −0.01 | 0.94 | −0.16 | 0.25 |
| Nasal | 0.17 | 0.23 | 0.01 | 0.93 |
| SLK score | −0.12 | 0.40 | −0.16 | 0.26 |
| Upper LWE score | 0.28 | 0.042 | 0.08 | 0.59 |
| Lower LWE score | 0.25 | 0.07 | 0.06 | 0.69 |
| FBUT | −0.12 | 0.41 | 0.04 | 0.77 |
| TMR | −0.003 | 0.98 | −0.01 | 0.92 |
| Schirmer 1 test | −0.009 | 0.95 | 0.17 | 0.23 |
| (n = 57) | Multivariate Analysis | Multivariate Analysis 2 | ||
|---|---|---|---|---|
| Variables | Logarithmic Value | p Value | Logarithmic Value | p Value |
| Age | 0.160 | 0.69 | 0.210 | 0.62 |
| Sex (female) | 0.055 | 0.88 | 0.030 | 0.93 |
| Central Corneal staining score | 1.386 | 0.04 | 1.430 | 0.04 |
| Conjunctival staining score | 0.112 | 0.77 | ||
| Upper LWE score | 0.940 | 0.11 | 0.913 | 0.12 |
| SLK score | 0.131 | 0.74 | ||
| FBUT | 0.769 | 0.17 | 0.883 | 0.13 |
| TMR | 0.565 | 0.27 | 0.626 | 0.24 |
| Pain Degree (PainVision®) | ||
|---|---|---|
| n = 36 | R | p Value |
| Corneal Staining Score (Total) | 0.58 | 0.0002 |
| Upper | 0.25 | 0.14 |
| Temporal | 0.61 | <0.0001 |
| Nasal | 0.60 | 0.0001 |
| Central | 0.69 | <0.0001 |
| Lower | 0.40 | 0.02 |
| Conjunctival Staining Score (Total) | 0.71 | <0.0001 |
| Temporal | 0.66 | <0.0001 |
| Nasal | 0.73 | <0.0001 |
| SLK Score | 0.43 | 0.009 |
| Upper LWE Score | 0.55 | 0.0005 |
| Lower LWE Score | 0.45 | 0.006 |
| FBUT | −0.71 | <0.0001 |
| TMR | −0.49 | 0.002 |
| Schirmer 1 test | ||
| n = 36 | Multivariate Analysis | Multivariate Analysis 2 | ||
|---|---|---|---|---|
| Variables | Logarithmic Value | p Value | Logarithmic Value | p Value |
| Age | 0.001 | 0.997 | 0.139 | 0.73 |
| Sex (female) | 0.404 | 0.39 | 0.231 | 0.59 |
| Corneal staining score | ||||
| Upper | 0.469 | 0.34 | ||
| Temporal | 0.100 | 0.79 | ||
| Nasal | 0.379 | 0.42 | ||
| Central | 2.020 | 0.0095 | 2.060 | 0.009 |
| Lower | 0.444 | 0.36 | ||
| Conjunctival staining score | ||||
| Temporal | 0.096 | 0.80 | ||
| Nasal | 0.798 | 0.16 | 0.953 | 0.11 |
| SLK score | 0.009 | 0.98 | ||
| Upper LWE score | 0.626 | 0.24 | 0.605 | 0.25 |
| Lower LWE score | 0.008 | 0.98 | ||
| FBUT | 0.736 | 0.18 | 0.441 | 0.36 |
| TMR | 1.466 | 0.03 | 1.456 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshikawa, Y.; Yokoi, N.; Kusada, N.; Kato, H.; Sakai, R.; Komuro, A.; Sonomura, Y.; Sotozono, C. Eye Pain Caused by Epithelial Damage in the Central Cornea in Aqueous-Deficient Dry Eye. Diagnostics 2024, 14, 30. https://doi.org/10.3390/diagnostics14010030
Yoshikawa Y, Yokoi N, Kusada N, Kato H, Sakai R, Komuro A, Sonomura Y, Sotozono C. Eye Pain Caused by Epithelial Damage in the Central Cornea in Aqueous-Deficient Dry Eye. Diagnostics. 2024; 14(1):30. https://doi.org/10.3390/diagnostics14010030
Chicago/Turabian StyleYoshikawa, Yamato, Norihiko Yokoi, Natsuki Kusada, Hiroaki Kato, Rieko Sakai, Aoi Komuro, Yukiko Sonomura, and Chie Sotozono. 2024. "Eye Pain Caused by Epithelial Damage in the Central Cornea in Aqueous-Deficient Dry Eye" Diagnostics 14, no. 1: 30. https://doi.org/10.3390/diagnostics14010030
APA StyleYoshikawa, Y., Yokoi, N., Kusada, N., Kato, H., Sakai, R., Komuro, A., Sonomura, Y., & Sotozono, C. (2024). Eye Pain Caused by Epithelial Damage in the Central Cornea in Aqueous-Deficient Dry Eye. Diagnostics, 14(1), 30. https://doi.org/10.3390/diagnostics14010030






