Rare Condition of Aberrant Arterial Supply to a Normal Lung: A Cases Series and Literature Review
Abstract
:1. Introduction
2. Cases
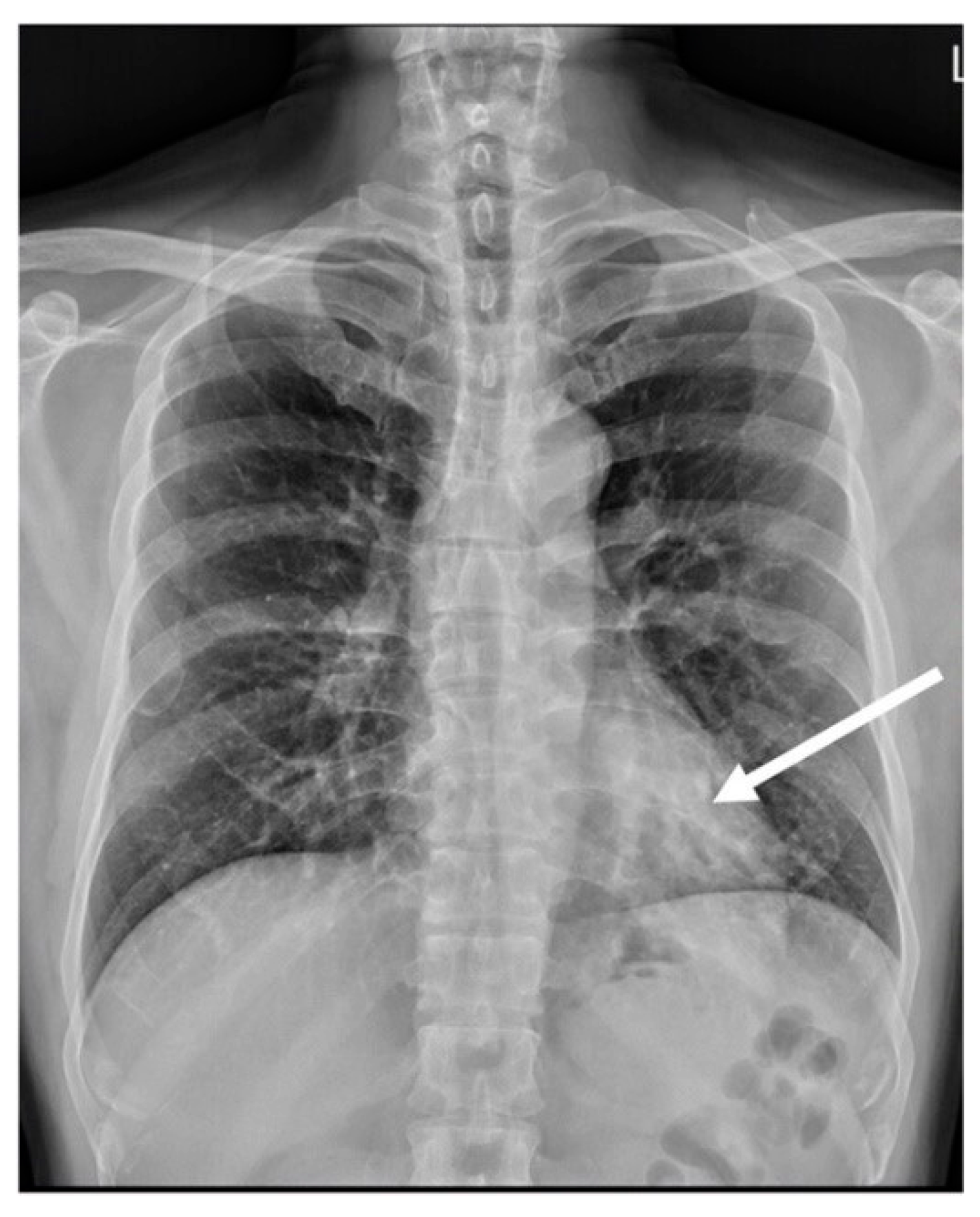
2.1. Case 1
2.2. Case 2
2.3. Case 3
3. Discussion
3.1. Classification of the Spectrum of Pulmonary Sequestration and Aberrant Arterial Supply to a Normal Lung
3.2. Embryology of the Systemic Arterial Supply to a Normal Lung
3.3. Manifestation of Systemic Arterial Supply to a Normal Lung
3.4. Diagnosis
3.5. Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Irodi, A.; Prabhu, S.M.; John, R.A.; Leena, R. Congenital bronchopulmonary vascular malformations, “sequestration” and beyond. Indian J. Radiol. Imaging 2015, 25, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.C., Jr.; Murney, J.A.; Dominy, D.E. Systemic arterial blood supply to a normal lung. JAMA 1962, 182, 497–499. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.P., 3rd; Perry, L.W. Systemic artery—Pulmonary vein fistulas, congenital and acquired left to left shunt. Am. J. Cardiol. 1969, 23, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Ernst, S.M.; Bruschke, A.V. An aberrant systemic artery to the right lung with normal pulmonary tissue. Chest 1971, 60, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Currarino, G.; Willis, K.; Miller, W. Congenital fistula between an aberrant systemic artery and a pulmonary vein without sequestration. A report of three cases. J. Pediatr. 1975, 87, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Kirks, D.R.; Kane, P.E.; Free, E.A.; Taybi, H. Systemic arterial supply to normal basilar segments of the left lower lobe. AJR Am. J. Roentgenol. 1976, 126, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Robida, A.; Eltohami, E.A.; Chaikhouni, A. Aberrant systemic artery-pulmonary venous fistula: Diagnosis with Doppler imaging. Int. J. Cardiol. 1992, 35, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Brühlmann, W.; Weishaupt, D.; Goebel, N.; Imhof, E. Therapeutic embolization of a systemic arterialization of lung without sequestration. Eur. Radiol. 1998, 8, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Chabbert, V.; Doussau-Thuron, S.; Otal, P.; Bouchard, L.; Didier, A.; Joffre, F.; Rousseau, H. Endovascular treatment of aberrant systemic arterial supply to normal basilar segments of the right lower lobe: Case report and review of the literature. Cardiovasc. Intervent. Radiol. 2002, 25, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Iizasa, T.; Haga, Y.; Hiroshima, K.; Fujisawa, T. Systemic arterial supply to the left basal segment without the pulmonary artery: Four consecutive cases. Eur. J. Cardiothorac. Surg. 2003, 23, 847–849. [Google Scholar] [CrossRef] [PubMed]
- Tatli, S.; Yucel, E.K.; Couper, G.S.; Henderson, J.M.; Colson, Y.L. Aneurysm of an aberrant systemic artery to the lung. AJR Am. J. Roentgenol. 2005, 184, 1241–1244. [Google Scholar] [CrossRef] [PubMed]
- Baek, W.K.; Cho, J.; Kim, J.T.; Yoon, Y.H.; Kim, K.H.; Lim, H.K.; Kim, L.; Lee, H.L. Systemic arterial supply to normal basal segments of the left lower lobe along with the pulmonary artery: Is lung resection warranted? J. Thorac. Cardiovasc. Surg. 2006, 131, 742–743. [Google Scholar] [CrossRef] [PubMed]
- Kosutic, J.; Minic, P.; Sovtic, A.; Prijic, S. Upper lung lobe systemic artery-pulmonary vein fistula with signs and symptoms of congestive heart failure: Successful treatment with coil embolization. J. Vasc. Interv. Radiol. 2007, 18, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.N.; Joshi, P.; Sim, K.H. Large anomalous systemic arterial supply to the left lung without pulmonary sequestration: A rare cause of heart failure in a child. Pediatr. Cardiol. 2009, 30, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Singhi, A.K.; Nicholson, I.; Francis, E.; Kumar, R.K.; Hawker, R. Anomalous systemic arterial supply to normal basal segment of the left lung. Heart Lung Circ. 2011, 20, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, A.S.; Gupta, P.; Mukund, A.; Kumar, A.; Gupta, M. Anomalous systemic artery to a normal lung: A rare cause of hemoptysis in adults. Oman Med. J. 2012, 27, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Noonan, P.M.; Ramchandani, B.; Barron, D.J.; Stumper, O. Catheter rehabilitation of occluded aberrant pulmonary artery. Interact. Cardiovasc. Thorac. Surg. 2013, 17, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Mautone, M.; Naidoo, P. A case of systemic arterial supply to the right lower lobe of the lung: Imaging findings and review of the literature. J. Radiol. Case Rep. 2014, 8, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Walsworth, M.K.; Yap, F.Y.; McWilliams, J.P. Aberrant systemic arterial supply to normal lung arising from the proper hepatic artery discovered during transarterial chemoembolization. J. Radiol. Case Rep. 2015, 9, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Hata, Y.; Otsuka, H.; Koezuka, S.; Okubo, Y.; Isobe, K.; Tochigi, N.; Shibuya, K.; Homma, S.; Iyoda, A. Simultaneous resection of bilateral anomalous systemic supply to the basal segments of the lungs: A case report. J. Cardiothorac. Surg. 2015, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Yamashita, Y.; Tsubokawa, N.; Takasaki, T.; Taniyama, D.; Kuraoka, K.; Toyota, N.; Mimura, T.; Harada, H. Anomalous Systemic Arterialization of a Normal Basal Lung Segment with Aneurysm of the Aberrant Artery. Kyobu Geka 2016, 69, 1003–1007. (In Japanese) [Google Scholar] [PubMed]
- Arvind, B.; Gupta, S.K.; Kothari, S.S. Isolated systemic arterial supply to normal lung—An unusual cause of extracardiac left-to-right shunt. Cardiol. Young 2020, 30, 1946–1950. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Khan, A.; Cheng, G. The abnormal systemic artery to the left lower lobe (ASALLL): A report of two cases. Radiol. Case Rep. 2020, 15, 1960–1964. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, T.; Hino, H.; Kuwauchi, S.; Zempo, N.; Ishida, K.; Maru, N.; Matsui, H.; Taniguchi, Y.; Saito, T.; Tsuta, K.; et al. Anomalous systemic arterial supply to the basal segment of the lung with giant aberrant artery: A case report. Surg. Case Rep. 2020, 6, 285. [Google Scholar] [CrossRef] [PubMed]
- Wee, N.K.; H’ng, M.W.C.; Punamiya, S. Isolated Systemic Arterial Supply to Normal Lung with Aneurysm Formation: A Rare Entity with An Even Rarer Complication. Am. J. Case Rep. 2020, 21, e926409. [Google Scholar] [CrossRef] [PubMed]
- Giancaspro, G.; Hahn, L.; Hsiao, A.; Kligerman, S.J. Anomalous Systemic Arterial Supply to Normal Basal Segments of the Lung. Radiol. Cardiothorac. Imaging 2022, 4, e220029. [Google Scholar] [CrossRef] [PubMed]
- Zylak, C.J.; Eyler, W.R.; Spizarny, D.L.; Stone, C.H. Developmental lung anomalies in the adult: Radiologic-pathologic correlation. Radiographics 2002, 22, S25–S43. [Google Scholar] [CrossRef] [PubMed]
- Biyyam, D.R.; Chapman, T.; Ferguson, M.R.; Deutsch, G.; Dighe, M.K. Congenital lung abnormalities: Embryologic features, prenatal diagnosis, and postnatal radiologic-pathologic correlation. Radiographics 2010, 30, 1721–1738. [Google Scholar] [CrossRef] [PubMed]






| Abnormality of Tracheobronchopulmonary Airway and/or Arterial Blood Supply | Examples of Recognized Entities | Venous Drainage |
|---|---|---|
| Bronchopulmonary abnormality (normal pulmonary artery blood supply) | Trachea: tracheal stenosis Bronchial: bronchial stenosis or atresia, bronchogenic cyst Parenchyma: congenital lung cyst, cystic adenomatoid malformation, lobar emphysema | Normal Anomalous Multiple Mixed Mismatched |
| Arterial blood supply abnormality (normal bronchopulmonary airway) | Aberrant artery supply to normal lung | |
| Both bronchopulmonary and arterial abnormality (abnormal bronchopulmonary airway with systemic arterial blood supply) | Bronchopulmonary abnormality is patent: congenital cystic bronchiectasis, lobar emphysema with systemic arterial supply, scimitar syndrome | |
| Bronchopulmonary connection absence: classical sequestration, congenital lung cysts with systemic arterial blood supply |
| Author, Year | Age (yr)/Sex | Symptom | Origin of System Aberrant Vessel/ Diagnosis Motility | Site of Drainage Lung | Management | Outcome |
|---|---|---|---|---|---|---|
| Campbell et al., 1962 [2] | 35/M | Incidental finding (CXR) | AA/OP | RLL | Lobectomy | Complicated with empyema. Completely recovered on half-year follow-up. |
| 1.2/M (Preterm) | Murmur | DTA/OP | LLL | Ligation | Recovered on one-year follow-up. | |
| Scott et al., 1968 [3] | 6.2/F | Murmur | DTA/Angiogram | LLL | Ligation and lobectomy | No complications |
| 7.2/F | Murmur | DTA (TOF)/Angiogram | Left lung | Ligation and TOF repair | Succumbed to uncontrolled bleeding after TOF repair | |
| Ernst et al., 1971 [4] | 3/M | Murmur | AA/Angiogram | RLL | Ligation | No symptoms |
| Currarino et al., 1975 [5] | 2.5 mo/M | Murmur | AA/Angiogram | RLL | Ligation | Deceased * |
| 3/F | Murmur | DTA/Angiogram | RLL | Ligation and partial lobectomy | Not mentioned | |
| 5.5/M | Murmur | DTA/Angiogram | LLL | None | Not mentioned | |
| Kirks et al., 1976 [6] | 6/M | Murmur | DTA/Angiogram | LLL | Ligation and Lobectomy | Not mentioned |
| 17 mo/F | Murmur | DTA/Angriogram | LLL | Lobectomy | Not mentioned | |
| Robida A et al., 1992 [7] | 6/F | Murmur | AA/US | RLL | Ligation | Not mentioned |
| Bruhlmann et al., 1997 [8] | 51/M | Hemoptysis | DTA/CT, Angiogram | LLL | Embolization (coils) | No symptoms in 10-month follow-up |
| Chabbert et al., 2002 [9] | 17/M | Chest pain | AA/CT, Angiogram | RLL | Embolization (coils) | No symptoms in 12-month follow-up |
| Toshihiko et al., 2003 [10] | 50/F | Hemoptysis | DTA/CT, Angiogram, bronchoscopy | LLL | Segmentectomy | Not mentioned |
| 17/M | Hemoptysis | DTA/as above | LLL | Segmentectomy | Not mentioned | |
| 43/F | Hemoptysis | DTA/as above | LLL | Segmentectomy | Not mentioned | |
| 20/M | Murmur | DTA/as above | LLL | Lobectomy | Not mentioned | |
| Servet et al., 2005 [11] | 47/F | Incidental finding (MRI) | AA/CTA | RLL | Lobectomy | Not mentioned |
| Baek et al., 2006 [12] | 17/M | Hemoptysis | DTA/CT, angiogram | LLL | Ligation | No symptoms in 1-year follow-up |
| Kosutic et al., 2007 [13] | 3 mo/M | Heart failure | DTA (two origins to two aberrant arteries)/CT, Angiogram | RUL | Embolization (Coil) | Recovered in 6-month follow-up |
| Wong et al., 2008 [14] | 10 mo/M | Heart failure | DTA/CT, Angiogram | LLL | Ligation and lobectomy | No complications |
| Singhi et al., 2012 [15] | 1 mo/M | Heart failure | DTA/CT | Left lung | Ligation | No complications |
| 90-day-old/U | Heart failure | AA/Angiogram | LLL | Embolization (Amplatzer vascular Plug, Gianturco coils) | No symptoms in 3-year follow-up | |
| 74 day-old/U | Heart failure | DTA/Angiogram | LLL | Embolization (Coil) | No symptoms in 6-month follow-up | |
| Bhalla et al., 2012 [16] | 41/M | Hemoptysis | DTA/CTA, Angiogram | LLL | Ligation | Not mentioned |
| 27/F | Hemoptysis | Celiac trunk/CTA, Angiogram | RML | Embolization (Glue) | Not mentioned | |
| 32/F | Hemoptysis | DTA/CTA | LLL | None | Not mentioned | |
| Noonan et al., 2013 [17] | 10/F | None | Right-sided ductus arteriosus/Angiogram | Right lung | Vessel repair using a stent graft | Complete recovery on 6-month follow-up |
| Mautone et al., 2014 [18] | 22/F | Hemoptysis | DTA/CTA | RML | None | Not mentioned |
| Walsworth et al., 2015 [19] | 53/M | Incidental abnormal (CXR) | Proper hepatic artery/Angiogram | LLL | None | Stable on 3-month follow-up |
| Makino et al., 2015 [20] | 33/M | None | DTA (One origin to two aberrant arteries)/CT | RLL, LLL | Segmentectomy | Stable on 12-month follow-up |
| Ando et al., 2016 [21] | 40/M | Incidental finding (CXR) | DTA (aneurysmal aberrant artery)/CT | LLL | Lobectomy | Not mentioned |
| Arvind et al., 2020 [22] | 21/F | Heart failure | DTA/CTA, Angiogram | LLL | Embolization (Vascular plug) | No symptoms on 3-month follow-up |
| Yan et al., 2020 [23] | 15/M | Hemoptysis | DTA/CT, angiogram | LLL | Embolization | Complications with hemoptysis, ischemic necrosis |
| 36/M | Hemoptysis Chest pain | DTA/CT | LLL | Lobectomy | Not mentioned | |
| Utsumi et al., 2020 [24] | 42/M | Incidental finding (CXR) | Celiac trunk/CT | LLL | Lobectomy | No symptoms on 6-month follow-up |
| Wee et al., 2022 [25] | 61/M | Incidental finding (CXR) | DTA (aneurysmal aberrant artery)/CTA | LLL | None | Not mentioned |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-Y.; Lai, Y.-J.; Huang, C.-C. Rare Condition of Aberrant Arterial Supply to a Normal Lung: A Cases Series and Literature Review. Diagnostics 2024, 14, 32. https://doi.org/10.3390/diagnostics14010032
Chang Y-Y, Lai Y-J, Huang C-C. Rare Condition of Aberrant Arterial Supply to a Normal Lung: A Cases Series and Literature Review. Diagnostics. 2024; 14(1):32. https://doi.org/10.3390/diagnostics14010032
Chicago/Turabian StyleChang, Yu-Yun, Yen-Jun Lai, and Chun-Chieh Huang. 2024. "Rare Condition of Aberrant Arterial Supply to a Normal Lung: A Cases Series and Literature Review" Diagnostics 14, no. 1: 32. https://doi.org/10.3390/diagnostics14010032





