18F-FDG PET Is Not Inferior to 68Ga-PSMA PET for Detecting Biochemical Recurrent Prostate Cancer with a High Gleason Score: A Head-to-Head Comparison Study
Abstract
:1. Introduction
2. Materials and Methods
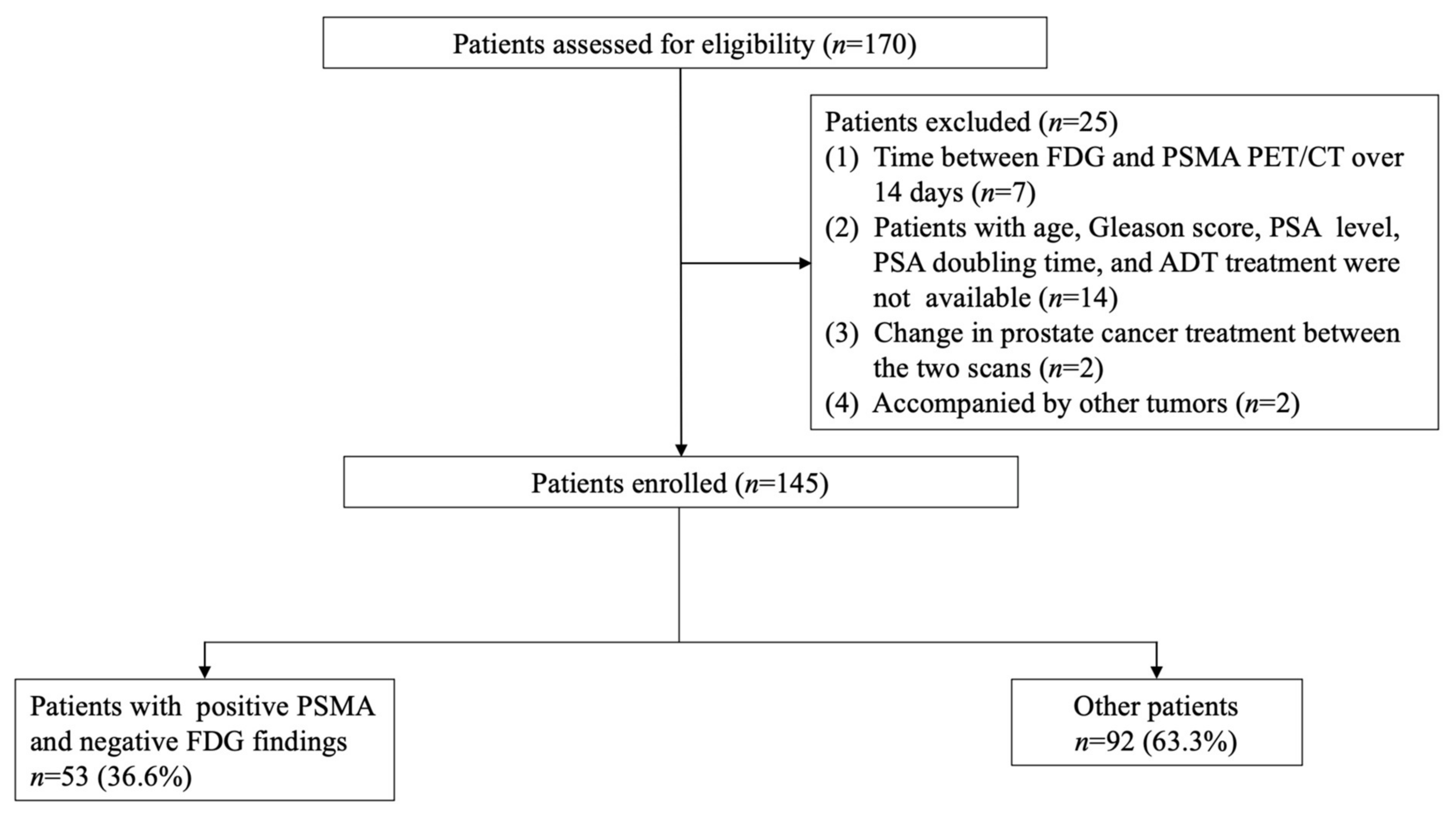
2.1. Patients
2.2. 18F-FDG and 68Ga-PSMA PET/CT
2.3. Image Evaluation
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Detection Rate of FDG and PSMA PET/CT
3.3. Differences in Clinical Features between FDG PET-Negative Patients with Positive PSMA PET Results and Other Patients
3.4. Comparison of Detection Rates of FDG and PSMA PET/CT Based on Lesion Region and Gleason Score
3.5. Semiquantitative Analysis of Concordantly Positive Lesions in Patients with a Gleason Score of 9
3.6. Inter-Reader Agreement and Validation of Positive Lesions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Boorjian, S.A.; Eastham, J.A.; Graefen, M.; Guillonneau, B.; Karnes, R.J.; Moul, J.W.; Schaeffer, E.M.; Stief, C.; Zorn, K.C. A critical analysis of the long-term impact of radical prostatectomy on cancer control and function outcomes. Eur. Urol. 2012, 61, 664–675. [Google Scholar] [CrossRef]
- Briganti, A.; Abdollah, F.; Nini, A.; Suardi, N.; Gallina, A.; Capitanio, U.; Bianchi, M.; Tutolo, M.; Passoni, N.M.; Salonia, A.; et al. Performance characteristics of computed tomography in detecting lymph node metastases in contemporary patients with prostate cancer treated with extended pelvic lymph node dissection. Eur. Urol. 2012, 61, 1132–1138. [Google Scholar] [CrossRef]
- Harisinghani, M.G.; Barentsz, J.; Hahn, P.F.; Deserno, W.M.; Tabatabaei, S.; van de Kaa, C.H.; de la Rosette, J.; Weissleder, R. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N. Engl. J. Med. 2003, 348, 2491–2499. [Google Scholar] [CrossRef]
- Hövels, A.M.; Heesakkers, R.A.; Adang, E.M.; Jager, G.J.; Strum, S.; Hoogeveen, Y.L.; Severens, J.L.; Barentsz, J.O. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: A meta-analysis. Clin. Radiol. 2008, 63, 387–395. [Google Scholar] [CrossRef]
- Perera, M.; Papa, N.; Christidis, D.; Wetherell, D.; Hofman, M.S.; Murphy, D.G.; Bolton, D.; Lawrentschuk, N. Sensitivity, Specificity, and Predictors of Positive 68Ga-Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2016, 70, 926–937. [Google Scholar] [CrossRef]
- Perera, M.; Papa, N.; Roberts, M.; Williams, M.; Udovicich, C.; Vela, I.; Christidis, D.; Bolton, D.; Hofman, M.S.; Lawrentschuk, N.; et al. Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer-Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-specific Membrane Antigen-avid Lesions: A Systematic Review and Meta-analysis. Eur. Urol. 2020, 77, 403–417. [Google Scholar]
- Afshar-Oromieh, A.; Zechmann, C.M.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Holland-Letz, T.; Hadaschik, B.A.; Giesel, F.L.; Debus, J.; et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Rayn, K.N.; Elnabawi, Y.A.; Sheth, N. Clinical implications of PET/CT in prostate cancer management. Transl. Androl. Urol. 2018, 7, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, H.; Karapolat, I. (18)F-fluorodeoxyglucose PET/CT for detection of disease in patients with prostate-specific antigen relapse following radical treatment of a local-stage prostate cancer. Oncol. Lett. 2016, 11, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Jadvar, H. Imaging evaluation of prostate cancer with 18F-fluorodeoxyglucose PET/CT: Utility and limitations. Eur. J. Nucl. Med. Mol. Imaging 2013, 40 (Suppl. 1), S5–S10. [Google Scholar] [CrossRef] [PubMed]
- Crocerossa, F.; Marchioni, M.; Novara, G.; Carbonara, U.; Ferro, M.; Russo, G.I.; Porpiglia, F.; Di Nicola, M.; Damiano, R.; Autorino, R.; et al. Detection Rate of Prostate Specific Membrane Antigen Tracers for Positron Emission Tomography/Computerized Tomography in Prostate Cancer Biochemical Recurrence: A Systematic Review and Network Meta-Analysis. J. Urol. 2021, 205, 356–369. [Google Scholar] [CrossRef]
- Fendler, W.P.; Calais, J.; Eiber, M.; Flavell, R.R.; Mishoe, A.; Feng, F.Y.; Nguyen, H.G.; Reiter, R.E.; Rettig, M.B.; Okamoto, S.; et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol. 2019, 5, 856–863. [Google Scholar] [CrossRef] [PubMed]
- McGeorge, S.; Kwok, M.; Jiang, A.; Emmett, L.; Pattison, D.A.; Thomas, P.A.; Yaxley, J.W.; Roberts, M.J. Dual-Tracer Positron-Emission Tomography Using Prostate-Specific Membrane Antigen and Fluorodeoxyglucose for Staging of Prostate Cancer: A Systematic Review. Adv. Urol. 2021, 2021, 1544208. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.A.; Rodríguez, M.; Rioja, J.; Peñuelas, I.; Martí-Climent, J.; Garrastachu, P.; Quincoces, G.; Zudaire, J.; García-Velloso, M.J. Dual tracer 11C-choline and FDG-PET in the diagnosis of biochemical prostate cancer relapse after radical treatment. Mol. Imaging Biol. 2010, 12, 210–217. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Y.; Shi, Y.; Zhu, Y.; Xu, L.; Huang, G.; Liu, J. Diagnostic value of 18F-FDG PET/CT in patients with biochemical recurrent prostate cancer and negative 68Ga-PSMA PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2970–2977. [Google Scholar] [CrossRef]
- Demirci, E.; Sahin, O.E.; Ocak, M.; Akovali, B.; Nematyazar, J.; Kabasakal, L. Normal distribution pattern and physiological variants of 68Ga-PSMA-11 PET/CT imaging. Nucl. Med. Commun. 2016, 37, 1169–1179. [Google Scholar] [CrossRef]
- Eiber, M.; Herrmann, K.; Calais, J.; Hadaschik, B.; Giesel, F.L.; Hartenbach, M.; Hope, T.; Reiter, R.; Maurer, T.; Weber, W.A.; et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): Proposed miTNM Classification for the Interpretation of PSMA-Ligand PET/CT. J. Nucl. Med. 2018, 59, 469–478. [Google Scholar] [CrossRef]
- Seifert, R.; Emmett, L.; Rowe, S.P.; Herrmann, K.; Hadaschik, B.; Calais, J.; Giesel, F.L.; Reiter, R.; Maurer, T.; Heck, M.; et al. Second Version of the Prostate Cancer Molecular Imaging Standardized Evaluation Framework Including Response Evaluation for Clinical Trials (PROMISE V2). Eur. Urol. 2023, 83, 405–412. [Google Scholar] [CrossRef]
- Hofman, M.S.; Hicks, R.J.; Maurer, T.; Eiber, M. Prostate-specific Membrane Antigen PET: Clinical Utility in Prostate Cancer, Normal Patterns, Pearls, and Pitfalls. Radiographics 2018, 38, 200–217. [Google Scholar] [CrossRef]
- Fendler, W.P.; Eiber, M.; Beheshti, M.; Bomanji, J.; Ceci, F.; Cho, S.; Giesel, F.; Haberkorn, U.; Hope, T.A.; Kopka, K.; et al. (68)Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.S.; Packard, A.T.; Johnson, D.R.; Johnson, G.B. Pitfalls of a Mixed Metabolic Response at PET/CT. Radiographics 2019, 39, 1461–1475. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, C.; Wei, Y.; Meng, J.; Zhang, Y.; Gan, H.; Xu, X.; Wan, F.; Pan, J.; Ma, X.; et al. A Prospective Trial of 68Ga-PSMA and 18F-FDG PET/CT in Nonmetastatic Prostate Cancer Patients with an Early PSA Progression During Castration. Clin. Cancer Res. 2020, 26, 4551–4558. [Google Scholar] [CrossRef] [PubMed]
- Calais, J.; Ceci, F.; Eiber, M.; Hope, T.A.; Hofman, M.S.; Rischpler, C.; Bach-Gansmo, T.; Nanni, C.; Savir-Baruch, B.; Elashoff, D.; et al. (18)F-fluciclovine PET-CT and (68)Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: A prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019, 20, 1286–1294. [Google Scholar] [CrossRef]
- Meziou, S.; Ringuette Goulet, C.; Hovington, H.; Lefebvre, V.; Lavallée, É.; Bergeron, M.; Brisson, H.; Champagne, A.; Neveu, B.; Lacombe, D.; et al. GLUT1 expression in high-risk prostate cancer: Correlation with (18)F-FDG-PET/CT and clinical outcome. Prostate Cancer Prostatic Dis. 2020, 23, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Mena, E.; Lindenberg, M.L.; Turkbey, I.B.; Shih, J.H.; Harmon, S.A.; Lim, I.; Lin, F.; Adler, S.; Eclarinal, P.; McKinney, Y.L.; et al. (18)F-DCFPyL PET/CT Imaging in Patients with Biochemically Recurrent Prostate Cancer After Primary Local Therapy. J. Nucl. Med. 2020, 61, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Knorr, K.; Spohn, F.; Will, L.; Maurer, T.; Flechsig, P.; Neels, O.; Schiller, K.; Amaral, H.; Weber, W.A.; et al. Detection Efficacy of (18)F-PSMA-1007 PET/CT in 251 Patients with Biochemical Recurrence of Prostate Cancer after Radical Prostatectomy. J. Nucl. Med. 2019, 60, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Mannas, M.P.; Lee, T.; Pourghiasian, M.; Wilson, D.C.; Black, P.C. Incidentalomas of the prostate detected by 18-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography. Can. Urol. Assoc. J. 2020, 14, E180–E184. [Google Scholar] [CrossRef]
- Bakht, M.K.; Lovnicki, J.M.; Tubman, J.; Stringer, K.F.; Chiaramonte, J.; Reynolds, M.R.; Derecichei, I.; Ferraiuolo, R.M.; Fifield, B.A.; Lubanska, D.; et al. Differential Expression of Glucose Transporters and Hexokinases in Prostate Cancer with a Neuroendocrine Gene Signature: A Mechanistic Perspective for 18F-FDG Imaging of PSMA-Suppressed Tumors. J. Nucl. Med. 2020, 61, 904–910. [Google Scholar] [CrossRef]
- Sheikhbahaei, S.; Afshar-Oromieh, A.; Eiber, M.; Solnes, L.B.; Javadi, M.S.; Ross, A.E.; Pienta, K.J.; Allaf, M.E.; Haberkorn, U.; Pomper, M.G.; et al. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2117–2136. [Google Scholar] [CrossRef]



| Characteristics | |
|---|---|
| Age (years) | |
| Mean ± SD | 68.5 ± 6.6 |
| Median (IQR) | 69.0 (64.0–73.0) |
| PSA doubling time (months) | |
| Mean ± SD | 8.4 ± 6.1 |
| Median (IQR) | 5.8 (3.0–9.1) |
| PSA level (ng/mL) | |
| Mean ± SD | 3.6 ± 0.9 |
| Median (IQR) | 0.87 (0.50–2.31) |
| Prior therapy | |
| None | 114 |
| Radiotherapy or ADT | 31 |
| Two scans interval (days) | |
| Mean ± SD | 5.5 ± 0.4 |
| Median (IQR) | 6.0 (1.0–8.0) |
| Gleason score | |
| 6 | 7 |
| 7 | 72 |
| 8 | 38 |
| 9 | 28 |
| Variable and Intercept | FDG PET/CT | PSMA PET/CT | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Logistic Regression | Multivariate Logistic Regression | Univariate Logistic Regression | Multivariate Logistic Regression | |||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Age (years) | 0.592 (0.204–1.715) | 0.334 | 0.333 (0.084–1.318) | 0.117 | 1.540 (0.572–4.145) | 0.393 | 1.473 (0.493–4.405) | 0.488 |
| PSA doubling time (months) (≥6 vs. <6) | 0.765 (0.542–1.080) | 0.262 | 0.742 (0.462–1.192) | 0.421 | 0.732 (0.348–1.540) | 0.381 | 0.836 (0.542–1.289) | 0.524 |
| PSA level (high vs. low) | 7.469 (2.697–20.687) | <0.001 | 11.026 (3.214–37.824) | <0.001 | 4.858 (2.379–9.923) | <0.001 | 4.862 (2.338–10.110) | <0.001 |
| Radiotherapy (present vs. absent) | 2.162 (0.346–13.492) | 0.409 | 5.269 (0.341–81.521) | 0.234 | 2.829 (0.308–25.971) | 0.358 | 3.760 (0.362–39.036) | 0.267 |
| ADT therapy (present vs. absent) | 0.931 (0.341–2.540) | 0.889 | 0.460 (0.110–1.918) | 0.287 | 0.630 (0.269–1.478) | 0.288 | 0.611 (0.230–1.620) | 0.322 |
| Two scans interval (days) | 1.083 (0.502–2.339) | 0.839 | 0.749 (0.274–2.046) | 0.573 | 0.933 (0.477–1.828) | 0.841 | 0.762 (0.356–1.630) | 0.484 |
| Gleason score (high vs. low) | 10.588 (4.184–26.793) | <0.001 | 20.227 (5.741–71.267) | <0.001 | 1.358 (0.903–2.012) | 0.144 | 2.013 (0.732–5.538) | 0.175 |
| Variable and Intercept | Univariate Logistic Regression | Multivariate Logistic Regression | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age (years) | 2.199 (0.684–7.065) | 0.186 | 2.340 (0.700–7.823) | 0.168 |
| PSA doubling time (months) | 0.834 (0.634–1.097) | 0.325 | 0.865 (0.532–1.406) | 0.436 |
| (≥6 vs. <6) | ||||
| PSA level | 1.459 (0.735–2.896) | 0.280 | 1.703 (0.815–3.558) | 0.157 |
| (high vs. low) | ||||
| Radiotherapy | 1.163 (0.188–7.195) | 0.871 | 1.307 (0.179–9.524) | 0.792 |
| (present vs. absent) | ||||
| ADT therapy | 0.585 (0.228–1.500) | 0.264 | 0.883 (0.312–2.496) | 0.815 |
| (present vs. absent) | ||||
| Two scans interval (days) | 1.008 (0.508–2.003) | 0.981 | 1.135 (0.537–2.400) | 0.741 |
| Gleason score | 0.100 (0.023–0.439) | 0.002 | 0.088 (0.019–0.401) | 0.002 |
| (high vs. low) | ||||
| Concordant Positive Region | n | Leison SUVmax | L/B Liver | L/B Aorta | L/B Muscle | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FDG | PSMA | p | FDG | PSMA | p | FDG | PSMA | p | FDG | PSMA | p | ||
| Overall | 18 | 4.8 ± 2.8 | 18.5 ± 7.1 | <0.001 | 1.9 ± 0.6 | 3.6 ± 1.3 | 0.635 | 2.0 ± 0.8 | 6.8 ± 3.0 | <0.001 | 6.7 ± 2.1 | 28.0 ± 12.4 | <0.001 |
| Prostate bed (T) | 8 | 4.6 ± 1.3 | 19.3 ± 6.3 | 0.007 | 1.5 ± 0.4 | 3.6 ± 1.2 | 0.130 | 2.1 ± 0.6 | 8.8 ± 2.9 | 0.038 | 6.5 ± 1.9 | 27.6 ± 9.0 | 0.038 |
| Pelvic LN (N) | 6 | 2.6 ± 0.3 | 17.1 ± 8.1 | 0.002 | 0.9 ± 0.1 | 3.2 ± 1.5 | 0.180 | 1.2 ± 0.1 | 7.8 ± 3.7 | 0.022 | 3.7 ± 0.4 | 24.4 ± 11.5 | 0.040 |
| Extrapelvic LN (M1a) | 3 | 6.9 ± 4.4 | 6.7 ± 4.8 | 0.995 | 2.4 ± 1.6 | 1.4 ± 1.0 | 0.649 | 3.0 ± 2.0 | 3.1 ± 2.1 | 0.976 | 9.1 ± 6.1 | 9.0 ± 5.9 | 0.985 |
| Bone (M1b) | 7 | 5.2 ± 1.9 | 21.6 ± 11.6 | 0.009 | 1.8 ± 0.8 | 3.8 ± 2.3 | 0.059 | 1.9 ± 1.0 | 5.0 ± 3.4 | 0.036 | 7.4 ± 2.7 | 30.9 ± 16.6 | 0.003 |
| Other organs (M1c) | 0 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Any extrapelvic lesions (M1) | 8 | 5.5 ± 2.8 | 18.4 ± 12.2 | 0.007 | 1.9 ± 0.9 | 3.5 ± 2.3 | 0.079 | 2.5 ± 1.3 | 8.3 ± 5.6 | 0.014 | 7.9 ± 4.1 | 26.2 ± 17.4 | 0.007 |
| Patient | PSA | Gleason Score | FDG Scan | FDG Final Diagnosis | PSMA Scan | PSMA Final Diagnosis | Region Validated | Lesion Validated | Validation Method |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.29 | 7 | T0N0M0 | FN | T0N1M0 | TP | N1 | Iliac lymph nodes | Histopathology |
| 2 | 0.86 | 8 | T0N0M0 | FN | T0N1M0 | TP | N1 | Iliac lymph nodes | Histopathology |
| 3 | 0.78 | 8 | T0N0M0 | FN | TrN0M0 | TP | Tr | Prostate Fossa | PSA decreases after focal radiotherapy |
| 4 | 1.86 | 7 | T0N0M0 | FN | T0N0M1b | TP | M1b | Bone | PSA decreases after focal radiotherapy |
| 5 | 0.49 | 8 | T0N0M0 | FN | T0N0M1b | TP | M1b | Bone | PSA decreases after focal radiotherapy |
| 6 | 7.90 | 9 | T0N0M1b | TP | T0N0M1b | TP | M1b | Bone | PSA decreases after focal radiotherapy |
| 7 | 0.83 | 7 | TrN0M0 | TP | TrN0M0 | TP | Tr | Prostate Fossa | PSA decreases after focal radiotherapy |
| 8 | 1.30 | 9 | T0N0M1b | TP | TrN1M1b | TP | Tr | Prostate Fossa | PSA decreases after focal radiotherapy |
| 9 | 0.46 | 8 | T0N0M0 | FN | T0N1M0 | TP | N1 | Iliac lymph nodes | PSA decreases after focal radiotherapy |
| 10 | 1.59 | 8 | T0N1M0 | TP | T0N1M0 | TP | N1 | Iliac lymph nodes | PSA decreases after focal radiotherapy |
| 11 | 0.56 | 7 | T0N0M0 | FN | TrN1M1b | TP | Tr | Prostate Fossa | PSA decreases after focal radiotherapy |
| 12 | 0.64 | 8 | T0N0M1b | TP | T0N0M1b | TP | M1b | Bone | PSA decreases after focal radiotherapy |
| 13 | 3.80 | 7 | T0N0M0 | FN | TrN0M0 | TP | Tr | Prostate Fossa | PSA decreases after focal radiotherapy |
| 14 | 0.92 | 7 | TrN0M0 | TP | TrN0M0 | TP | Tr | Prostate Fossa | PSA decreases after focal radiotherapy |
| 15 | 12.72 | 8 | T0N0M0 | FN | TrN0M0 | TP | Tr | Prostate Fossa | PSA decreases after focal radiotherapy |
| 16 | 0.22 | 8 | T0N0M0 | FN | T0N1M0 | TP | N1 | Iliac lymph nodes | PSA decreases after focal radiotherapy |
| 17 | 1.55 | 9 | T0N1M0 | TP | T0N1M0 | TP | N1 | Iliac lymph nodes | PSA decreases after focal radiotherapy |
| 18 | 6.19 | 9 | T0N0M0 | FN | T0N1M0 | TP | N1 | Iliac lymph nodes | PSA decreases after focal radiotherapy |
| 19 | 0.66 | 9 | T0N0M0 | FN | TrN0M0 | TP | Tr | Prostate Fossa | PSA decreases after focal radiotherapy |
| 20 | 1.53 | 7 | TrN0M0 | TP | TrN0M0 | TP | Tr | Prostate Fossa | PSA decreases after focal radiotherapy |
| 21 | 0.81 | 9 | TrN0M0 | TP | TrN0M0 | TP | Tr | Prostate Fossa | PSA decreases after focal radiotherapy |
| 22 | 3.28 | 7 | T0N0M0 | FN | T0N0M1b | TP | M1b | Bone | PSA decreases after focal radiotherapy |
| 23 | 1.14 | 7 | TrN0M0 | TP | TrN0M0 | TP | Tr | Prostate Fossa | PSA decreases after focal radiotherapy |
| Patients | FDG | PSMA | |||||
|---|---|---|---|---|---|---|---|
| TP | FN | Sensitivity | TP | FN | Sensitivity | p | |
| Total patients (n = 23) | 10 | 13 | 43.5% | 23 | 0 | 100.0% | <0.001 |
| Low Gleason score (n = 17) | 6 | 11 | 35.3% | 17 | 0 | 100.0% | <0.001 |
| High Gleason score (n = 6) | 4 | 2 | 66.7% | 6 | 0 | 100.0% | 0.455 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Chen, R.; Yu, X.; Liu, J.; Wang, Y. 18F-FDG PET Is Not Inferior to 68Ga-PSMA PET for Detecting Biochemical Recurrent Prostate Cancer with a High Gleason Score: A Head-to-Head Comparison Study. Diagnostics 2024, 14, 7. https://doi.org/10.3390/diagnostics14010007
Xu L, Chen R, Yu X, Liu J, Wang Y. 18F-FDG PET Is Not Inferior to 68Ga-PSMA PET for Detecting Biochemical Recurrent Prostate Cancer with a High Gleason Score: A Head-to-Head Comparison Study. Diagnostics. 2024; 14(1):7. https://doi.org/10.3390/diagnostics14010007
Chicago/Turabian StyleXu, Lian, Ruohua Chen, Xiaofeng Yu, Jianjun Liu, and Yuetao Wang. 2024. "18F-FDG PET Is Not Inferior to 68Ga-PSMA PET for Detecting Biochemical Recurrent Prostate Cancer with a High Gleason Score: A Head-to-Head Comparison Study" Diagnostics 14, no. 1: 7. https://doi.org/10.3390/diagnostics14010007




