The Predictors of Early Treatment Effectiveness of Intravitreal Bevacizumab Application in Patients with Diabetic Macular Edema
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, T.E.; Wong, T.Y. Diabetic retinopathy: Looking forward to 2030. Front. Endocrinol. 2023, 13, 1077669. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Jiao, B.; Han, Q. Effect of intravitreal anti-vascular growth factor agents with or without macular photocoagulation on diabetic macular edema: A systematic review and meta-analysis. Diabetes Ther. 2019, 10, 1283–1296. [Google Scholar] [CrossRef] [PubMed]
- Valle, M.S.; Russo, C.; Malaguarnera, L. Protective role of vitamin D against oxidative stress in diabetic retinopathy. Diabetes/Metab. Res. Rev. 2021, 37, e3447. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lo, A.C.Y. Diabetic retinopathy: Pathophysiology and treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.J.; Zhang, W.F. The relationship of blood cell-associated inflammatory indices and diabetic retinopathy: A meta-analysis and systematic review. Int. J. Ophthalmol. 2019, 12, 312. [Google Scholar] [PubMed]
- Wang, J.R.; Chen, Z.; Yang, K.; Yang, H.J.; Tao, W.Y.; Li, Y.P.; Jiang, Z.J.; Bai, C.F.; Yin, Y.C.; Duan, J.M.; et al. Association between neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and diabetic retinopathy among diabetic patients without a related family history. Diabetol. Metab. Syndr. 2020, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Tecilazich, F.; Formenti, A.M.; Giustina, A. Role of vitamin D in diabetic retinopathy: Pathophysiological and clinical aspects. Rev. Endocr. Metab. Disord. 2021, 22, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, J.; Hu, Y.; Ding, Y.; Zhu, J.; Zhuang, C. Relationship between serum apolipoproteins levels and retinopathy risk in subjects with type 2 diabetes mellitus. Acta Diabetol. 2018, 55, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Sędziak-Marcinek, B.; Teper, S.; Chełmecka, E.; Wylęgała, A.; Marcinek, M.; Bas, M.; Wylęgała, E. Diabetic macular edema treatment with bevacizumab does not depend on the retinal nonperfusion presence. J. Diabetes Res. 2021, 2021, 6620122. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Zhang, C.; Zhang, J.; Gu, L.; Luo, D.; Qiu, Q. Diabetic macular edema: Current understanding, molecular mechanisms and therapeutic implications. Cells 2022, 11, 3362. [Google Scholar] [CrossRef]
- Jhaveri, C.D.; Glassman, A.R.; Ferris, F.L., III; Liu, D.; Maguire, M.G.; Allen, J.B.; Baker, C.W.; Browning, D.; Cunningham, M.A.; Friedman, S.M.; et al. DRCR Retina Network. Aflibercept monotherapy or bevacizumab first for diabetic macular edema. N. Engl. J. Med. 2022, 387, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Bahr, T.A.; Bakri, S.J. Update on the management of diabetic retinopathy: Anti-VEGF agents for the prevention of complications and progression of nonproliferative and proliferative retinopathy. Life 2023, 13, 1098. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, C.P.; Ferris, F.L., III; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T.; et al. Global diabetic retinopathy project group. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Song, S.; Zhang, Y.; Jin, K.; Ye, J. OCT-based biomarkers are associated with systemic inflammation in patients with treatment-naïve diabetic macular edema. Ophthalmol. Ther. 2022, 11, 2153–2167. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, F.; Morescalchi, F.; Cancarini, A.; Russo, A.; Rezzola, S.; Costagliola, C. Diabetic retinopathy, a vascular and inflammatory disease: Therapeutic implications. Diabetes Metab. 2019, 45, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Wong, T.Y.; Sabanayagam, C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015, 2, 17. [Google Scholar] [CrossRef]
- Yalinbas Yeter, D.; Eroglu, S.; Sariakcali, B.; Bozali, E.; Vural Ozec, A.; Erdogan, H. The usefulness of monocyte-to-high density lipoprotein and neutrophil-to-lymphocyte ratio in diabetic macular edema prediction and early anti-VEGF treatment response. Ocul. Immunol. Inflamm. 2022, 30, 901–906. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, Y.; Xu, X.; Yang, B.; Mei, F.; Zhou, Q.; Yan, L.; Wang, J.; Wu, X. Pretreatment neutrophil-to-lymphocyte ratio predicts prognosis in patients with diabetic macular edema treated with ranibizumab. BMC Ophthalmol. 2019, 19, 194. [Google Scholar] [CrossRef]
- Karimi, S.; Arabi, A.; Shahraki, T.; Safi, S. Association of white blood cell counts, leukocyte ratios, and serum uric acid with clinical outcome of intravitreal bevacizumab in diabetic macular edema. Korean J. Ophthalmol. 2022, 36, 244–252. [Google Scholar] [CrossRef]
- Bambace, N.M.; Levis, J.E.; Holmes, C.E. The effect of P2Y-mediated platelet activation on the release of VEGF and endostatin from platelets. Platelets 2010, 21, 85–93. [Google Scholar] [CrossRef]
- Elbeyli, A.; Kurtul, B.E.; Ozcan, S.C.; Ozarslan Ozcan, D. The diagnostic value of systemic immune-inflammation index in diabetic macular oedema. Clin. Exp. Optom. 2022, 105, 831–835. [Google Scholar] [CrossRef]
- Özata Gündoğdu, K.; Doğan, E.; Çelik, E.; Alagöz, G. Serum inflammatory marker levels in serous macular detachment secondary to diabetic macular edema. Eur. J. Ophthalmol. 2022, 32, 3637–3643. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Chen, M.; Feng, Q.; Wan, H.; Wang, J.; Yang, F.; Cao, H. The Platelet-to-Lymphocyte Ratio Predicts Diabetic Retinopathy in Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 3617–3626. [Google Scholar] [CrossRef] [PubMed]
- Dascalu, A.M.; Serban, D.; Tanasescu, D.; Vancea, G.; Cristea, B.M.; Stana, D.; Nicolae, V.A.; Serboiu, C.; Tribus, L.C.; Tudor, C.; et al. The Value of White Cell Inflammatory Biomarkers as Potential Predictors for Diabetic Retinopathy in Type 2 Diabetes Mellitus (T2DM). Biomedicines 2023, 11, 2106. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, X.; Fu, M.; Ke, X. Optical Coherence Tomography-Based Grading of Diabetic Macular Edema Is Associated with Systemic Inflammatory Indices and Imaging Biomarkers. Ophthalmic Res. 2024, 67, 96–106. [Google Scholar]
- Tall, A.R. Plasma high density lipoproteins: Therapeutic targeting and links to atherogenic inflammation. Atherosclerosis 2018, 276, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.; Jalali, J.A.; Johnston, T.P.; Koulen, P. Emerging roles of dyslipidemia and hyperglycemia in diabetic retinopathy: Molecular mechanisms and clinical perspectives. Front. Endocrinol. 2021, 12, 620045. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.; Ma, J.; Su, X.; Zhong, Y. Emerging insights into the relationship between hyperlipidemia and the risk of diabetic retinopathy. Lipids Health Dis. 2020, 19, 241. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Ferri, N.; Macchi, C.; Corsini, A.; Sirtori, C.R. Lipid lowering drugs and inflammatory changes: An impact on cardiovascular outcomes? Ann. Med. 2018, 50, 461–484. [Google Scholar] [CrossRef]
- Ezhilvendhan, K.; Sathiyamoorthy, A.; Prakash, B.J.; Bhava, B.S.; Shenoy, A. Association of dyslipidemia with diabetic retinopathy in type 2 diabetes mellitus patients: A hospital-based study. J. Pharm. Bioallied Sci. 2021, 13 (Suppl. S2), S1062–S1067. [Google Scholar]
- Tomić, M.; Vrabec, R.; Bulum, T.; Ljubić, S. HDL cholesterol is a protective predictor in the development and progression of retinopathy in type 1 diabetes: A 15-year follow-up study. Diabetes Res. Clin. Pract. 2022, 186, 109814. [Google Scholar] [CrossRef]
- Gitay, M.N.; Sohail, A.; Arzoo, Y.; Shakir, M.A. Changes in serum lipids with the onset and progression of diabetic retinopathy in type-II diabetes mellitus. Pak. J. Med. Sci. 2023, 39, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Soedarman, S.; Kurnia, K.H.; Prasetya, A.D.B.; Sasongko, M.B. Cholesterols, apolipoproteins, and their associations with the presence and severity of diabetic retinopathy: A systematic review. Vision 2022, 6, 77. [Google Scholar] [CrossRef]
- Storti, F.; Raphael, G.; Griesser, V.; Klee, K.; Drawnel, F.; Willburger, C.; Scholz, R.; Langmann, T.; von Eckardstein, A.; Fingerle, J.; et al. Regulated efflux of photoreceptor outer segment-derived cholesterol by human RPE cells. Exp. Eye Res. 2017, 165, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Sasongko, M.B.; Wong, T.Y.; Nguyen, T.T.; Kawasaki, R.; Jenkins, A.; Shaw, J.; Wang, J.J. Serum apolipoprotein AI and B are stronger biomarkers of diabetic retinopathy than traditional lipids. Diabetes Care 2011, 34, 474–479. [Google Scholar] [CrossRef]
- Ankit, B.S.; Mathur, G.; Agrawal, R.P.; Mathur, K.C. Stronger relationship of serum apolipoprotein A-1 and B with diabetic retinopathy than traditional lipids. Indian J. Endocrinol. Metab. 2017, 21, 102–105. [Google Scholar]
- Crosby Nwaobi, R.; Chatziralli, I.; Sergentanis, T.; Dew, T.; Forbes, A.; Sivaprasad, S. Cross talk between lipid metabolism and inflammatory markers in patients with diabetic retinopathy. J. Diabetes Res. 2015, 2015, 191382. [Google Scholar] [CrossRef] [PubMed]
- Walldius, G.; Jungner, I. The apoB/apoA-I ratio: A strong, new risk factor for cardiovascular disease and a target for lipid-lowering therapy—A review of the evidence. J. Intern. Med. 2006, 259, 493–519. [Google Scholar] [CrossRef]
- Moosaie, F.; Davatgari, R.M.; Firouzabadi, F.D.; Esteghamati, S.; Deravi, N.; Meysamie, A.; Khaloo, P.; Nakhjavani, M.; Esteghamati, A. Lipoprotein(a) and apolipoproteins as predictors for diabetic retinopathy and its severity in adults with type 2 diabetes: A case-cohort study. Can. J. Diabetes 2020, 44, 414–421. [Google Scholar] [CrossRef]
- Pludowski, P.; Grant, W.B.; Konstantynowicz, J.; Holick, M.F. Editorial: Classic and pleiotropic actions of vitamin D. Front. Endocrinol. 2019, 10, 341. [Google Scholar] [CrossRef]
- Palomer, X.; González Clemente, J.M.; Blanco Vaca, F.; Mauricio, D. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes. Metab. 2008, 10, 185–197. [Google Scholar] [CrossRef]
- Alcubierre, N.; Valls, J.; Rubinat, E.; Cao, G.; Esquerda, A.; Traveset, A.; Granado-Casas, M.; Jurjo, C.; Mauricio, D. Vitamin D deficiency is associated with the presence and severity of diabetic retinopathy in type 2 diabetes mellitus. J. Diabetes Res. 2015, 2015, 374178. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.A.; Gao, F.; Qin, L.L. The association between vitamin D deficiency and diabetic retinopathy in type 2 diabetes: A meta-analysis of observational studies. Nutrients 2017, 9, 307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Upala, S.; Sanguankeo, A. Relationship between vitamin D deficiency and diabetic retinopathy: A meta-analysis. Can. J. Ophthalmol. 2017, 52, 219–224. [Google Scholar] [CrossRef]
- Ashinne, B.; Rajalakshmi, R.; Anjana, R.M.; Narayan, K.V.; Jayashri, R.; Mohan, V.; Hendrick, A.M. Association of serum vitamin D levels and diabetic retinopathy in Asian Indians with type 2 diabetes. Diabetes Res. Clin. Pract. 2018, 139, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Navaei, S.; Nazemi, S.; Emamian, M.H.; Hashemi, H.; Fotouhi, A. Vitamin D deficiency and diabetic retinopathy risk. J. Français d’Ophtalmol. 2023, 46, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Nadri, G.; Saxena, S.; Mahdi, A.A.; Kaur, A.; Ahmad, M.K.; Garg, P.; Meyer, C.H. Serum vitamin D is a biomolecular biomarker for proliferative diabetic retinopathy. Int. J. Retin. Vitr. 2019, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Albert, D.M.; Scheef, E.A.; Wang, S.; Mehraein, F.; Darjatmoko, S.R.; Sorenson, C.M.; Sheibani, N. Calcitriol is a potent inhibitor of retinal neovascularization. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Ben Shoshan, M.; Amir, S.; Dang, D.T.; Dang, L.H.; Weisman, Y.; Mabjeesh, N.J. 1alpha,25-dihydroxyvitamin D3 (Calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol. Cancer Ther. 2007, 6, 1433–1439. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.; Wang, Y. Intravitreous vascular endothelial growth factor and hypoxia-inducible factor 1a in patients with proliferative diabetic retinopathy. Am. J. Ophthalmol. 2009, 148, 883–889. [Google Scholar] [CrossRef]
| Parameters | Total Population (n = 78) | Responders (n = 40) | Non-Responders (n = 38) | p-Value |
|---|---|---|---|---|
| Age (years) | 67.2 ± 8.6 | 67.4 ± 7.4 | 67.0 ± 9.8 | 0.848 * |
| Female sex, n (%) | 36 (46) | 14 (35) | 22 (58) | 0.044 † |
| Smoking, n (%) | 20 (26) | 12 (30) | 8 (21) | 0.369 † |
| Duration of DM (years) | 17.4 ± 10.1 | 17.6 ± 10.1 | 17.1 ± 10.2 | 0.847 * |
| Arterial hypertension, n (%) | 59 (75.6) | 25 (62.5) | 34 (89.5) | 0.006 † |
| Chronic renal failure, n (%) | 18 (23.1) | 9 (22.5) | 9 (23.7) | 0.902 † |
| eGFR (mL/min/1.73 m2) | 67.9 ± 23.4 | 67.7 ± 23.3 | 68.1 ± 23.7 | 0.941 * |
| BMI (kg/m2) | 26.9 (25.5–29.4) | 26.3 (25.0–29.2) | 27.6 (25.9–29.8) | 0.143 ‡ |
| HbA1c (%) | 7.5 (6.6–8.4) | 7.6 (6.6–8.6) | 7.4 (6.4–8.0) | 0.345 ‡ |
| HDL-C (mmol/L) | 1.3 (1.1–1.6) | 1.3 (1.0–1.5) | 1.4 (1.1–1.6) | 0.609 ‡ |
| LDL-C (mmol/L) | 2.4 (1.6–3.1) | 2.3 (1.5–2.9) | 2.5 (1.8–3.2) | 0.522 ‡ |
| ApoA-I (g/L) | 1.49 ± 0.28 | 1.47 ± 0.27 | 1.50 ± 0.29 | 0.609 * |
| ApoB (g/L) | 0.93 ± 0.25 | 0.93 ± 0.25 | 0.94 ± 0.25 | 0.769 * |
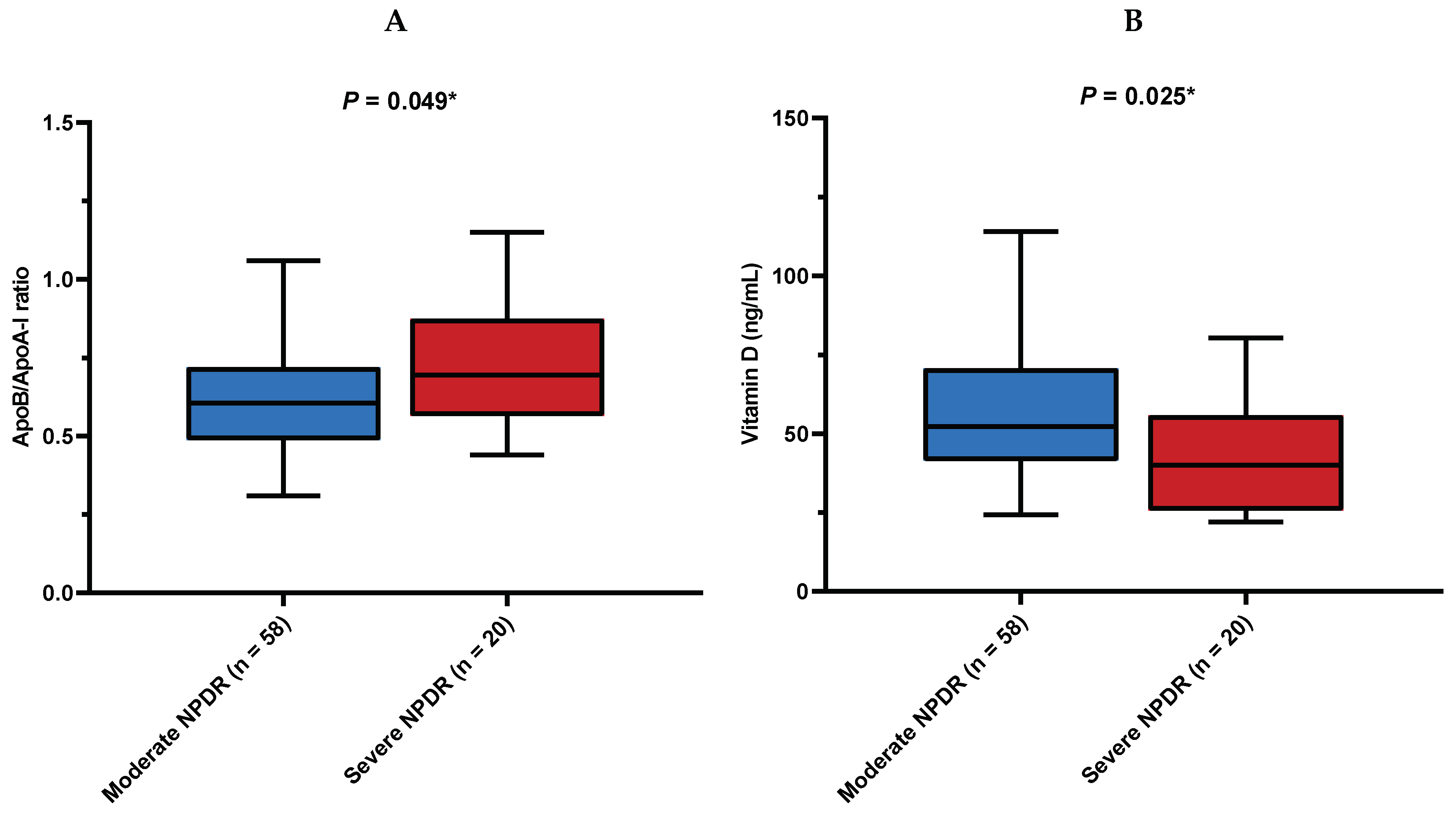
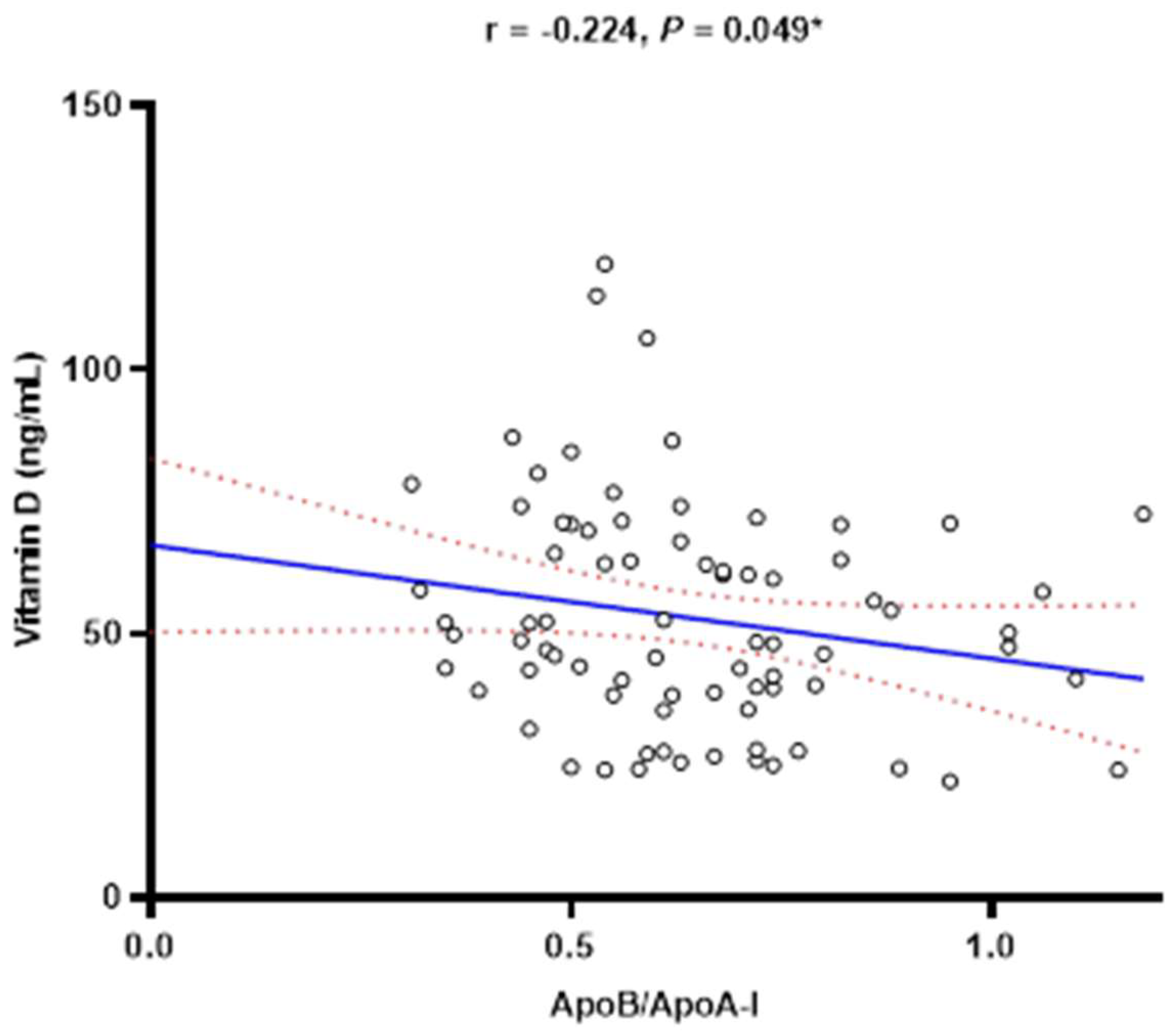
| ApoB/ApoA-I | 0.62 (0.50–0.74) | 0.62 (0.51–0.72) | 0.62 (0.48–0.74) | 1.000 ‡ |
| Neutrophils (×109/L) | 4.6 ± 1.2 | 4.2 ± 0.9 | 4.9 ± 1.3 | 0.006 * |
| Lymphocytes (×109/L) | 2.1 ± 0.6 | 2.2 ± 0.5 | 1.9 ± 0.6 | 0.031 * |
| Monocytes (×109/L) | 0.49 ± 0.14 | 0.48 ± 0.11 | 0.51 ± 0.17 | 0.466 * |
| Platelets (×109/L) | 229 (186–259) | 226 (175–267) | 235 (193–258) | 0.628 ‡ |
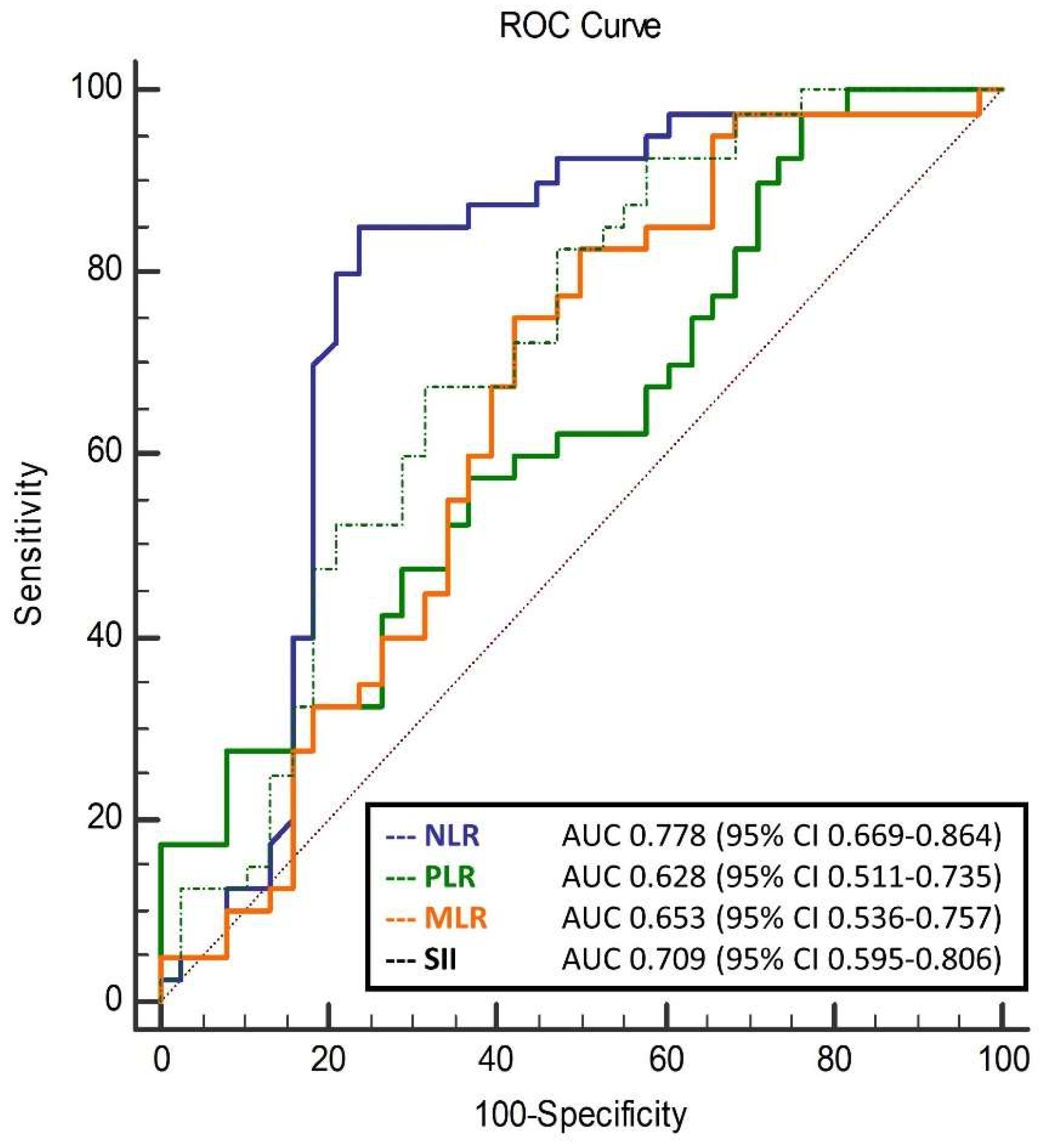
| NLR | 1.96 ± 0.67 | 2.03 ± 0.70 | 2.80 ± 1.08 | <0.001 * |
| MLR | 0.22 ± 0.06 | 0.23 ± 0.06 | 0.28 ± 0.10 | 0.011 * |
| PLR | 103.6 ± 37.1 | 107.4 ± 37.3 | 135.8 ± 58.0 | 0.013 * |
| SII | 423.9 ± 163.9 | 445.3 ± 166.3 | 675.3 ± 334.0 | <0.001 * |
| Monocyte/ApoA-I ratio | 0.35 (0.26–0.42) | 0.33 (0.26–0.41) | 0.34 (0.26–0.42) | 0.873 ‡ |
| HDL/ApoB ratio | 1.4 (1.1–1.9) | 1.4 (1.1–1.9) | 1.4 (1.1–1.9) | 0.893 ‡ |
| Vitamin D (nmol/L) | 49.3 (38.8–67.5) | 46.4 (27.9–63.4) | 52.1 (41.4–70.6) | 0.225 ‡ |
| Therapy, n (%) | ||||
| Statins | 49 (62.8) | 26 (65.0) | 23 (60.5) | 0.685 † |
| Oral antidiabetics | 65 (83.3) | 32 (80.0) | 33 (86.8) | 0.421 † |
| Insulin | 41 (52.6) | 20 (50.0) | 21 (55.3) | 0.644 † |
| GLP-1RA | 9 (11.5) | 4 (10.0) | 5 (13.2) | 0.665 † |
| Parameters | Total Population (n = 78) | Responders (n = 40) | Non-Responders (n = 38) | p-Value |
|---|---|---|---|---|
| Baseline BCVA (logMAR) | 0.40 (0.30–1.00) | 0.56 (0.35–1.30) | 0.35 (0.22–0.70) | 0.020 ‡ |
| Final BCVA (logMAR) | 0.35 (0.20–0.70) | 0.35 (0.18–0.65) | 0.33 (0.22–0.70) | 0.409 ‡ |
| ΔBCVA (logMAR) | −0.09 (−0.18 to 0.00) | −0.18 (−0.48 to −0.10) | 0.00 (−0.06 to 0.00) | <0.001 ‡ |
| Baseline CMT (μm) | 421 (352–481) | 460 (370–504) | 409 (350–448) | 0.032 ‡ |
| Final CMT (μm) | 372 (303–430) | 344 (291–391) | 404 (334–450) | 0.006 ‡ |
| ΔCMT (μm) | −44 (−88 to −14) | −88 (−137 to −65) | −12 (−23 to 7) | <0.001 ‡ |
| Pseudophakia, n (%) | 18 (23.1) | 8 (20.0) | 10 (26.3) | 0.511 ‡ |
| Type of macular edema, n (%) | ||||
| CME | 41 (52.6) | 23 (57.5) | 18 (47.4) | 0.549 † |
| SLDRT | 34 (43.6) | 15 (37.5) | 19 (50.0) | |
| SRF | 3 (3.8) | 2 (5.0) | 1 (2.6) | |
| Number of previous anti-VEGF injections | 3 (0–5) | 2 (0–4) | 3 (0–6) | 0.038 ‡ |
| Diabetic retinopathy severity, n (%) | ||||
| Moderate NPDR | 58 (74.4) | 33 (82.5) | 25 (65.8) | 0.093 † |
| Severe NPDR | 20 (25.6) | 7 (17.5) | 13 (34.2) |
| Variable | Univariable Model | Multivariable Model | ||
|---|---|---|---|---|
| r-Correlation Coefficient | p-Value | β ± SE | p-Value | |
| NLR | 0.48 | <0.001 | 7.58 ± 1.59 | <0.001 |
| MLR | 0.49 | <0.001 | 98.4 ± 17.9 | <0.001 |
| PLR | 0.35 | 0.002 | 0.11 ± 0.03 | 0.002 |
| SII | 0.47 | <0.001 | 0.03 ± 0.01 | <0.001 |
| Variable | Multivariable Model | |
|---|---|---|
| OR (95% CI) | p-Value | |
| NLR | 0.297 (0.138–0.642) | 0.002 |
| MLR | 0.001 (0.001–0.240) | 0.017 |
| PLR | 0.987 (0.977–0.998) | 0.018 |
| SII | 0.996 (0.994–0.999) | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katić, K.; Katić, J.; Kumrić, M.; Božić, J.; Tandara, L.; Šupe Domić, D.; Bućan, K. The Predictors of Early Treatment Effectiveness of Intravitreal Bevacizumab Application in Patients with Diabetic Macular Edema. Diagnostics 2024, 14, 992. https://doi.org/10.3390/diagnostics14100992
Katić K, Katić J, Kumrić M, Božić J, Tandara L, Šupe Domić D, Bućan K. The Predictors of Early Treatment Effectiveness of Intravitreal Bevacizumab Application in Patients with Diabetic Macular Edema. Diagnostics. 2024; 14(10):992. https://doi.org/10.3390/diagnostics14100992
Chicago/Turabian StyleKatić, Karla, Josip Katić, Marko Kumrić, Joško Božić, Leida Tandara, Daniela Šupe Domić, and Kajo Bućan. 2024. "The Predictors of Early Treatment Effectiveness of Intravitreal Bevacizumab Application in Patients with Diabetic Macular Edema" Diagnostics 14, no. 10: 992. https://doi.org/10.3390/diagnostics14100992
APA StyleKatić, K., Katić, J., Kumrić, M., Božić, J., Tandara, L., Šupe Domić, D., & Bućan, K. (2024). The Predictors of Early Treatment Effectiveness of Intravitreal Bevacizumab Application in Patients with Diabetic Macular Edema. Diagnostics, 14(10), 992. https://doi.org/10.3390/diagnostics14100992







