Reproducibility of Portable OCT and Comparison with Conventional OCT
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Study Population
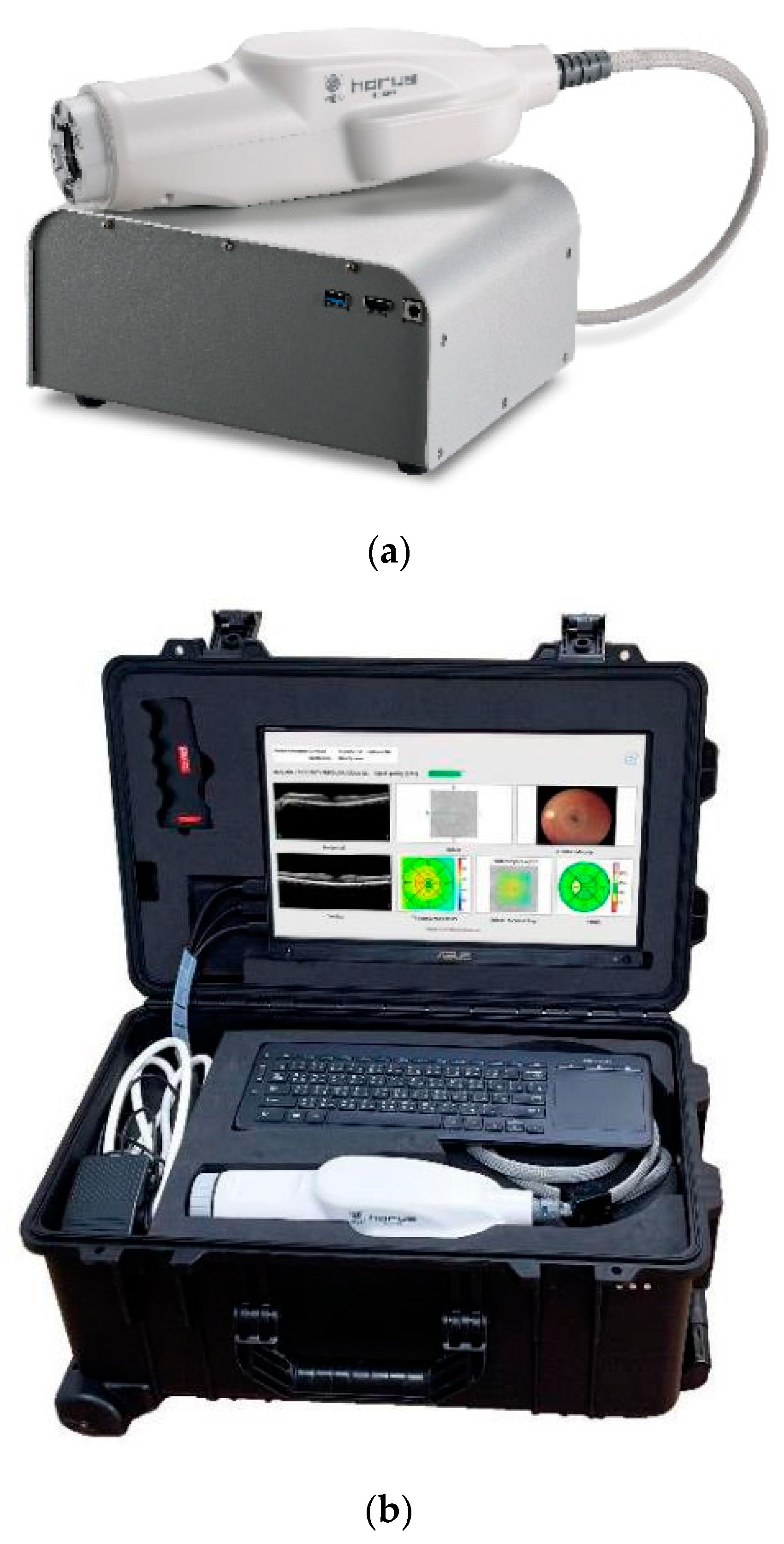
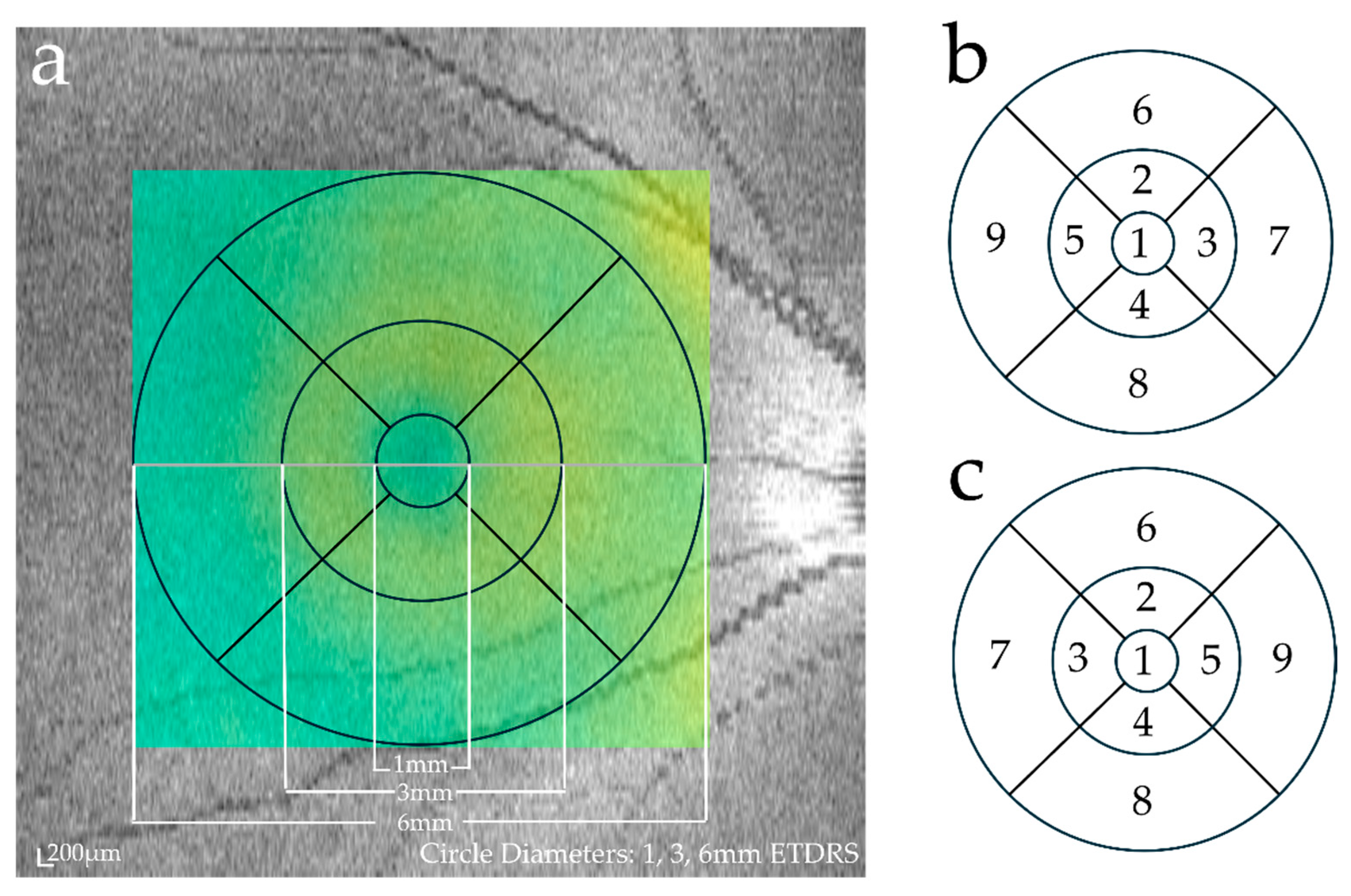
2.2. Image Acquisition and Evaluation
2.3. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adhi, M.; Duker, J. Optical coherence tomography—Current and future applications. Curr. Opin. Ophthalmol. 2013, 24, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Iesato, Y.; Toriyama, Y.; Imai, A.; Murata, T. Detection of fovea-threatening diabetic macular edema by optical coherence tomography to maintain good vision by prophylactic treatment. Ophthalmic Res. 2014, 52, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Iesato, Y.; Imai, A.; Hirano, T.; Toriyama, Y.; Murata, T. Effect of leaking capillaries and microaneurysms in the perifoveal capillary network on resolution of macular edema by anti-vascular endothelial growth factor treatment. Jpn. J. Ophthalmol. 2016, 60, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Kashani, A.H.; Chen, C.L.; Gahm, J.K.; Zheng, F.; Richter, G.M.; Rosenfeld, P.J.; Shi, Y.; Wang, R.K. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Prog. Retin. Eye Res. 2017, 60, 66–100. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Klimscha, S.; Waldstein, S.M.; Bogunović, H. A view of the current and future role of optical coherence tomography in the management of age-related macular degeneration. Eye 2017, 31, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Toriyama, Y.; Iesato, Y.; Imai, A.; Hirabayashi, K.; Nagaoka, T.; Takamura, Y.; Sugimoto, M.; Murata, T. Effect of leaking perifoveal microaneurysms on resolution of diabetic macular edema treated by combination therapy using anti-vascular endothelial growth factor and short pulse focal/grid laser photocoagulation. Jpn. J. Ophthalmol. 2017, 61, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Puliafito, C.A.; Hee, M.R.; Lin, C.P.; Reichel, E.; Schuman, J.S.; Duker, J.S.; Izatt, J.A.; Swanson, E.A.; Fujimoto, J.G. Imaging of macular diseases with optical coherence tomography. Ophthalmology 1995, 102, 217–229. [Google Scholar] [CrossRef]
- Massin, P.; Vicaut, E.; Haouchine, B.; Erginay, A.; Paques, M.; Gaudric, A. Reproducibility of retinal mapping using optical coherence tomography. Arch. Ophthalmol. 2001, 119, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Hee, M.R.; Puliafito, C.A.; Wong, C.; Duker, J.S.; Reichel, E.; Rutledge, B.; Schuman, J.S.; Swanson, E.A.; Fujimoto, J.G. Quantitative assessment of macular edema with optical coherence tomography. Arch. Ophthalmol. 1995, 113, 1019–1029. [Google Scholar] [CrossRef]
- Cho, C.; Liu, M.; Channa, R.; Zhang, Y.; Quigley, H.; Jefferys, J.; Scott, A. Detection of age-related macular degeneration by portable optical coherence tomography operated by nonexpert personnel. Potential use for screenings. J. Vitr. Dis. 2019, 3, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Muni, R.; Kohly, R.; Sohn, E.; Lee, T. Hand-held spectral domain optical coherence tomography finding in shaken-baby syndrome. Retina 2010, 30, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, Y.; Cheng, Y.; Qu, Y. Assessment of the effect of age on macular layer thickness in a healthy Chinese cohort using spectral-domain optical coherence tomography. BMC Ophthalmol. 2018, 18, 169. [Google Scholar] [CrossRef] [PubMed]
- Wykoff, C.C.; Garweg, J.G.; Regillo, C.; Souied, E.; Wolf, S.; Dhoot, D.S.; Agostini, H.T.; Chang, A.; Laude, A.; Wachtlin, J.; et al. Kestrel and KITE Phase 3 studies: 100-week results with brolucizumab in patients with diabetic macular edema. Am. J. Ophthalmol. 2024, 260, 70–83. [Google Scholar] [CrossRef]
- Wong, T.Y.; Haskova, Z.; Asik, K.; Baumal, C.R.; Csaky, K.G.; Eter, N.; Ives, J.A.; Jaffe, G.J.; Korobelnik, J.F.; Lin, H.; et al. Faricimab treat-and-extend for diabetic macular edema: Two-year results from the randomized Phase 3 YOSEMITE and RHINE trials. Ophthalmology 2023. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Clark, W.L.; Boyer, D.S.; Heier, J.S.; Brown, D.M.; Vitti, R.; Kazmi, H.; Berliner, A.J.; Erickson, K.; Chu, K.W.; et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: The 24-week results of the VIBRANT study. Ophthalmology 2015, 122, 538–544. [Google Scholar] [CrossRef]
- Heier, J.S.; Khanani, A.M.; Quezada Ruiz, C.; Basu, K.; Ferrone, P.J.; Brittain, C.; Figueroa, M.S.; Lin, H.; Holz, F.G.; Patel, V.; et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and Lucerne): Two randomized, double-masked, phase 3, non-inferiority trials. Lancet 2022, 399, 729–740. [Google Scholar] [CrossRef]
- Dugel, P.U.; Koh, A.; Ogura, Y.; Jaffe, G.J.; Schmidt-Erfurth, U.; Brown, D.M.; Gomes, A.V.; Warburton, J.; Weichselberger, A.; Holz, F.G.; et al. HAWK and HARRIER: Phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology 2020, 127, 72–84. [Google Scholar] [CrossRef]
- Tan, C.S.; Chan, J.C.; Cheong, K.X.; Ngo, W.K.; Sadda, S.R. Comparison of retinal thicknesses measured using swept-source and spectral-domain optical coherence tomography devices. Ophthalmic Surg. Lasers Imaging Retin. 2015, 46, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Wolf-Schnurrbusch, U.E.; Ceklic, L.; Brinkmann, C.K.; Iliev, M.E.; Frey, M.; Rothenbuehler, S.P.; Enzmann, V.; Wolf, S. Macular thickness measurements in healthy eyes using six different optical coherence tomography instruments. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3432–3437. [Google Scholar] [CrossRef]
- Han, I.C.; Jaffe, G.J. Comparison of spectral- and time-domain optical coherence tomography for retinal thickness measurements in healthy and diseased eyes. Am. J. Ophthalmol. 2009, 147, 847–858.e1. [Google Scholar] [CrossRef]
- Suzuma, K.; Yamada, Y.; Liu, M.; Tsuiki, E.; Fujikawa, A.; Kitaoka, T. Comparing central retinal thickness in diabetic macular edema measured by two different spectral-domain optical coherence tomography devices. Jpn. J. Ophthalmol. 2011, 55, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Sayanagi, K.; Sharma, S.; Kaiser, P.K. Comparison of retinal thickness measurements between three-dimensional and radial scans on spectral-domain optical coherence tomography. Am. J. Ophthalmol. 2009, 148, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Giani, A.; Cigada, M.; Choudhry, N.; Deiro, A.P.; Oldani, M.; Pellegrini, M.; Invernizzi, A.; Duca, P.; Miller, J.W.; Staurenghi, G. Reproducibility of retinal thickness measurements on normal and pathologic eyes by different optical coherence tomography instruments. Am. J. Ophthalmol. 2010, 150, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Waldstein, S.M.; Gerendas, B.S.; Montuoro, A.; Simader, C.; Schmidt-Erfurth, U. Quantitative comparison of macular segmentation performance using identical retinal regions across multiple spectral-domain optical coherence tomography instruments. Br. J. Ophthalmol. 2015, 99, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudinezhad, G.; Mohammadzadeh, V.; Amini, N.; Toriz, V.; Pourhomayoun, M.; Heydarzadeh, S.; Mylavarapu, A.; Morales, E.; Caprioli, J.; Nouri-Mahdavi, K. Local macular thickness relationships between 2 OCT devices. Ophthalmol. Glaucoma 2021, 4, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, A.; Edington, M.; Morjaria, R.; Chong, N.V. Comparing macular thickness measurements in patients with diabetic macular edema with the Optos spectral OCT/SLO and Heidelberg Spectralis HRA + OCT. Vision 2016, 1, 2. [Google Scholar] [CrossRef]
- Kakinoki, M.; Miyake, T.; Sawada, O.; Sawada, T.; Kawamura, H.; Ohji, M. Comparison of macular thickness in diabetic macular edema using spectral-domain optical coherence tomography and time-domain optical coherence tomography. J. Ophthalmol. 2012, 2012, 959721. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Stone, L.G.; Devenport, A.; Stratton, I.M.; Talks, J.S. Macula service evaluation and assessing priorities for anti-VEGF treatment in the light of COVID-19. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 2639–2645. [Google Scholar] [CrossRef]
- Chiku, Y.; Hirano, T.; Hoshiyama, K.; Iesato, Y.; Murata, T. Impact of local COVID-19 alert levels on rhegmatogenous retinal detachment. Jpn. J. Ophthalmol. 2023, 67, 255–263. [Google Scholar] [CrossRef]
- Tsuboi, K.; You, Q.; Guo, Y.; Wang, J.; Flaxel, C.; Bailey, S.; Huang, D.; Jia, Y.; Hwang, T. Automated macular fluid volume as a treatment indicator for diabetic macular edema. J. Vitreoretin. Dis. 2023, 29, 226–231. [Google Scholar] [CrossRef] [PubMed]
| Cirrus | ACT100 | |
|---|---|---|
| Manufacturer | Carl Zeiss | MiiS |
| Principles of OCT | Spectral-domein OCT | Spectral-domein OCT |
| Scan speed (A-scan/s) | 27,000–68,000 | 80,000 |
| Light source wavelength (nm) | 840 | 840 |
| Lateral resolution (µm) | 15 | 20 |
| Longitudinal resolution (µm) | 5 | 10 |
| Mean Retinal Thickness (μm) | Pearson’s Correlation Coefficient | |||||
|---|---|---|---|---|---|---|
| Cirrus | ACT100 | Difference | p | Pearson’s Correlation Coefficient | p | |
| Fovea | 257.9 | 245.8 | 12.1 | <0.0001 | 0.9295 | <0.0001 |
| Pericentral superior | 330.1 | 289.5 | 40.6 | <0.0001 | 0.8271 | <0.0001 |
| Pericentral temporal | 316.8 | 284.2 | 32.5 | <0.0001 | 0.6086 | 0.0001 |
| Pericentral nasal | 322.9 | 282.6 | 40.4 | <0.0001 | 0.8350 | <0.0001 |
| Pericentral inferior | 330.5 | 296.5 | 34.0 | <0.0001 | 0.8175 | <0.0001 |
| Peripheral superior | 286.0 | 259.5 | 26.6 | <0.0001 | 0.3794 | 0.0246 |
| Peripheral temporal | 266.2 | 244.5 | 21.7 | <0.0001 | 0.7903 | <0.0001 |
| Peripheral inferior | 268.1 | 255.1 | 13.0 | <0.0001 | 0.6985 | <0.0001 |
| Peripheral nasal | 304.1 | 281.9 | 22.2 | <0.0001 | 0.6030 | 0.0001 |
| ICC | CV | ||||
|---|---|---|---|---|---|
| Cirrus | ACT100 | Cirrus | ACT100 | p | |
| Fovea | 0.998 | 0.986 | 5.4 | 13.6 | <0.0001 |
| Pericentral superior | 0.99 | 0.947 | 5.6 | 13 | <0.0001 |
| Pericentral temporal | 0.994 | 0.965 | 4.2 | 12.4 | <0.0001 |
| Pericentral nasal | 0.995 | 0.926 | 4.1 | 18.9 | <0.0001 |
| Pericentral inferior | 0.992 | 0.933 | 5.4 | 18.1 | <0.0001 |
| Peripheral superior | 0.95 | 0.686 | 9.4 | 23.6 | 0.0036 |
| Peripheral temporal | 0.993 | 0.921 | 5.6 | 19 | <0.0001 |
| Peripheral inferior | 0.985 | 0.717 | 6.5 | 26.1 | <0.0001 |
| Peripheral nasal | 0.994 | 0.795 | 4.5 | 24.9 | <0.0001 |
| Mean Retinal Thickness (µm) | Pearson’s Correlation Coefficient | |||||
|---|---|---|---|---|---|---|
| Cirrus | ACT100 | Difference | p | Pearson’s Correlation Coefficient | p | |
| Fovea | 258.6 | 247.5 | 11.1 | <0.0001 | 0.8970 | <0.0001 |
| Pericentral superior | 329.5 | 291.5 | 38.0 | <0.0001 | 0.8644 | <0.0001 |
| Pericentral temporal | 316.0 | 285.9 | 30.1 | <0.0001 | 0.8179 | <0.0001 |
| Pericentral nasal | 323.4 | 283.8 | 39.6 | <0.0001 | 0.8469 | <0.0001 |
| Pericentral inferior | 331.9 | 299.6 | 32.3 | <0.0001 | 0.8885 | <0.0001 |
| Peripheral superior | 284.5 | 262.0 | 22.5 | <0.0001 | 0.8042 | <0.0001 |
| Peripheral temporal | 264.3 | 247.0 | 17.2 | <0.0001 | 0.7142 | <0.0001 |
| Peripheral inferior | 267.3 | 251.0 | 16.3 | <0.0001 | 0.7640 | <0.0001 |
| Peripheral nasal | 303.7 | 280.9 | 22.8 | <0.0001 | 0.8703 | <0.0001 |
| ICC | CV | ||||
|---|---|---|---|---|---|
| Cirrus | ACT100 | Cirrus | ACT100 | p | |
| Fovea | 0.998 | 0.983 | 4.4 | 13.8 | <0.0001 |
| Pericentral superior | 0.997 | 0.961 | 3.2 | 13.8 | <0.0001 |
| Pericentral temporal | 0.996 | 0.972 | 3.5 | 11.3 | <0.0001 |
| Pericentral nasal | 0.995 | 0.955 | 3.9 | 15.2 | <0.0001 |
| Pericentral inferior | 0.997 | 0.959 | 3.0 | 13.6 | <0.0001 |
| Peripheral superior | 0.988 | 0.965 | 5.5 | 12.3 | 0.0036 |
| Peripheral temporal | 0.984 | 0.906 | 5.1 | 19.4 | <0.0001 |
| Peripheral inferior | 0.996 | 0.907 | 4.8 | 18.2 | <0.0001 |
| Peripheral nasal | 0.998 | 0.964 | 2.9 | 10.3 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, M.; Hirano, T.; Chiku, Y.; Takahashi, Y.; Miyasaka, H.; Kakihara, S.; Hoshiyama, K.; Murata, T. Reproducibility of Portable OCT and Comparison with Conventional OCT. Diagnostics 2024, 14, 1320. https://doi.org/10.3390/diagnostics14131320
Nakamura M, Hirano T, Chiku Y, Takahashi Y, Miyasaka H, Kakihara S, Hoshiyama K, Murata T. Reproducibility of Portable OCT and Comparison with Conventional OCT. Diagnostics. 2024; 14(13):1320. https://doi.org/10.3390/diagnostics14131320
Chicago/Turabian StyleNakamura, Marie, Takao Hirano, Yoshiaki Chiku, Yoshiaki Takahashi, Hideki Miyasaka, Shinji Kakihara, Ken Hoshiyama, and Toshinori Murata. 2024. "Reproducibility of Portable OCT and Comparison with Conventional OCT" Diagnostics 14, no. 13: 1320. https://doi.org/10.3390/diagnostics14131320
APA StyleNakamura, M., Hirano, T., Chiku, Y., Takahashi, Y., Miyasaka, H., Kakihara, S., Hoshiyama, K., & Murata, T. (2024). Reproducibility of Portable OCT and Comparison with Conventional OCT. Diagnostics, 14(13), 1320. https://doi.org/10.3390/diagnostics14131320






