Persisting Hypercalcemia and Hyperparathyroidism after Kidney Transplantation Have a Negative Impact on Graft and Patient Survival
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Set
2.2. Patient Characteristics
2.3. Exclusion Criteria
2.4. Parathormone and Calcium Levels
2.5. Kidney Function
2.6. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Natural History of Hyperparathyroidism from Transplantation to Last FU
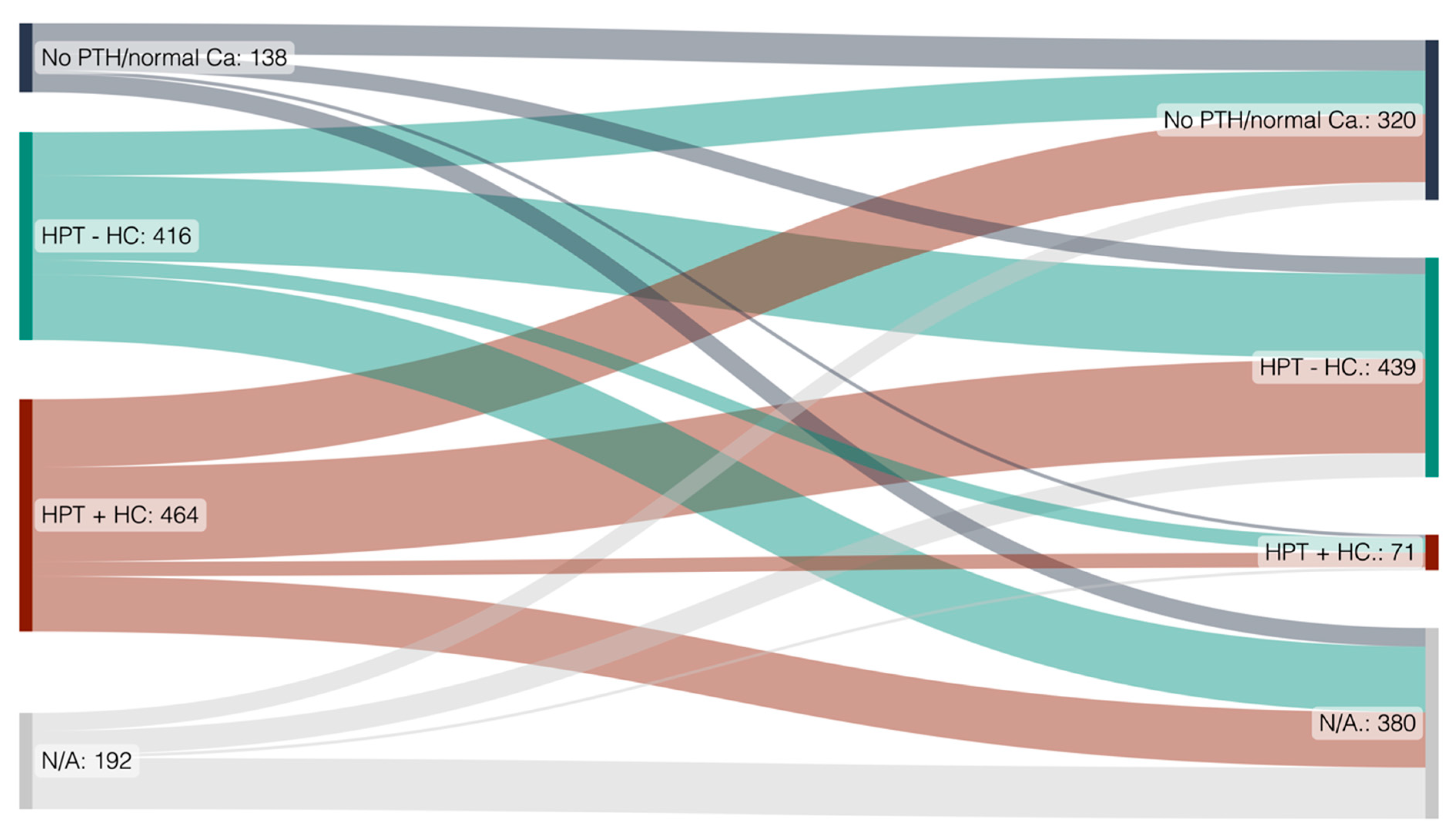
3.2.1. High Rates of Secondary Hyperparathyroidism with and without Hypercalcemia at Timepoint of Transplantation
3.2.2. A High Rate of Transplant Recipients with Hypercalcemia at Transplant Show Normalization of PTH and Calcium after Transplantation
3.3. Medication History with Potential Impact on Calcemia
3.3.1. Vitamin D and Vitamin D Analogs
3.3.2. Calcimimetics
3.4. Risk Factors for Hyperparathyroidis- and Hypercalcemia Development and Persistence after Kidney Transplantation
3.5. Role of Parathyroidectomy in the Kidney Transplant Cohort
3.6. Hyperparathyroidism in Living versus Deceased Kidney Donor Recipients and Its Effect on Patient and Graft Survival
3.6.1. Parathyroid Hormone Is Lower in Patients after Living Compared to Deceased Kidney Donation despite Comparable Graft Function
3.6.2. Living Donor KTRs Have a Higher Rate of PTH Normalization and an Overall Lower Rate of HPT with Hypercalcemia Than Deceased Donor KTRs (after KT)
3.6.3. Living Donor KTRs Have the Same Death-Censored Graft Loss, but Overall Better Survival than Deceased Donor KTRs
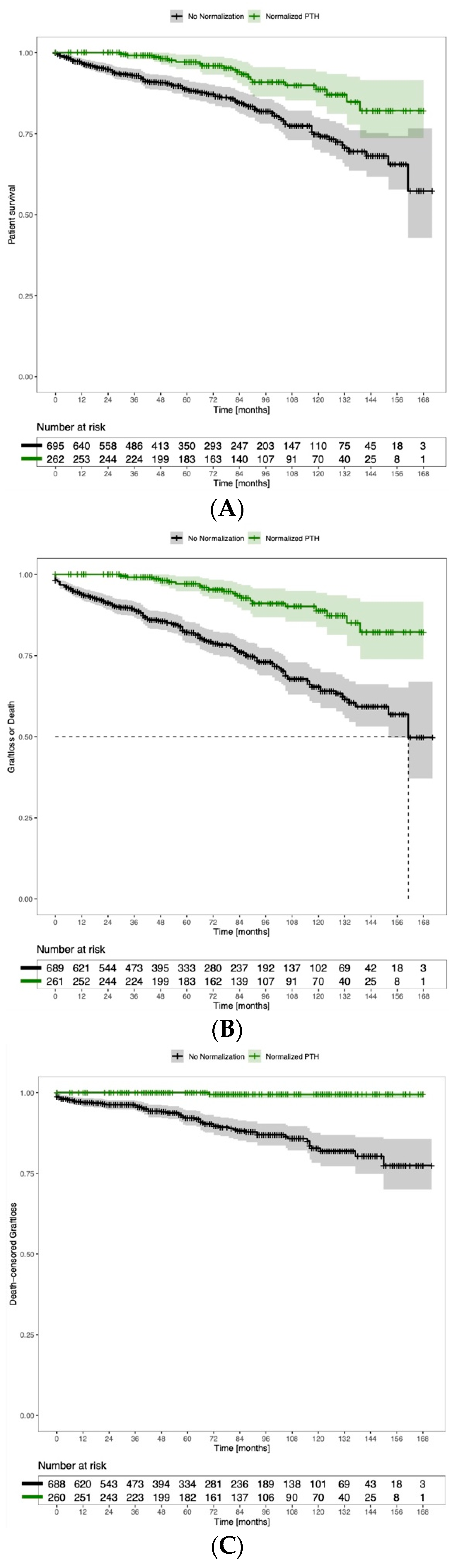
3.7. PTH Normalization after Kidney Transplantation Has a Positive Association with Graft and Overall Survival
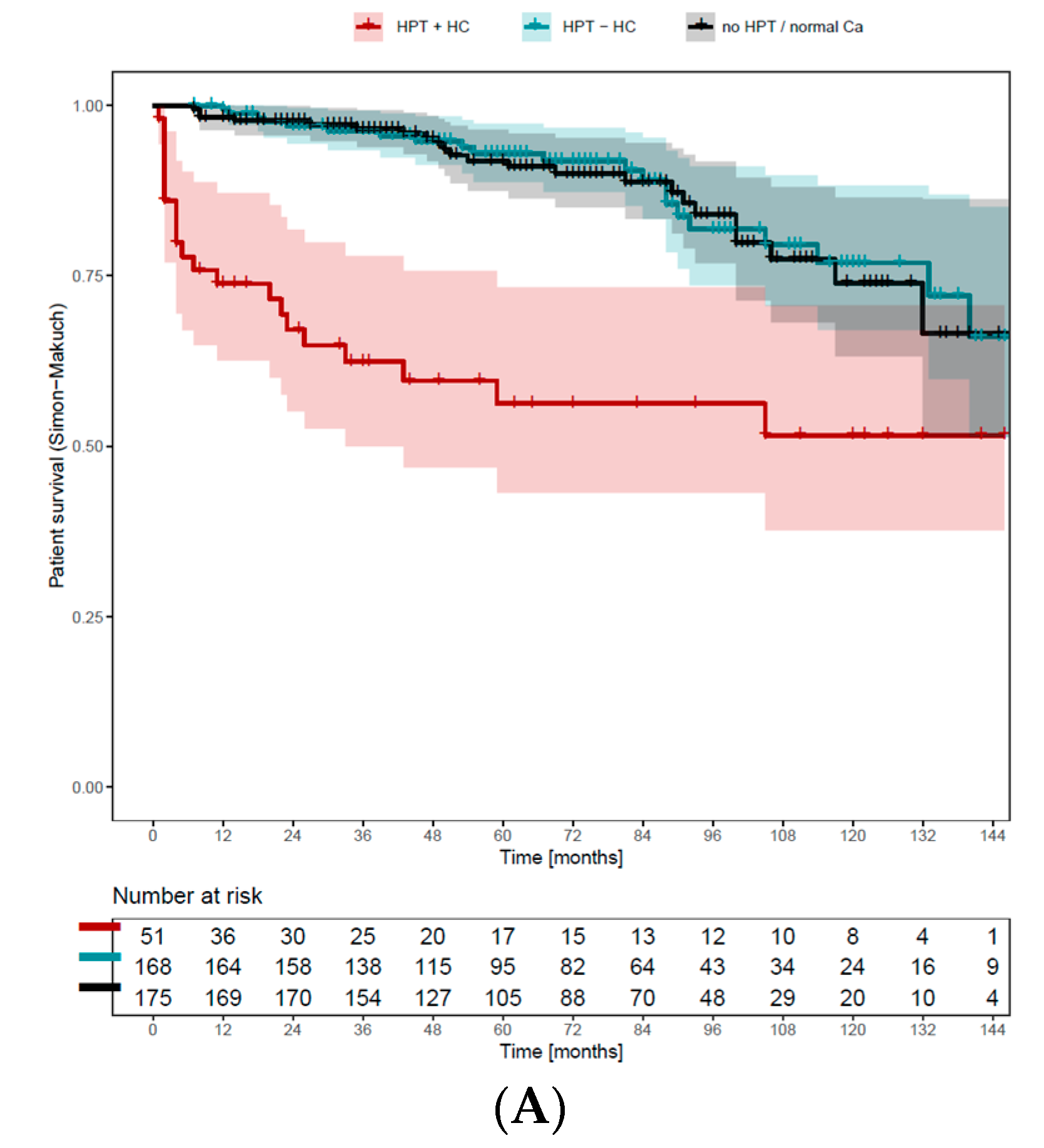
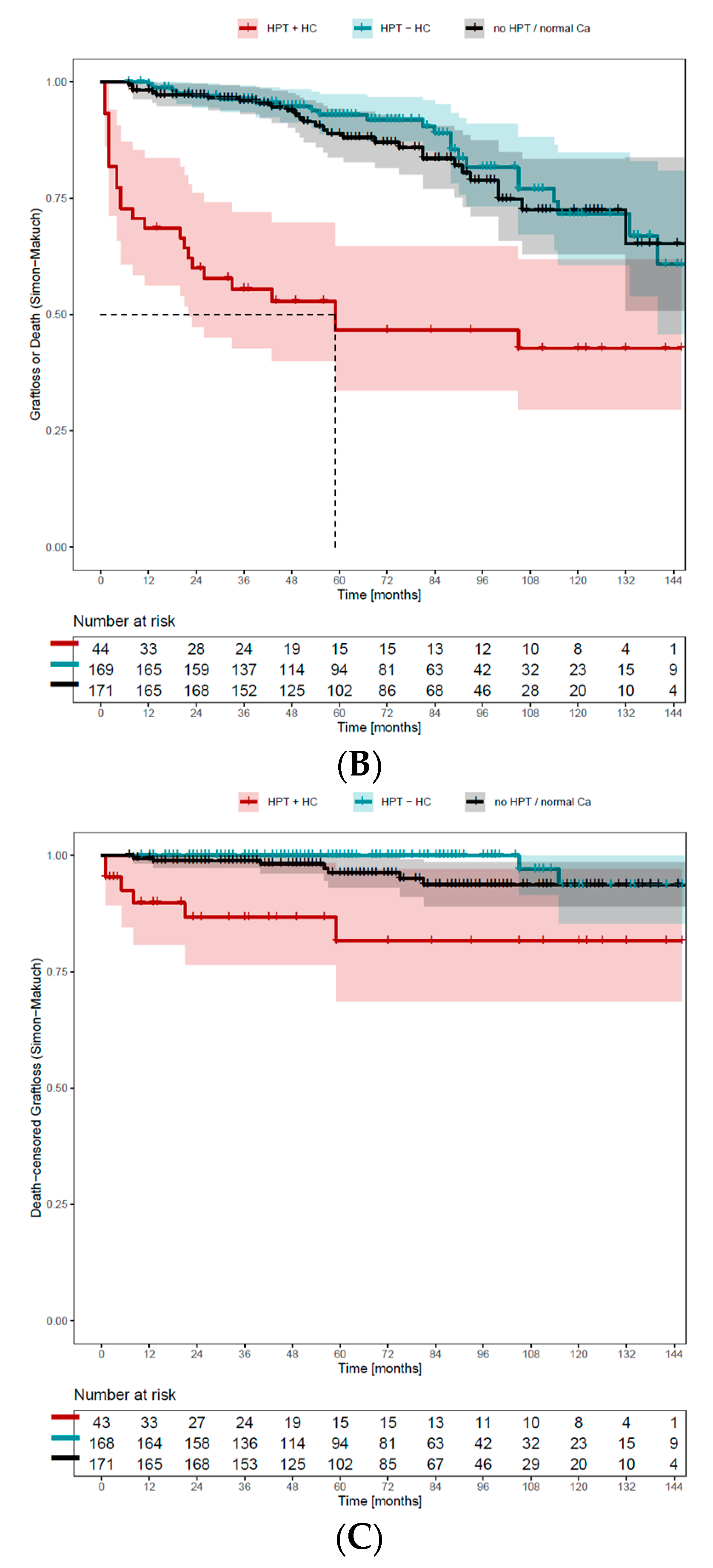
3.8. Persisting Hypercalcemia Is Associated with Negative Graft and Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2009, 76, S1–S130. [Google Scholar] [CrossRef]
- Bureo, J.C.; Arevalo, J.C.; Anton, J.; Adrados, G.; Jimenez Morales, J.L.; Robles, N.R.; en representación de los participantes en el estudio MIPTH. Prevalence of secondary hyperparathyroidism in patients with stage 3 and 4 chronic kidney disease seen in internal medicine. Endocrinol. Nutr. 2015, 62, 300–305. [Google Scholar] [CrossRef]
- Levin, A.; Bakris, G.L.; Molitch, M.; Smulders, M.; Tian, J.; Williams, L.A.; Andress, D.L. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int. 2007, 71, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Andress, D.L.; Coyne, D.W.; Kalantar-Zadeh, K.; Molitch, M.E.; Zangeneh, F.; Sprague, S.M. Management of secondary hyperparathyroidism in stages 3 and 4 chronic kidney disease. Endocr. Pract. 2008, 14, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Horl, W.H. The clinical consequences of secondary hyperparathyroidism: Focus on clinical outcomes. Nephrol. Dial. Transplant. 2004, 19 (Suppl. S5), V2–V8. [Google Scholar] [CrossRef]
- Kamycheva, E.; Sundsfjord, J.; Jorde, R. Serum parathyroid hormone levels predict coronary heart disease: The Tromsø Study. Eur. J. Cardiovasc. Prev. Rehabil. 2004, 11, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, S.K.; Stack, A.G.; Levin, N.W.; Hulbert-Shearon, T.; Port, F.K. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J. Am. Soc. Nephrol. 2001, 12, 2131–2138. [Google Scholar] [CrossRef]
- De Boer, I.H.; Gorodetskaya, I.; Young, B.; Hsu, C.Y.; Chertow, G.M. The severity of secondary hyperparathyroidism in chronic renal insufficiency is GFR-dependent, race-dependent, and associated with cardiovascular disease. J. Am. Soc. Nephrol. 2002, 13, 2762–2769. [Google Scholar] [CrossRef] [PubMed]
- Komaba, H.; Taniguchi, M.; Wada, A.; Iseki, K.; Tsubakihara, Y.; Fukagawa, M. Parathyroidectomy and survival among Japanese hemodialysis patients with secondary hyperparathyroidism. Kidney Int. 2015, 88, 350–359. [Google Scholar] [CrossRef]
- Block, G.A.; Klassen, P.S.; Lazarus, J.M.; Ofsthun, N.; Lowrie, E.G.; Chertow, G.M. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J. Am. Soc. Nephrol. 2004, 15, 2208–2218. [Google Scholar] [CrossRef]
- Cunningham, J.; Locatelli, F.; Rodriguez, M. Secondary hyperparathyroidism: Pathogenesis, disease progression, and therapeutic options. Clin. J. Am. Soc. Nephrol. 2011, 6, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Lou, I.; Foley, D.; Odorico, S.K.; Leverson, G.; Schneider, D.F.; Sippel, R.; Chen, H. How Well Does Renal Transplantation Cure Hyperparathyroidism? Ann. Surg. 2015, 262, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Heaf, J.; Tvedegaard, E.; Kanstrup, I.L.; Fogh-Andersen, N. Hyperparathyroidism and long-term bone loss after renal transplantation. Clin. Transplant. 2003, 17, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Perrin, P.; Caillard, S.; Javier, R.M.; Braun, L.; Heibel, F.; Borni-Duval, C.; Muller, C.; Olagne, J.; Moulin, B. Persistent hyperparathyroidism is a major risk factor for fractures in the five years after kidney transplantation. Am. J. Transplant. 2013, 13, 2653–2663. [Google Scholar] [CrossRef] [PubMed]
- Pihlstrom, H.; Dahle, D.O.; Mjoen, G.; Pilz, S.; Marz, W.; Abedini, S.; Holme, I.; Fellstrom, B.; Jardine, A.G.; Holdaas, H. Increased risk of all-cause mortality and renal graft loss in stable renal transplant recipients with hyperparathyroidism. Transplantation 2015, 99, 351–359. [Google Scholar] [CrossRef]
- Littbarski, S.A.; Kaltenborn, A.; Gwiasda, J.; Beneke, J.; Arelin, V.; Schwager, Y.; Stupak, J.V.; Marcheel, I.L.; Emmanouilidis, N.; Jager, M.D.; et al. Timing of parathyroidectomy in kidney transplant candidates with secondary hyperparathryroidism: Effect of pretransplant versus early or late post-transplant parathyroidectomy. Surgery 2018, 163, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Collaud, S.; Staub-Zahner, T.; Trombetti, A.; Clerici, T.; Marangon, N.; Binet, I.; Myers, P.O.; Rizzoli, R.; Martin, P.Y.; Robert, J.H.; et al. Increase in bone mineral density after successful parathyroidectomy for tertiary hyperparathyroidism after renal transplantation. World J. Surg. 2008, 32, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Pasieka, J.L.; Parsons, L.L. A prospective surgical outcome study assessing the impact of parathyroidectomy on symptoms in patients with secondary and tertiary hyperparathyroidism. Surgery 2000, 128, 531–539. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Hu, J.; Xu, X.; Zhang, K.; Li, Y.; Zheng, J.; Chen, W.; Wang, X. Comparison of estimated glomerular filtration rates in Chinese patients with chronic kidney disease among serum creatinine-, cystatin-C- and creatinine-cystatin-C-based equations: A retrospective cross-sectional study. Clin. Chim. Acta 2020, 505, 34–42. [Google Scholar] [CrossRef]
- Al-Shraideh, Y.; Farooq, U.; Farney, A.C.; Palanisamy, A.; Rogers, J.; Orlando, G.; Buckley, M.R.; Reeves-Daniel, A.; Doares, W.; Kaczmorski, S.; et al. Influence of recipient age on deceased donor kidney transplant outcomes in the expanded criteria donor era. Clin. Transplant. 2014, 28, 1372–1382. [Google Scholar] [CrossRef]
- Muirhead, N.; Zaltman, J.S.; Gill, J.S.; Churchill, D.N.; Poulin-Costello, M.; Mann, V.; Cole, E.H. Hypercalcemia in renal transplant patients: Prevalence and management in Canadian transplant practice. Clin. Transplant. 2014, 28, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, W.; Bartelworth, H.; Jockenhovel, F.; Schmidt-Gayk, H.; Witzke, O.; Wagner, K.; Heemann, U.W.; Reinwein, D.; Philipp, T.; Mann, K. Sequential changes of biochemical bone parameters after kidney transplantation. Nephrol. Dial. Transplant. 1998, 13, 436–442. [Google Scholar] [CrossRef]
- Evenepoel, P.; Bammens, B.; Claes, K.; Kuypers, D.; Meijers, B.K.; Vanrenterghem, Y. Measuring total blood calcium displays a low sensitivity for the diagnosis of hypercalcemia in incident renal transplant recipients. Clin. J. Am. Soc. Nephrol. 2010, 5, 2085–2092. [Google Scholar] [CrossRef][Green Version]
- David, D.S.; Sakai, S.; Brennan, B.L.; Riggio, R.A.; Cheigh, J.; Stenzel, K.H.; Rubin, A.L.; Sherwood, L.M. Hypercalcemia after renal transplantation. Long-term follow-up data. N. Engl. J. Med. 1973, 289, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Cundy, T.; Kanis, J.A.; Heynen, G.; Morris, P.J.; Oliver, D.O. Calcium metabolism and hyperparathyroidism after renal transplantation. Q. J. Med. 1983, 52, 67–78. [Google Scholar]
- Leca, N.; Laftavi, M.; Gundroo, A.; Kohli, R.; Min, I.; Karam, J.; Sridhar, N.; Blessios, G.; Venuto, R.; Pankewycz, O. Early and severe hyperparathyroidism associated with hypercalcemia after renal transplant treated with cinacalcet. Am. J. Transplant. 2006, 6, 2391–2395. [Google Scholar] [CrossRef]
- Egbuna, O.I.; Taylor, J.G.; Bushinsky, D.A.; Zand, M.S. Elevated calcium phosphate product after renal transplantation is a risk factor for graft failure. Clin. Transplant. 2007, 21, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, M.; Einollahi, B.; Asl, M.A.; Nafar, M.; Pourfarziani, V.; Moradi, M.; Samadpour, A.; Alghasi, M.; Davoudi, F. Calcium and phosphorus metabolism disturbances after renal transplantation. Transplant. Proc. 2007, 39, 1033–1035. [Google Scholar] [CrossRef]
- Evenepoel, P.; Van Den Bergh, B.; Naesens, M.; De Jonge, H.; Bammens, B.; Claes, K.; Kuypers, D.; Vanrenterghem, Y. Calcium metabolism in the early posttransplantation period. Clin. J. Am. Soc. Nephrol. 2009, 4, 665–672. [Google Scholar] [CrossRef]
- McCormick, F.; Held, P.J.; Chertow, G.M. The Terrible Toll of the Kidney Shortage. J. Am. Soc. Nephrol. 2018, 29, 2775–2776. [Google Scholar] [CrossRef] [PubMed]
- Israni, A.K.; Zaun, D.; Rosendale, J.D.; Schaffhausen, C.; Snyder, J.J.; Kasiske, B.L. OPTN/SRTR 2017 Annual Data Report: Deceased Organ Donation. Am J Transplant 2019, 19 (Suppl. 2), 485–516. [Google Scholar] [CrossRef] [PubMed]
- Fakhr Yasseri, A.M.; Namdari, F.; Gooran, S.; Ahmadi, A.; Dehghani, S.; Asadi, M.; Mehrsay, A.; Pourmand, G.; Dialameh, H. Living versus deceased kidney transplantation: Comparison of complications. Urologia 2021, 88, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Cianciolo, G.; Capelli, I.; Cappuccilli, M.; Schillaci, R.; Cozzolino, M.; La Manna, G. Calcifying circulating cells: An uncharted area in the setting of vascular calcification in CKD patients. Clin. Kidney J. 2016, 9, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Isakov, O.; Ghinea, R.; Beckerman, P.; Mor, E.; Riella, L.V.; Hod, T. Early persistent hyperparathyroidism post-renal transplantation as a predictor of worse graft function and mortality after transplantation. Clin. Transplant. 2020, 34, e14085. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.; Ramalho, J.A.M.; Elias, R.M.; Jorgetti, V.; Nahas, W.; Custodio, M.; Moyses, R.M.A.; David-Neto, E. Persistent hyperparathyroidism as a risk factor for long-term graft failure: The need to discuss indication for parathyroidectomy. Surgery 2018, 163, 1144–1150. [Google Scholar] [CrossRef]
- Okada, M.; Hiramitsu, T.; Ichimori, T.; Goto, N.; Narumi, S.; Watarai, Y.; Sato, T.; Tominaga, Y. Comparison of Pre- and Post-transplant Parathyroidectomy in Renal Transplant Recipients and the Impact of Parathyroidectomy Timing on Calcium Metabolism and Renal Allograft Function: A Retrospective Single-Center Analysis. World J. Surg. 2020, 44, 498–507. [Google Scholar] [CrossRef]

| Total Cohort (n = 1212) | Deceased (n = 848) | Living Donors (n = 364) | p | Missing | |
|---|---|---|---|---|---|
| Basic patient data | |||||
| Age at KT, median (IQR) | 52.1 (39.6–60.7) | 53.4 (41.9–62) | 46.9 (34.5–57.7) | <0.001 | - |
| Male gender, n (%) | 755 (62.3) | 527 (62.1) | 228 (62.6) | 0.872 | - |
| FU-time (months), median (IQR) | 62 (28–105) | 57 (26–97) | 79 (37–121) | <0.001 | 4.0% |
| Calcium at last FU, median (IQR) | 2.32 (2.24–2.41) | 2.32 (2.24–2.41) | 2.31 (2.24–2.39) | 0.447 | 47.9% |
| Creatinine at last FU, median (IQR) | 121 (93–169.5) | 121 (93–169.5) | 124.5 (102–164.8) | 0.153 | 33.9% |
| Phosphate at last FU, median (IQR) | 0.93 (0.80–1.11) | 0.93 (0.80–1.11) | 0.95 (0.79–1.11) | 0.972 | 41.7% |
| 1,25-dihydroxyvitamin D, median (IQR) | 32 (24.2–38.9) | 32 (24.2–38.9) | 32.8 (25.9–40.2) | 0.255 | 69.5% |
| Transplantation related | |||||
| Time on waitlist (months), median (IQR) Dialysis before transplantation, n (%) No dialysis HD CAPD CCPD Other or combination Duration of dialysis (months), median (IQR) Number of KT, n (%) | 22 (9–42) 231 (19.1) 810 (66.8) 162 (13.4) 2 (0.2) 4 (0.3) 33 (17–55) | 28 (14–48) 94 (11.1) 629 (74.3) 120 (14.2) 2 (0.2) 2 (0.2) 39 (23–60) | 8 (4–15) 137 (37.8) 181 (50.0) 42 (11.6) 2 (0.6) 0 (0.0) 12 (5–27) | <0.001 <0.001 <0.001 0.429 | 8.5% 0.2% 0.3% - |
| 1 | 1038 (85.6) | 718 (84.7) | 320 (87.9) | ||
| 2 | 152 (12.5) | 114 (13.4) | 38 (10.4) | ||
| 3 | 20 (1.7) | 15 (1.8) | 5 (1.4) | ||
| 4 | 2 (0.2) | 1 (0.1) | 1 (0.3) | ||
| Living-Donor KT Warm ischemia (min), median (IQR) Cold ischemia (h), median (IQR) | 364 (30.0) 30 (20–36) 9.5 (7.1–12.3) | - 27 (17–37) 9.9 (7.4–12.5) | 364 (100) 30 (30–35) 2.1 (2.1–2.3) | <0.001 0.038 <0.001 | - 88.8% 27.1% |
| Rejection before organ failure, n (%) | 91 (7.9) | 63 (7.7) | 28 (8.4) | 0.728 | 5.3% |
| Time from TPL to rejection (months), median (IQR) | 39 (4.3–69.9) | 35.6 (1.7–66.5) | 52.3 (15.9–77.7) | 0.082 | - |
| Primary non-function, n (%) | 22 (1.8) | 18 (2.1) | 4 (1.1) | 0.221 | - |
| Patient and graft survival | |||||
| Patient survival, % | |||||
| 1-year | 96.6 | 95.6 | 98.8 | <0.001 | 0.7% |
| 2-year | 95.1 | 94.0 | 97.9 | ||
| 5-year | 89.8 | 87.5 | 94.8 | ||
| 10-year | 77.2 | 74.2 | 83.5 | ||
| Graft survival, % | |||||
| 1-year | 94.2 | 92.9 | 97.6 | 0.004 | 2.3% |
| 2-year | 92.2 | 90.9 | 95.7 | ||
| 5-year | 84.7 | 82.5 | 90.0 | ||
| 10-year | 70.1 | 67.8 | 75.1 | ||
| Death-censored graft survival, % | |||||
| 1-year | 97.1 | 96.6 | 98.1 | 0.461 | 5.4% |
| 2-year | 96.5 | 96.2 | 97.2 | ||
| 5-year | 93.4 | 93.1 | 94.0 | ||
| 10-year | 87.2 | 86.6 | 88.3 |
| No 2HPT | PTH not Available Normal Calcium | PTH not Available Hypocalcemia | 2HPT Normo- or Hypocalcemia |
PTH Elevated Calcium not Available |
PTH not Available Hypercalcemia |
PTH Elevated Hypercalcemia | N/A (missing data) | |
|---|---|---|---|---|---|---|---|---|
| Tx | 69 (5.7) | 69 (5.7) | 20 (1.7) | 396 (32.7) | 8 (0.7) | 10 (0.8) | 448 (37.0) | 192 (15.8) |
| 6 months | 262 (21.6) | 61 (5.0) | 1 (0.1) | 603 (49.8) | 21 (1.7) | 13 (1.1) | 101 (8.3) | 150 (12.4) |
| 12 months | 316 (26.1) | 46 (3.8) | 3 (0.2) | 536 (44.2) | 20 (1.7) | 5 (0.4) | 74 (6.1) | 212 (17.5) |
| 24 months | 261 (21.5) | 59 (4.9) | 2 (0.2) | 437 (36.1) | 15 (1.2) | 3 (0.2) | 55 (4.5) | 380 (31.4) |
| 5 years | 196 (16.2) | 57 (4.7) | 5 (0.4) | 255 (21.0) | 5 (0.4) | 3 (0.2) | 21 (1.7) | 670 (55.3) |
| last FU | 146 (12.0) | 190 (15.7) | 34 (2.8) | 224 (18.5) | 23 (1.9) | 34 (2.8) | 9 (0.7) | 552 (45.5) |
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| p | HR | 95% CI | p | HR | 95% CI | |
| Female sex | 0.148 | 0.76 | 0.53–1.10 | 0.171 | 0.75 | 0.50–1.13 |
| Donor age | 0.798 | 1.00 | 0.99–1.01 | 0.988 | 1.00 | 0.99–1.01 |
| Time on waiting list (months) | <0.001 | 1.02 | 1.02–1.03 | 0.014 | 1.01 | 1.00–1.02 |
| Dialysis time (months) | <0.001 | 1.01 | 1.01–1.02 | 0.020 | 1.01 | 1.00–1.02 |
| LDKT | <0.001 | 0.43 | 0.27–0.70 | 0.342 | 0.684 | 0.31–1.50 |
| PTH not Normalized n = 696 | PTH Normalized n = 263 | p | Missing | |
|---|---|---|---|---|
| GFR (mL/min), median (IQR) | ||||
| KT | 8 (6–10) | 8 (6–11) | 0.076 | 6.2% |
| 6 months | 51 (37–64) | 55 (44–68) | <0.001 | 4.2% |
| 12 months | 53 (40–67) | 58 (48–73) | <0.001 | 8.7% |
| 24 months | 53 (39–67) | 61 (48–79) | <0.001 | 21.8% |
| 36 months | 52 (39–66) | 60 (47–76) | <0.001 | 33.1% |
| 48 months | 52 (38–66) | 59 (49–76) | <0.001 | 42.6% |
| 5 years | 53 (38–68) | 59 (50–75) | <0.001 | 51.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egli, H.; Burla, N.; Breuer, E.; Baron, C.; Hübel, K.; de Rougemont, O.; Seeger, H.; Vetter, D. Persisting Hypercalcemia and Hyperparathyroidism after Kidney Transplantation Have a Negative Impact on Graft and Patient Survival. Diagnostics 2024, 14, 1358. https://doi.org/10.3390/diagnostics14131358
Egli H, Burla N, Breuer E, Baron C, Hübel K, de Rougemont O, Seeger H, Vetter D. Persisting Hypercalcemia and Hyperparathyroidism after Kidney Transplantation Have a Negative Impact on Graft and Patient Survival. Diagnostics. 2024; 14(13):1358. https://doi.org/10.3390/diagnostics14131358
Chicago/Turabian StyleEgli, Hannes, Naomi Burla, Eva Breuer, Camilla Baron, Kerstin Hübel, Olivier de Rougemont, Harald Seeger, and Diana Vetter. 2024. "Persisting Hypercalcemia and Hyperparathyroidism after Kidney Transplantation Have a Negative Impact on Graft and Patient Survival" Diagnostics 14, no. 13: 1358. https://doi.org/10.3390/diagnostics14131358
APA StyleEgli, H., Burla, N., Breuer, E., Baron, C., Hübel, K., de Rougemont, O., Seeger, H., & Vetter, D. (2024). Persisting Hypercalcemia and Hyperparathyroidism after Kidney Transplantation Have a Negative Impact on Graft and Patient Survival. Diagnostics, 14(13), 1358. https://doi.org/10.3390/diagnostics14131358






