External Validation of the Machine Learning-Based Thermographic Indices for Rheumatoid Arthritis: A Prospective Longitudinal Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recruited Patients
2.2. Thermography Tests
2.3. Clinical and Laboratory Assessments
2.4. Calculation of ThermoJIS, ThermoDAI and ThermoDAI-CRP
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
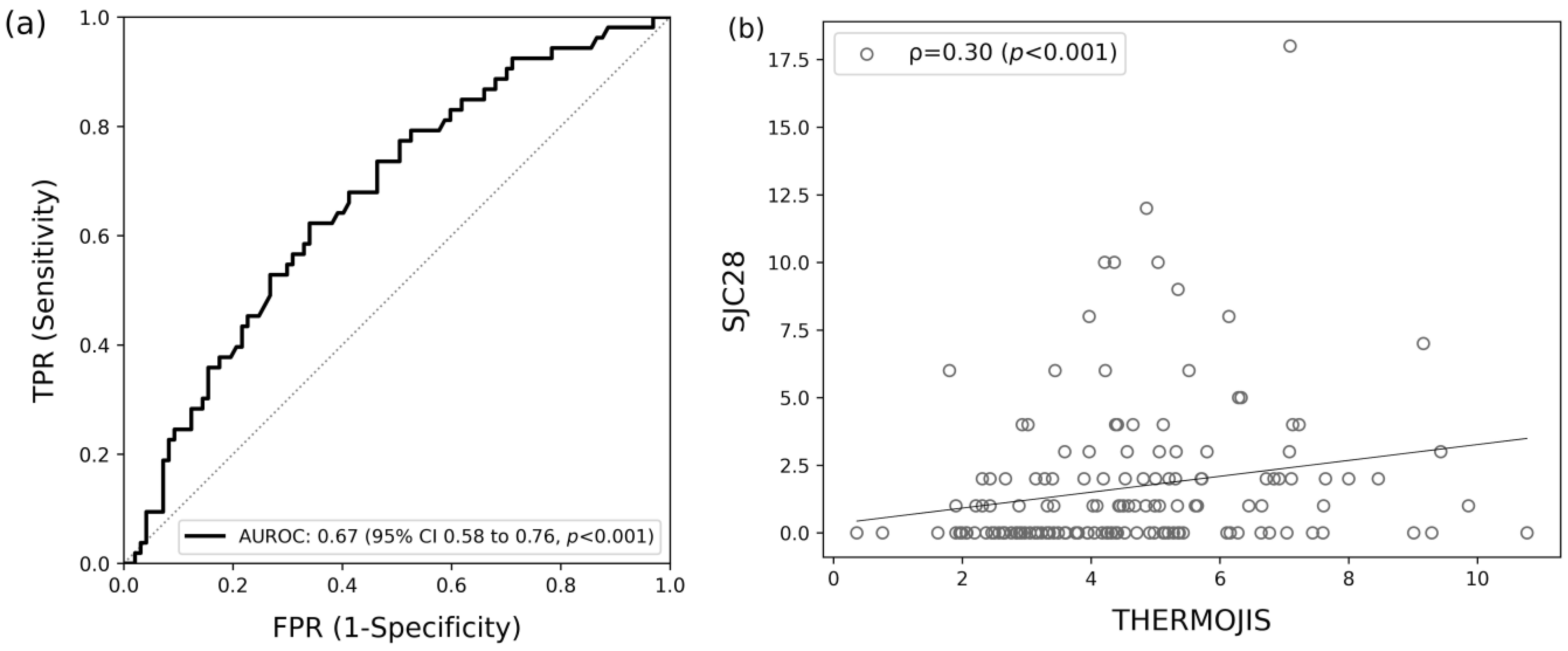
3.2. Association of ThermoJIS with Swollen Joint Count
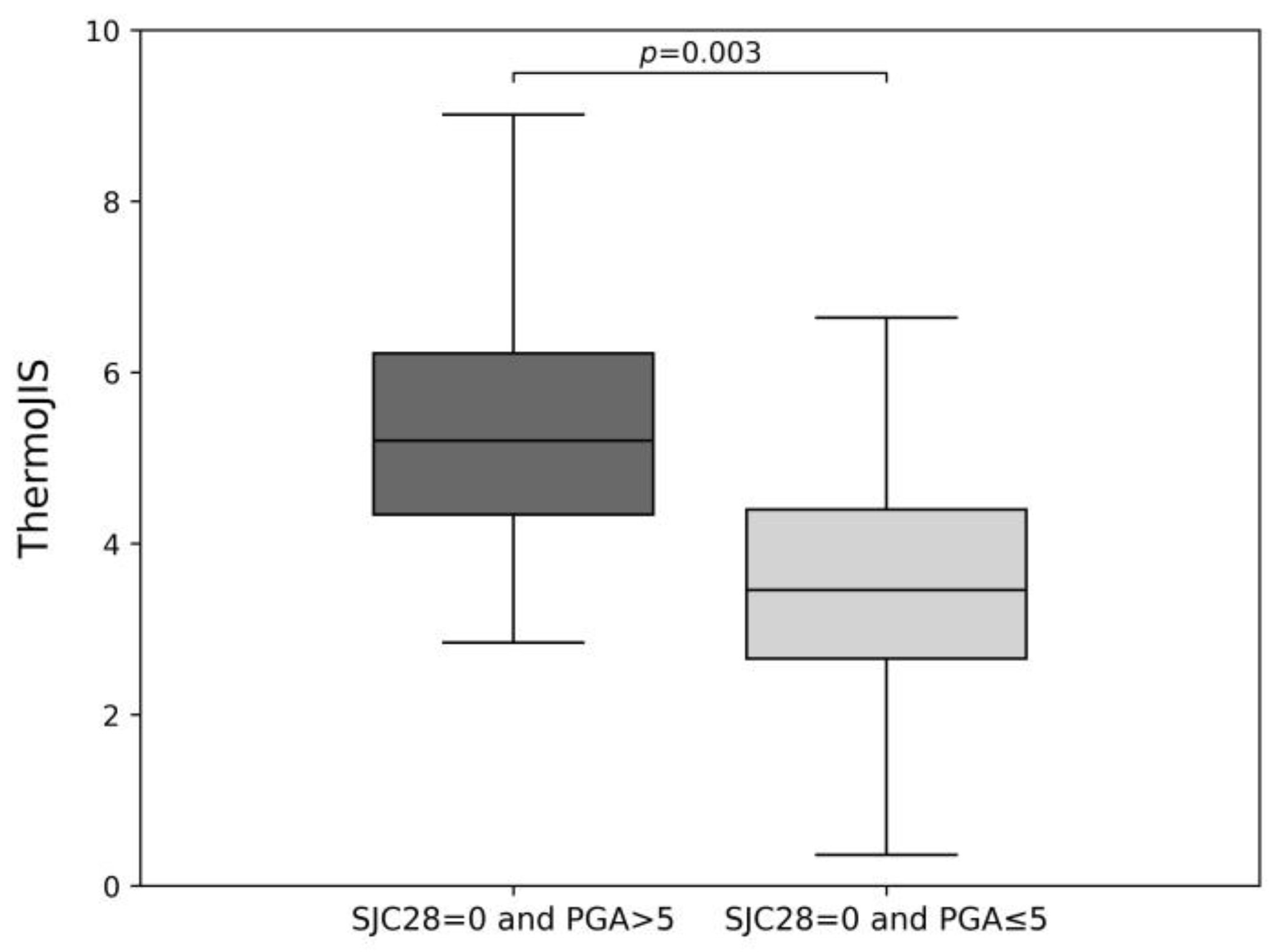
3.3. Sensitive Detection of Synovitis
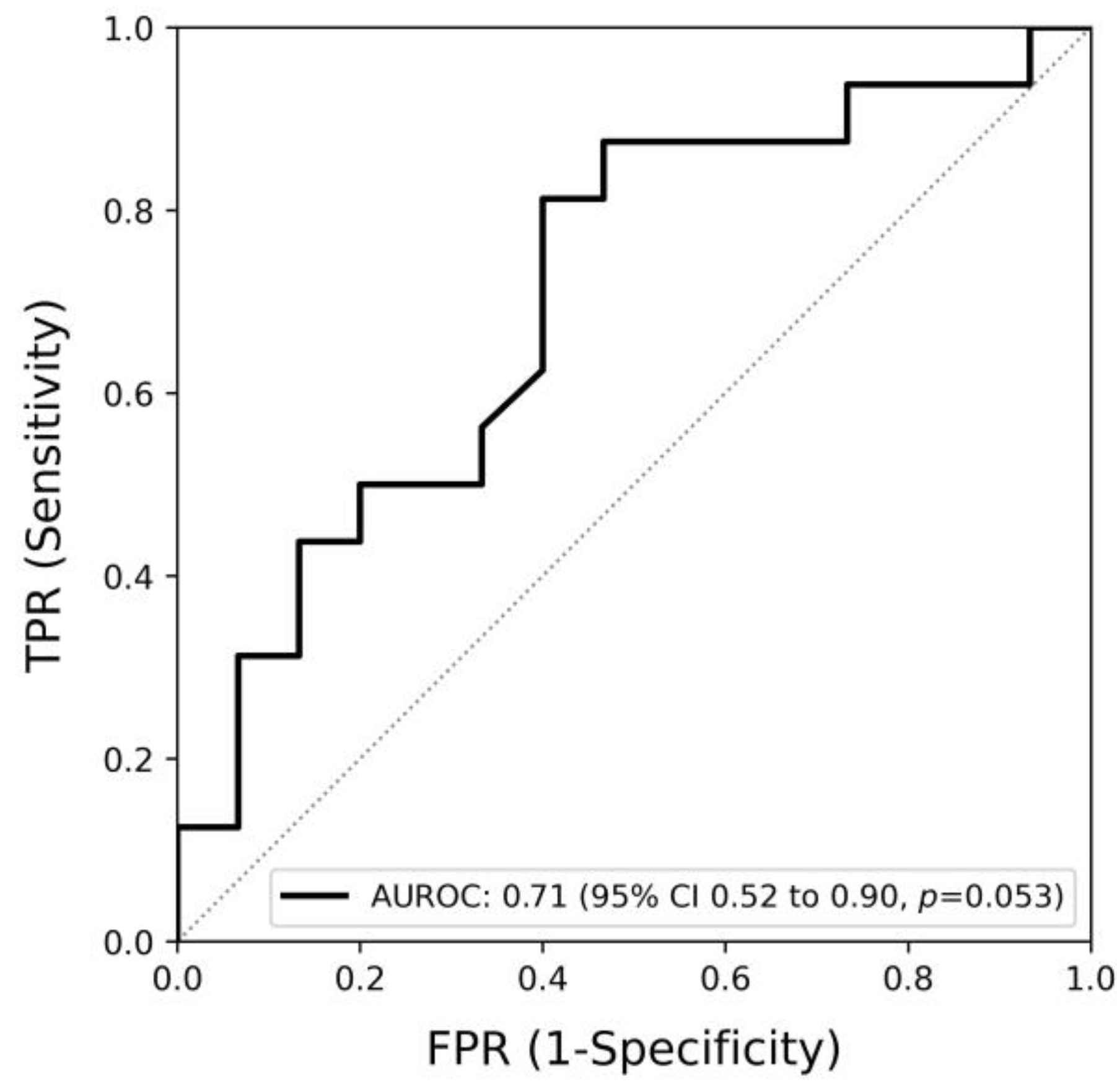
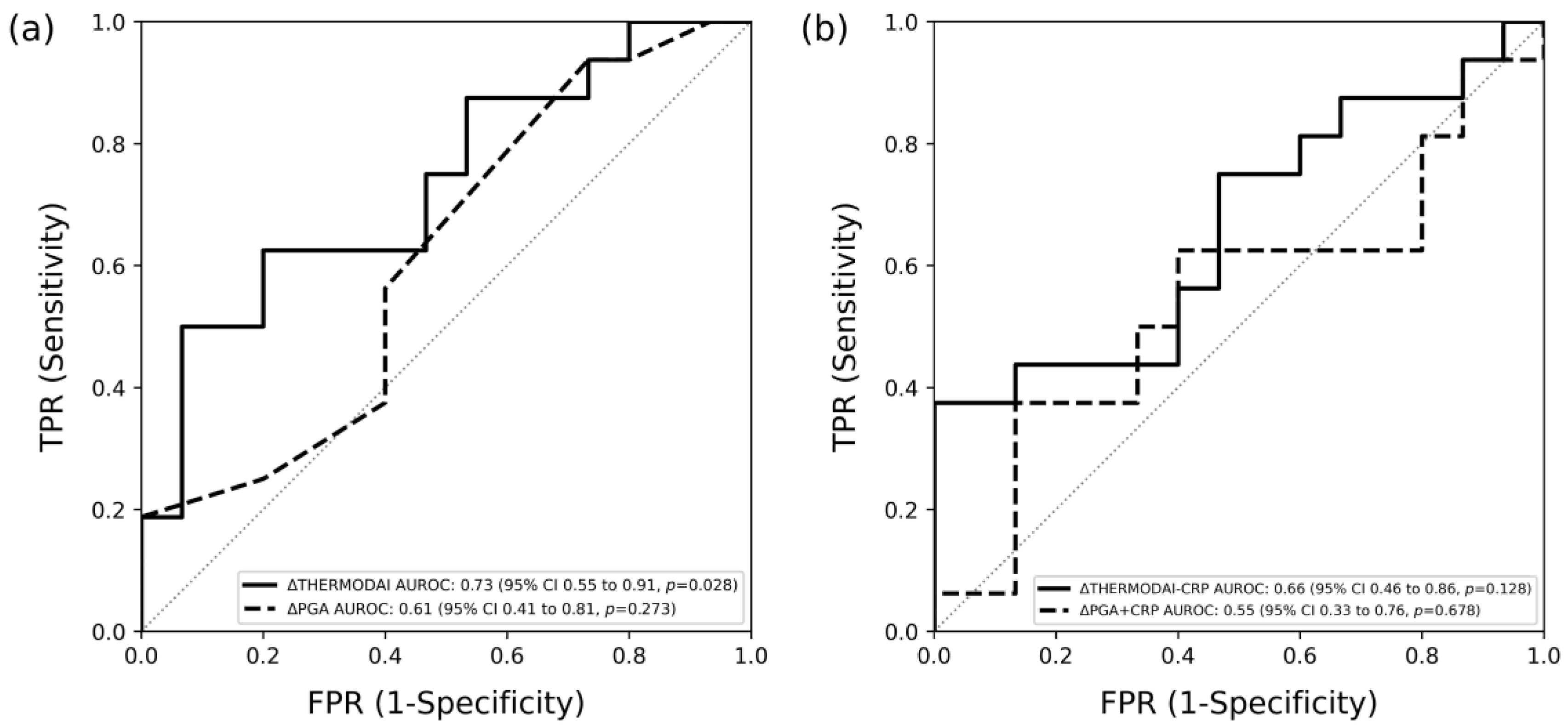
3.4. Association of the Change of ThermoJIS with the Change of Swollen Joint Count
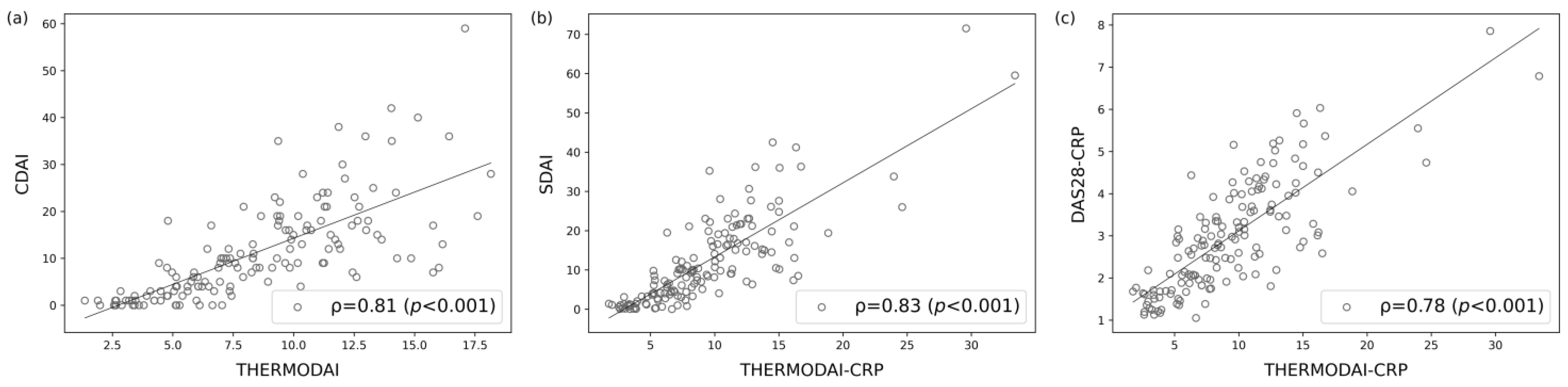
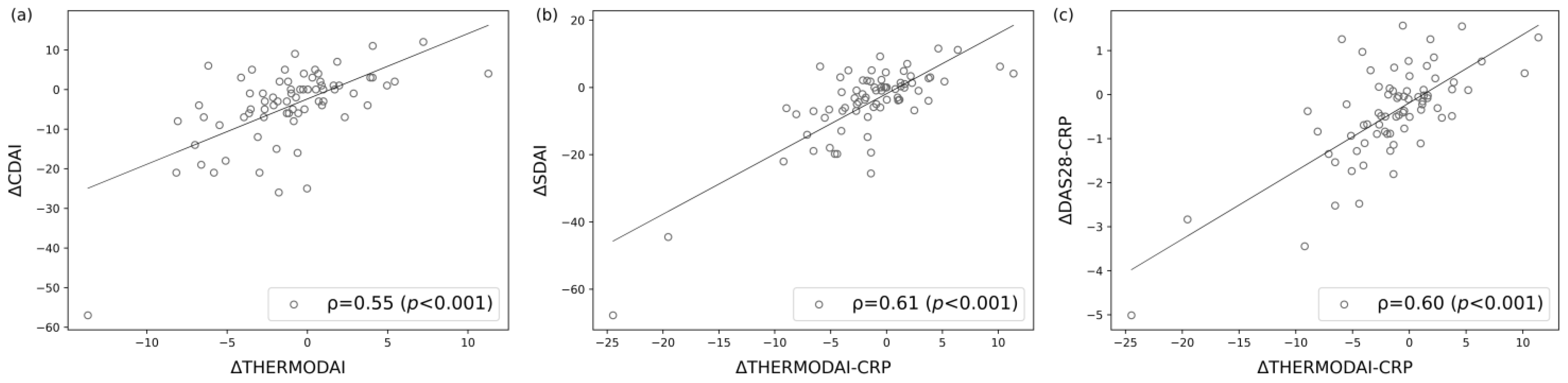
3.5. Correlation of ThermoDAI and ThermoDAI-CRP with CDAI, SDAI, and DAS28-CRP
3.6. Correlations of the Change of ThermoDAI and ThermoDAI-CRP with the Change of CDAI, SDAI, and DAS28-CRP
3.7. Contribution of ThermoJIS Component in ThermoDAI and ThermoDAI-CRP Indices
3.8. Detection of Patients Who Met Treatment Response Using ThermoDAI and ThermoDAI-CRP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Previous Development of the Machine Learning Model
References
- Grigor, C.; Capell, H.; Stirling, A.; McMahon, A.D.; Lock, P.; Vallance, R.; Kincaid, W.; Porter, D. Effect of a Treatment Strategy of Tight Control for Rheumatoid Arthritis (the TICORA Study): A Single-Blind Randomised Controlled Trial. Lancet 2004, 364, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Verstappen, S.M.M.; Jacobs, J.W.G.; van der Veen, M.J.; Heurkens, A.H.M.; Schenk, Y.; ter Borg, E.J.; Blaauw, A.a.M.; Bijlsma, J.W.J. Utrecht Rheumatoid Arthritis Cohort study group Intensive Treatment with Methotrexate in Early Rheumatoid Arthritis: Aiming for Remission. Computer Assisted Management in Early Rheumatoid Arthritis (CAMERA, an Open-Label Strategy Trial). Ann. Rheum. Dis. 2007, 66, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid Arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR Recommendations for the Management of Rheumatoid Arthritis with Synthetic and Biological Disease-Modifying Antirheumatic Drugs: 2019 Update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.M.; Weinblatt, M.E. Rheumatoid Arthritis. Lancet 2001, 358, 903–911. [Google Scholar] [CrossRef]
- Brenner, M.; Braun, C.; Oster, M.; Gulko, P.S. Thermal Signature Analysis as a Novel Method for Evaluating Inflammatory Arthritis Activity. Ann. Rheum. Dis. 2006, 65, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, B.M.; Lesch, M.; Brammer, D.; Bove, S.E.; Thiel, M.; Kilgore, K.S. Use of a Portable Thermal Imaging Unit as a Rapid, Quantitative Method of Evaluating Inflammation and Experimental Arthritis. J. Pharmacol. Toxicol. Methods 2008, 57, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Snekhalatha, U.; Anburajan, M.; Sowmiya, V.; Venkatraman, B.; Menaka, M. Automated Hand Thermal Image Segmentation and Feature Extraction in the Evaluation of Rheumatoid Arthritis. Proc. IMechE 2015, 229, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Spalding, S.J.; Kwoh, C.K.; Boudreau, R.; Enama, J.; Lunich, J.; Huber, D.; Denes, L.; Hirsch, R. Three-Dimensional and Thermal Surface Imaging Produces Reliable Measures of Joint Shape and Temperature: A Potential Tool for Quantifying Arthritis. Arthritis Res. Ther. 2008, 10, R10. [Google Scholar] [CrossRef]
- Tan, Y.K.; Hong, C.; Li, H.; Allen, J.C.; Thumboo, J. Thermography in Rheumatoid Arthritis: A Comparison with Ultrasonography and Clinical Joint Assessment. Clin. Radiol. 2020, 75, 963.e17–963.e22. [Google Scholar] [CrossRef]
- Gatt, A.; Mercieca, C.; Borg, A.; Grech, A.; Camilleri, L.; Gatt, C.; Chockalingam, N.; Formosa, C. A Comparison of Thermographic Characteristics of the Hands and Wrists of Rheumatoid Arthritis Patients and Healthy Controls. Sci. Rep. 2019, 9, 17204. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllias, K.; Clasen, M.; De Blasi, M.; Berres, M.; Nikolodimos, E.; Schwarting, A. Performance of a Novel High-Resolution Infrared Thermography Marker in Detecting and Assessing Joint Inflammation: A Comparison with Joint Ultrasound. Clin. Exp. Rheumatol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Vasdev, V.; Singh, R.; Aggarwal, V.; Bhatt, S.; Kartik, S.; Hegde, A.; Kumar, A.; Bhaskar, S.V. Thermal Imaging in Rheumatoid Arthritis Knee Joints and Its Correlation with Power Doppler Ultrasound. Med. J. Armed Forces India 2023, 79, S189–S195. [Google Scholar] [CrossRef]
- Tan, Y.K.; Sultana, R.; Thumboo, J. Thermography at the Elbow Among Patients with Rheumatoid Arthritis: A Comparison with Ultrasound-Detected Joint Inflammation Findings. Rheumatol. Ther. 2024, 11, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.K.; Lim, G.H. Assessment of Joint Inflammation at the Wrist of Patients with Rheumatoid Arthritis: Thermography Findings Closely Mirror Those from Ultrasonography. Clin. Exp. Rheumatol. 2024, 42, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.K.; Lim, G.H. Subclinical Joint Inflammation in Rheumatoid Arthritis: Comparing Thermal and Ultrasound Imaging at the Metacarpophalangeal Joint. Adv. Rheumatol. 2024, 64, 36. [Google Scholar] [CrossRef] [PubMed]
- Chojnowski, M. Infrared Thermal Imaging in Connective Tissue Diseases. Reumatologia 2017, 55, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Kesztyüs, D.; Brucher, S.; Wilson, C.; Kesztyüs, T. Use of Infrared Thermography in Medical Diagnosis, Screening, and Disease Monitoring: A Scoping Review. Medicina 2023, 59, 2139. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, B.B.; Bagavathiappan, S.; Jayakumar, T.; Philip, J. Medical Applications of Infrared Thermography: A Review. Infrared Phys. Technol. 2012, 55, 221–235. [Google Scholar] [CrossRef]
- Branco, J.H.L.; Branco, R.L.L.; Siqueira, T.C.; de Souza, L.C.; Dalago, K.M.S.; Andrade, A. Clinical Applicability of Infrared Thermography in Rheumatic Diseases: A Systematic Review. J. Therm. Biol. 2022, 104, 103172. [Google Scholar] [CrossRef]
- Schiavon, G.; Capone, G.; Frize, M.; Zaffagnini, S.; Candrian, C.; Filardo, G. Infrared Thermography for the Evaluation of Inflammatory and Degenerative Joint Diseases: A Systematic Review. Cartilage 2021, 13, 1790S–1801S. [Google Scholar] [CrossRef] [PubMed]
- Kow, J.; Tan, Y.K. An Update on Thermal Imaging in Rheumatoid Arthritis. Jt. Bone Spine 2023, 90, 105496. [Google Scholar] [CrossRef] [PubMed]
- Morales-Ivorra, I.; Narváez, J.; Gómez-Vaquero, C.; Moragues, C.; Nolla, J.M.; Narváez, J.A.; Marín-López, M.A. Assessment of Inflammation in Patients with Rheumatoid Arthritis Using Thermography and Machine Learning: A Fast and Automated Technique. RMD Open 2022, 8, e002458. [Google Scholar] [CrossRef]
- Morales-Ivorra, I.; Narváez, J.; Gómez-Vaquero, C.; Moragues, C.; Nolla, J.M.; Narváez, J.A.; Marín-López, M.A. A Thermographic Disease Activity Index for Remote Assessment of Rheumatoid Arthritis. RMD Open 2022, 8, e002615. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid Arthritis Classification Criteria: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Esteve-Vives, J.; Batlle-Gualda, E.; Reig, A. Spanish Version of the Health Assessment Questionnaire: Reliability, Validity and Transcultural Equivalency. Grupo Para La Adaptación Del HAQ a La Población Española. J. Rheumatol. 1993, 20, 2116–2122. [Google Scholar] [PubMed]
- Fries, J.F.; Spitz, P.W.; Young, D.Y. The Dimensions of Health Outcomes: The Health Assessment Questionnaire, Disability and Pain Scales. J. Rheumatol. 1982, 9, 789–793. [Google Scholar]
- Felson, D.T.; Smolen, J.S.; Wells, G.; Zhang, B.; van Tuyl, L.H.D.; Funovits, J.; Aletaha, D.; Allaart, C.F.; Bathon, J.; Bombardieri, S.; et al. American College of Rheumatology/European League against Rheumatism Provisional Definition of Remission in Rheumatoid Arthritis for Clinical Trials. Ann. Rheum. Dis. 2011, 70, 404–413. [Google Scholar] [CrossRef]
- van Gestel, A.M.; Prevoo, M.L.; van ’t Hof, M.A.; van Rijswijk, M.H.; van de Putte, L.B.; van Riel, P.L. Development and Validation of the European League Against Rheumatism Response Criteria for Rheumatoid Arthritis. Comparison with the Preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 1996, 39, 34–40. [Google Scholar] [CrossRef]
- Backhaus, M.; Burmester, G.R.; Sandrock, D.; Loreck, D.; Hess, D.; Scholz, A.; Blind, S.; Hamm, B.; Bollow, M. Prospective Two Year Follow up Study Comparing Novel and Conventional Imaging Procedures in Patients with Arthritic Finger Joints. Ann. Rheum. Dis. 2002, 61, 895–904. [Google Scholar] [CrossRef]
- Kane, D.; Balint, P.V.; Sturrock, R.D. Ultrasonography Is Superior to Clinical Examination in the Detection and Localization of Knee Joint Effusion in Rheumatoid Arthritis. J. Rheumatol. 2003, 30, 966–971. [Google Scholar] [PubMed]
- Karim, Z.; Wakefield, R.J.; Quinn, M.; Conaghan, P.G.; Brown, A.K.; Veale, D.J.; O’Connor, P.; Reece, R.; Emery, P. Validation and Reproducibility of Ultrasonography in the Detection of Synovitis in the Knee: A Comparison with Arthroscopy and Clinical Examination. Arthritis Rheum. 2004, 50, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Szkudlarek, M.; Klarlund, M.; Narvestad, E.; Court-Payen, M.; Strandberg, C.; Jensen, K.E.; Thomsen, H.S.; Østergaard, M. Ultrasonography of the Metacarpophalangeal and Proximal Interphalangeal Joints in Rheumatoid Arthritis: A Comparison with Magnetic Resonance Imaging, Conventional Radiography and Clinical Examination. Arthritis Res. Ther. 2006, 8, R52. [Google Scholar] [CrossRef] [PubMed]
- Hammer, H.B.; Michelsen, B.; Sexton, J.; Haugen, I.K.; Provan, S.A.; Haavardsholm, E.A.; Uhlig, T.; Kvien, T.K. Swollen, but Not Tender Joints, Are Independently Associated with Ultrasound Synovitis: Results from a Longitudinal Observational Study of Patients with Established Rheumatoid Arthritis. Ann. Rheum. Dis. 2019, 78, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Hammer, H.B.; Michelsen, B.; Provan, S.A.; Sexton, J.; Lampa, J.; Uhlig, T.; Kvien, T.K. Tender Joint Count and Inflammatory Activity in Patients With Established Rheumatoid Arthritis: Results From a Longitudinal Study. Arthritis Care Res (Hoboken) 2020, 72, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Prevoo, M.L.; van ’t Hof, M.A.; Kuper, H.H.; van Leeuwen, M.A.; van de Putte, L.B.; van Riel, P.L. Modified Disease Activity Scores That Include Twenty-Eight-Joint Counts. Development and Validation in a Prospective Longitudinal Study of Patients with Rheumatoid Arthritis. Arthritis Rheum. 1995, 38, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Terslev, L.; Brahe, C.H.; Hetland, M.L.; Georgiadis, S.; Ellegaard, K.; Juul, L.; Huynh, T.; Døhn, U.M.; Fana, V.; Møller, T.; et al. Doppler Ultrasound Predicts Successful Discontinuation of Biological DMARDs in Rheumatoid Arthritis Patients in Clinical Remission. Rheumatology 2021, 60, keab276. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.F.; Tan, Y.K.; Conaghan, P.G. Monitoring in Established RA: Role of Imaging and Soluble Biomarkers. Best. Pract. Res. Clin. Rheumatol. 2015, 29, 566–579. [Google Scholar] [CrossRef]
- Carstensen, S.M.D.; Terslev, L.; Jensen, M.P.; Østergaard, M. Future Use of Musculoskeletal Ultrasonography and Magnetic Resonance Imaging in Rheumatoid Arthritis. Curr. Opin. Rheumatol. 2020, 32, 264–272. [Google Scholar] [CrossRef]
- Baker, J.F.; Conaghan, P.G.; Gandjbakhch, F. Update on Magnetic Resonance Imaging and Ultrasound in Rheumatoid Arthritis. Clin. Exp. Rheumatol. 2018, 36 (Suppl. S114), 16–23. [Google Scholar]

| Baseline Visit (n = 77) | Week 12 Visit (n = 73) | p-Values | |
|---|---|---|---|
| Age (years) | 56 ± 13 | 56 ± 13 | 1.0 |
| Female | 60 (77.9%) | 58 (79.5%) | 1.0 |
| Prednisone ≥ 7.5 mg | 10 (13%) | 6 (8.2%) | 0.21 |
| cDMARD | 50 (64.9%) | 52 (71.2%) | 0.16 |
| bDMARD/tsDMARD | 35 (45.5%) | 33 (45.2%) | 0.32 |
| TJC28 | 1 (0, 4) | 1 (0, 3) | <0.05 |
| SJC28 | 1 (0, 3) | 0 (0, 2) | <0.05 |
| SJC28 > 1 | 31 (40.3%) | 22 (30.1%) | 0.05 |
| PGA | 5 (2, 6) | 3 (1, 5) | <0.05 |
| EGA | 3 (1, 5) | 2 (0, 5) | <0.05 |
| HAQ-DI | 0.2 (0.0, 1.0) | 0.1 (0.0, 0.6) | <0.05 |
| ESR (mm/h) | 19 (10, 36) | 18 (10, 32) | 0.08 |
| CRP (mg/L) | 3.8 (1.2, 8.0) | 2.8 (1.0, 6.7) | 0.52 |
| DAS28 | 3.7 ± 1.4 | 3.3 ± 1.1 | <0.05 |
| CDAI | 13.0 ± 11.9 | 9.2 ± 8.0 | <0.05 |
| SDAI | 14.2 ± 13.4 | 9.9 ± 8.3 | <0.05 |
| ThermoJIS | 4.7 ± 2.1 | 4.4 ± 1.7 | 0.35 |
| ThermoDAI | 8.9 ± 4.0 | 7.9 ± 3.6 | <0.05 |
| ThermoDAI-CRP | 10.1 ± 5.5 | 8.5 ± 4.1 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Ivorra, I.; Taverner, D.; Codina, O.; Castell, S.; Fischer, P.; Onken, D.; Martínez-Osuna, P.; Battioui, C.; Marín-López, M.A. External Validation of the Machine Learning-Based Thermographic Indices for Rheumatoid Arthritis: A Prospective Longitudinal Study. Diagnostics 2024, 14, 1394. https://doi.org/10.3390/diagnostics14131394
Morales-Ivorra I, Taverner D, Codina O, Castell S, Fischer P, Onken D, Martínez-Osuna P, Battioui C, Marín-López MA. External Validation of the Machine Learning-Based Thermographic Indices for Rheumatoid Arthritis: A Prospective Longitudinal Study. Diagnostics. 2024; 14(13):1394. https://doi.org/10.3390/diagnostics14131394
Chicago/Turabian StyleMorales-Ivorra, Isabel, Delia Taverner, Oriol Codina, Sonia Castell, Peter Fischer, Derek Onken, Píndaro Martínez-Osuna, Chakib Battioui, and Manuel Alejandro Marín-López. 2024. "External Validation of the Machine Learning-Based Thermographic Indices for Rheumatoid Arthritis: A Prospective Longitudinal Study" Diagnostics 14, no. 13: 1394. https://doi.org/10.3390/diagnostics14131394





