Alcohol Toxicity in the Developing Cerebellum
Abstract
:1. Introduction
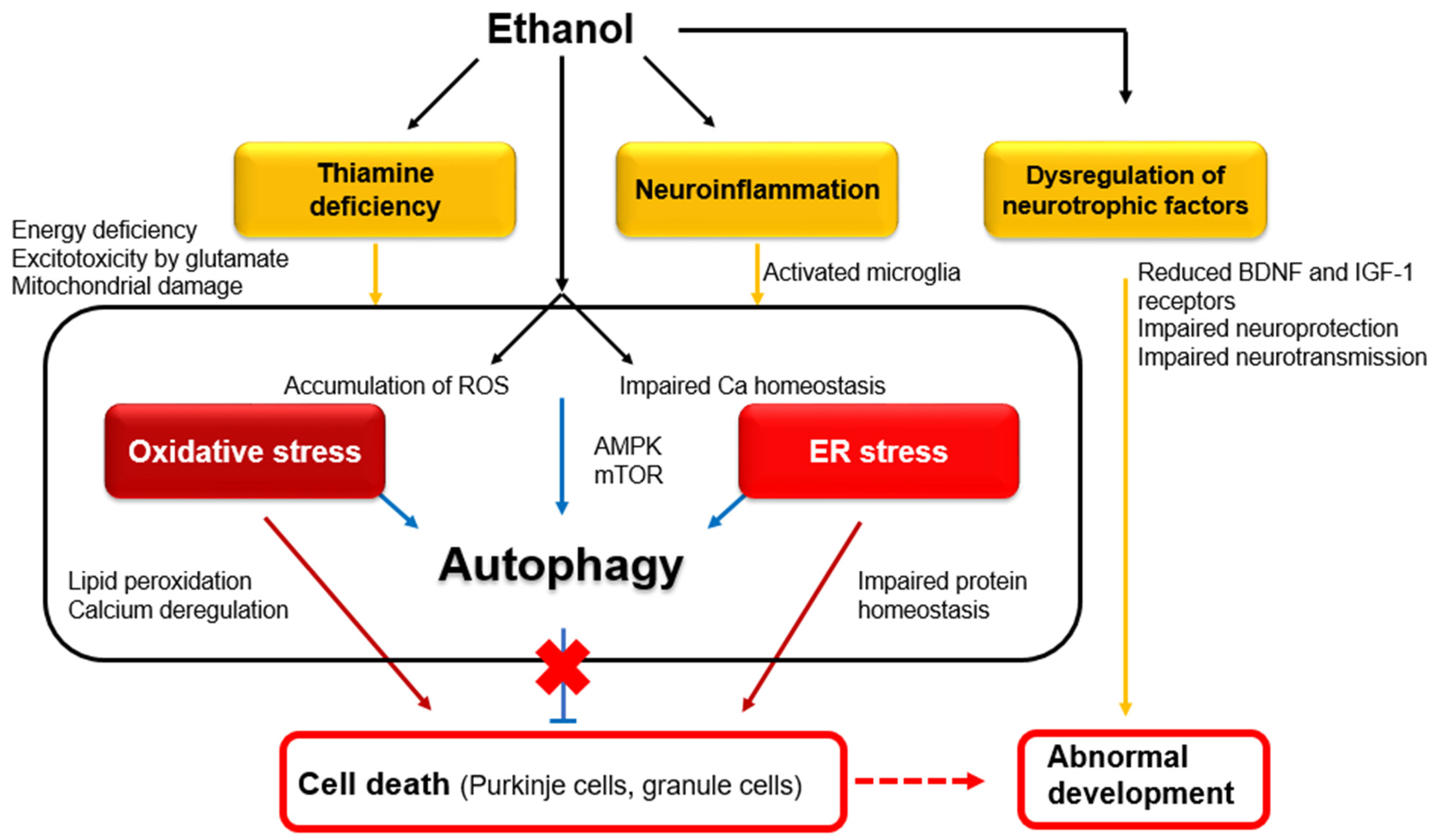
1.1. Cellular and Molecular Mechanisms Underlying Alcohol-Induced Functional Disorders and Degeneration
1.2. Effects of Alcohol on the Developing Cerebellum
2. Clinical Profiles of FASD
2.1. Pathology
2.2. Cognitive, Behavioral, and Motor Manifestations
| Neurological Symptoms | Study Design | Ref. |
|---|---|---|
| Intellectual deficits | IQ comparison FASD: n = 41; mean age 13.7 (sd 3.47); mean IQ 91.6 (sd 14.37) Control: n = 46; mean age 13.3 (sd 3.64); mean IQ 110.0 (sd 12.09) FASD group exhibited significantly lower IQ. | [30] |
| Deficits in attention, especially visually presented information | Sustained attention was measured with “AK” subtests from a commercially available Continuous Performance Task program. FASD: n = 128; control: n = 53 Both groups: mean age 15.12 (sd 0.92) FASD group exhibited significantly more errors. | [39] |
| Deficits in visual perception and construction task | Visual hierarchical stimuli consisting of large (global) letters or shapes constructed from the arrangement of numerous smaller (local) letters or shapes FASD: n = 14; control: n = 14 Both group: age 9~16 years FASD subjects exhibited impairment in recalling local features relative to global features. | [40] |
| Deficits in executive functions, e.g., verbal fluency, response inhibition, problem-solving and planning, concept formation, and working memory | Neuropsychological test battery FASD: n = 10, age 13 years Control: n = 10, age 12 years 9 months FASD subjects exhibited greater difficulty than controls in tasks that involved the manipulation of information and goal management in working memory (e.g., planning, controlled oral word association, etc.). | [44] |
| Deficits in language, especially in word order, sentence combining, and grammatical comprehension | Formal communication skill assessments FASD: n = 8, age 4.5~9 years Control: n = 8, age 3.5~6 years FASD subjects exhibited mental age-inconsistent abilities in the comprehension and use of grammatical markers both in repetition and in spontaneous language tasks. | [45] |
| Deficits in adaptive functioning, e.g., communication, socialization, and daily living skills | Short Sensory Profile scores, Adaptive Behavior Assessment System Second Edition scores, and Wechsler Intelligence Scale Fourth Edition/Wechsler Preschool and Primary Scale of Intelligence Third Edition scores FASD and non-FASD: n = 46, age 3~14 years FASD subjects showed significantly lower scores on adaptative behavior than non-FASD subjects. No correlation was observed between IQ scores and adaptive behavior scores. | [46] |
| Deficits in fine motor controls and balance | Motor coordination test (balance; finger, hand, and foot coordination) Prospective longitudinal study of adults in two groups: adults previously diagnosed with FASD (n = 90) and adults who were exposed to varying levels of alcohol (n = 402). Only subjects who had been previously identified as having a diagnosis on FASD in childhood still showed deficits on motor tasks as adults. | [47] |
3. Role of the Cerebellum in Neurodevelopmental Disorders
3.1. Closed and Reciprocal Cerebro-Cerebellar Circuits
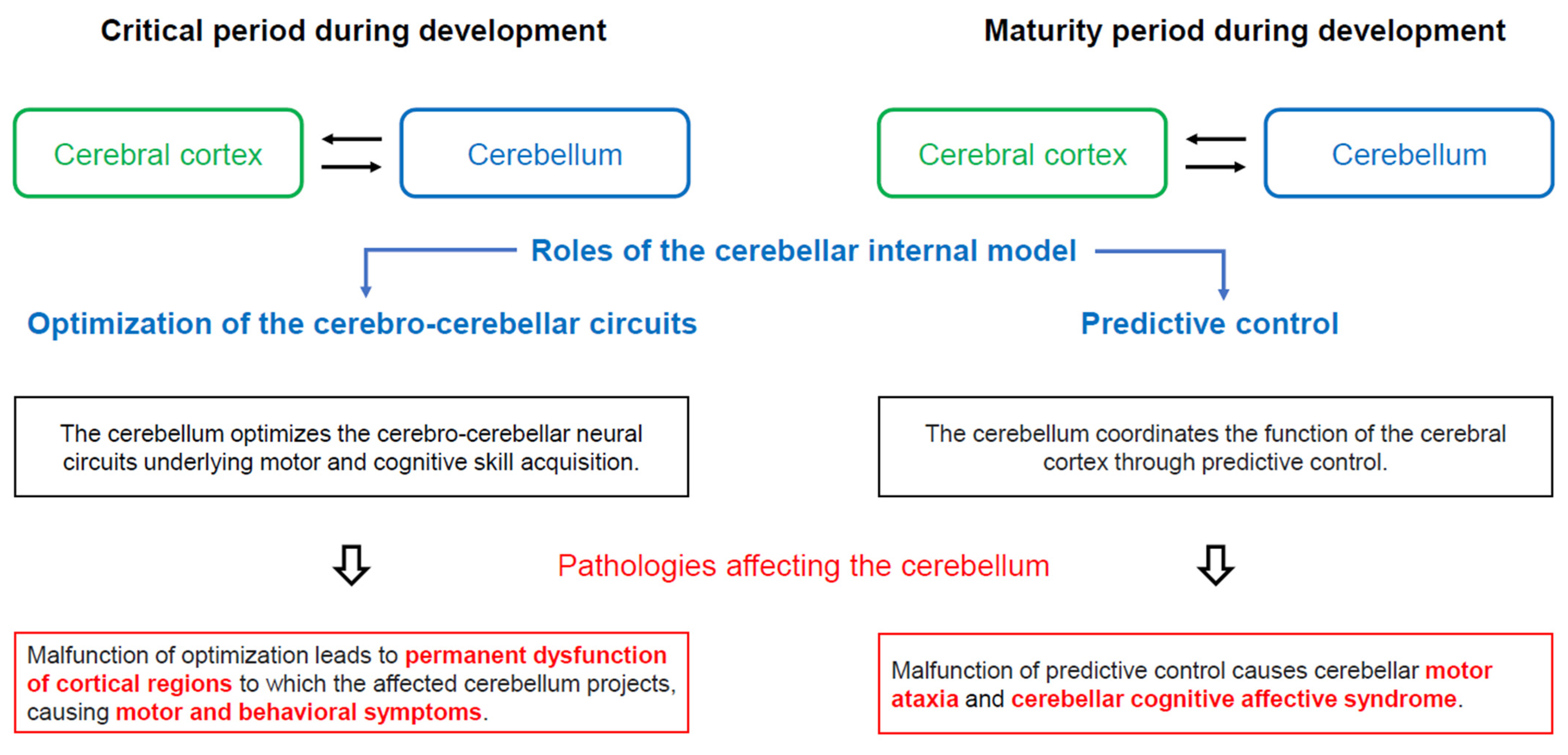
3.2. Functional Diaschisis and Sensitive Period
3.3. Autism Spectrum Disorder and Attention-Deficit-Hyperactivity Disorder as Neurodevelopmental Disorders
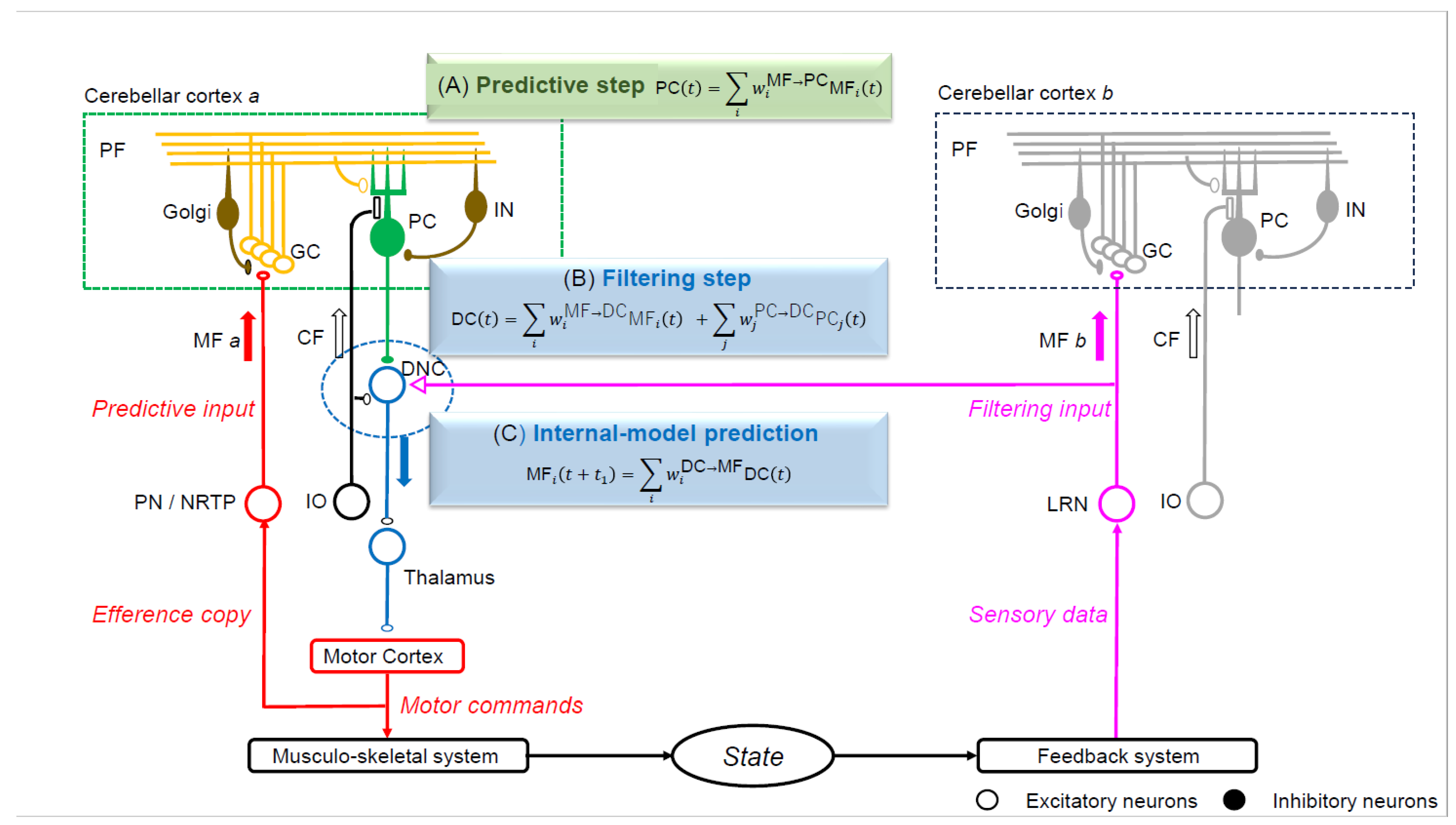
3.4. The Internal Model within the Cerebellum
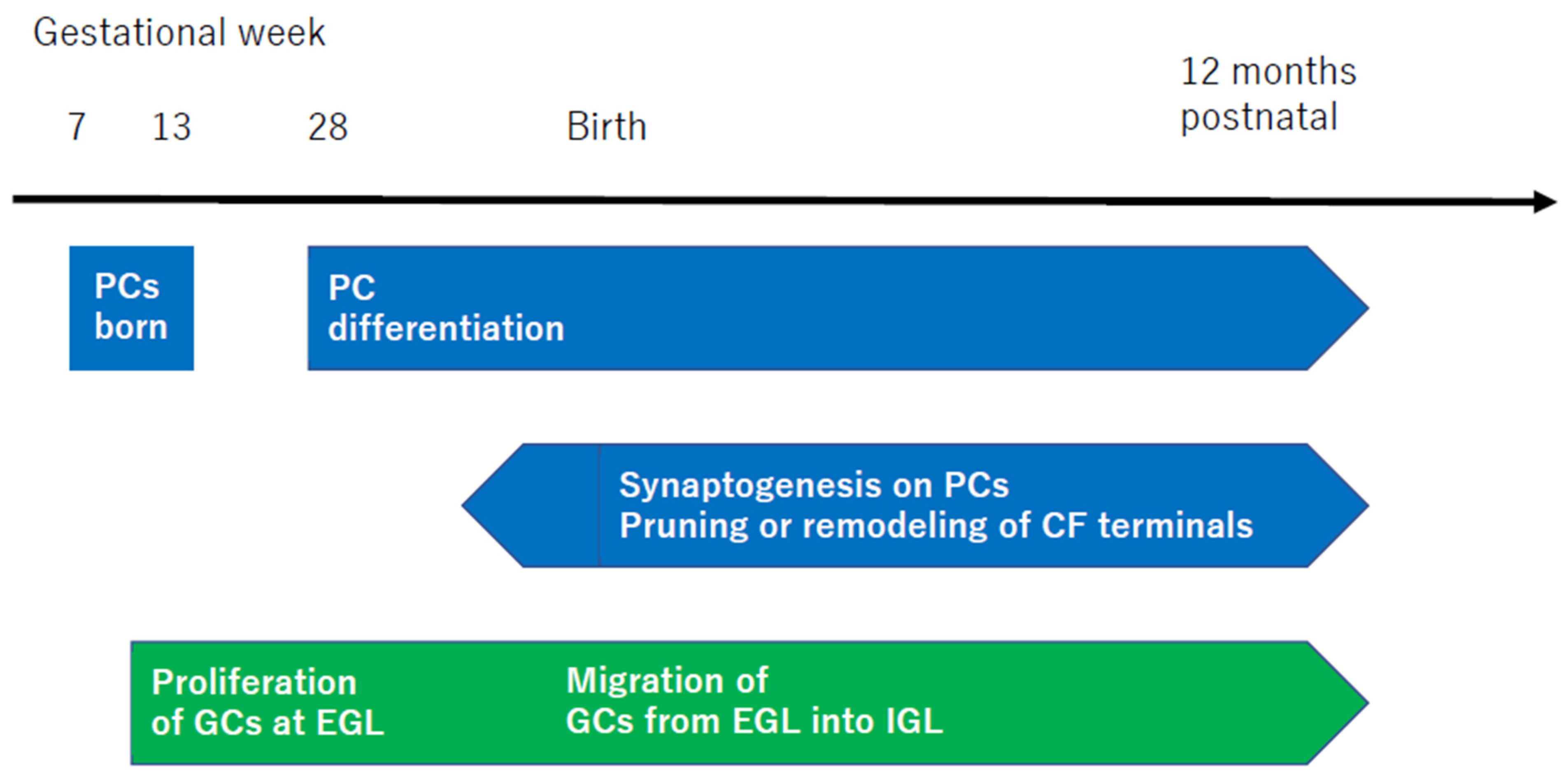
3.5. Neural Development of the Cerebellar Cortex and Failure of the Internal Model
4. Malfunctioning Connectivity between the Cerebral Cortex and Cerebellum in FASD
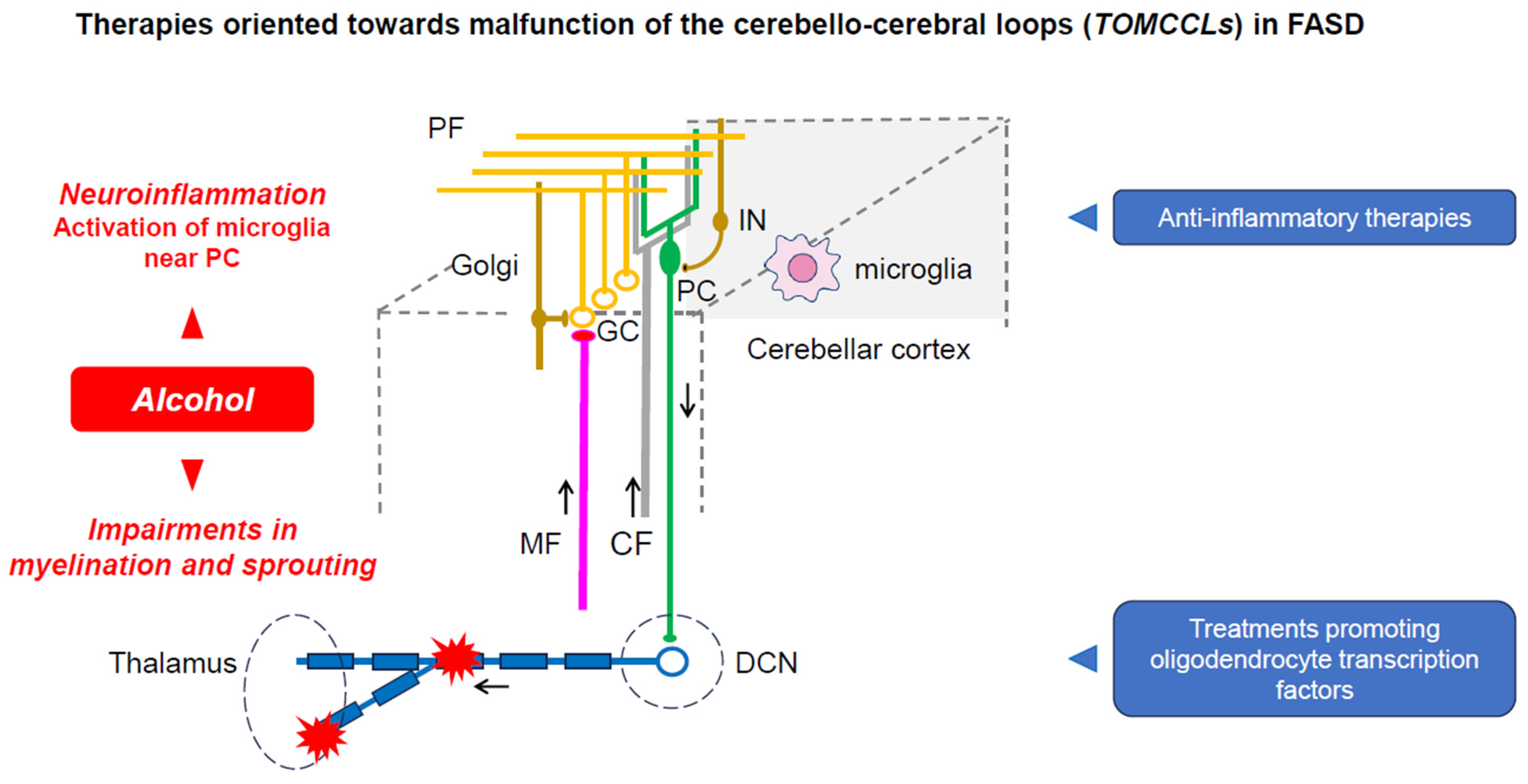
5. TOMCCLs: Therapies Oriented towards the Malfunctioning of the Cerebello-Cerebral Loops
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manto, M.U.; Jacquy, J. Alcohol toxicity in the cerebellum: Clinical aspects. In The Cerebellum and Its Disorders; Manto, M.U., Pandolfo, M., Eds.; Cambridge University Press: Cambridge, UK, 2002; pp. 336–341. [Google Scholar]
- Mitoma, H.; Manto, M.; Shaikh, A.G. Mechanisms of ethanol-induced cerebellar ataxia: Understanding of neuronal death in the cerebellum. Int. J. Environ. Res. Public Health 2021, 18, 8678. [Google Scholar] [CrossRef] [PubMed]
- Laureno, R. Nutritional cerebellar degeneration, with comments on its relationship to Wernicke disease and alcoholism. In Handbook of Clinical Neurology. Vol 103 (3rd Series) Ataxic Disorders; Subramony, S.H., Dürr, A., Eds.; Elsevier: Amsterdam, The Netherland, 2012; pp. 175–187. [Google Scholar]
- Dar, M.S. Ethanol-induced cerebellar ataxia: Cellular and molecular mechanisms. Cerebellum 2015, 14, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Victor, M.; Adams, R.D.; Mancall, E.L. A restricted form of cerebellar cortical degeneration occurring in alcoholic patients: Cerebellar cortical degenerations. Arch. Neurol. 1959, 1, 579–688. [Google Scholar] [CrossRef]
- Johnson-Greene, D.; Adams, K.M.; Gilman, S.; Kluin, K.J.; Junck, L.; Martorello, S.; Heumann, M. Impaired upper limb coordination in alcoholic cerebellar degeneration. Ann. Neurol. 1997, 54, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Mattson, S.N.; Roesch, S.C.; Glass, L.; Deweese, B.N.; Coles, C.D.; Kable, J.A.; May, P.A.; Kalberg, W.O.; Sowell, E.R.; Adnams, C.M.; et al. Further development of a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol. Clin. Exp. Res. 2013, 37, 517–528. [Google Scholar] [CrossRef]
- May, P.A.; Baete, A.; Russo, J.; Elliott, A.J.; Blankenship, J.; Kalberg, W.O.; Buckley, D.; Brooks, M.; Hasken, J.; Abdul-Rahman, O.; et al. Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics 2014, 134, 855–866. [Google Scholar] [CrossRef]
- West, J.R. Acute and long-term changes in the cerebellum following developmental exposure to ethanol. Alcohol. Alcohol. Suppl. 1993, 2, 199–202. [Google Scholar]
- Norman, A.L.; Crocker, N.; Mattson, S.N.; Riley, E.P. Neuroimaging and fetal alcohol spectrum disorders. Dev. Disabil. Res. Rev. 2009, 15, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Schmahmann, J.D.; Caplan, D. Cognition, emotion and the cerebellum. Brain 2006, 129, 290–292. [Google Scholar] [CrossRef]
- Hoche, F.; Guell, X.; Vangel, M.G.; Sherman, J.C.; Schmahmann, J.D. The cerebellar cognitive affective/Schmahmann syndrome scale. Brain 2018, 141, 248–270. [Google Scholar] [CrossRef]
- Luo, J. Effects of ethanol on the cerebellum: Advances and prospects. Cerebellum 2015, 14, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, C.J. The cerebellum and neurodevelopmental disorders. Cerebellum 2016, 15, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Riley, E.P.; McGee, C.L. Fetal alcohol spectrum disorders; An overview with emphasis on changes in brain and behavior. Exp. Biol. Med. 2005, 230, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Mattson, S.N.; Bernes, G.A.; Doyle, L.R. Fetal alcohol spectrum disorders: A review of the neurobehavioral deficits associated with prenatal alcohol exposure. Alcohol. Clin. Exp. Res. 2019, 43, 1046–1062. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, J.R.; Riley, E.P.; Charness, M.E. Clinical presentation, diagnosis, and management of fetal alcohol spectrum disorder. Lancet Neurol. 2019, 18, 760–770. [Google Scholar] [CrossRef]
- Sans-Fitó, A.; Solerdelcoll, A.; Boix-Lluch, C.; Serra-Amaya, C.; Serra-Grabulosa, J.M.; Caldú, X. Fetal alcohol spectrum disorder. An underdiagnosed neuro-development disorder of uncertain prognosis. Medicina (B Aires) 2019, 79 (Suppl. S1), 62–67. [Google Scholar]
- Jańczewska, I.; Wierzba, J.; Cichoń-Kotek, M.; Jańczewska, A. Fetal alcohol spectrum disorders—Diagnostic difficulties in the neonatal period and new diagnostic approaches. J. Mother Child 2019, 23, 60–66. [Google Scholar]
- Maya-Enero, S.; Ramis-Fernández, S.M.; Astals-Vizcaino, M.; García-Algar, Ó. Neurocognitive and behavioral profile of fetal alcohol spectrum disorder. An. Pediatr. (Engl. Ed.) 2021, 95, 208.e1–208.e9. [Google Scholar] [CrossRef]
- Gomez, D.A.; Abdul-Rahman, O.A. Fetal alcohol spectrum disorders: Current state of diagnosis and treatment. Curr. Opin. Pediatr. 2021, 33, 570–575. [Google Scholar] [CrossRef]
- Popova, S.; Dozet, D.; Shield, K.; Rehm, J.; Burd, L. Alcohol’s Impact on the Fetus. Nutrients 2021, 13, 3452. [Google Scholar] [CrossRef]
- Kautz-Turnbull, C.; Petrenko, C.L.M. A meta-analytic review of adaptive functioning in fetal alcohol spectrum disorders, and the effect of IQ, executive functioning, and age. Alcohol. Clin. Exp. Res. 2021, 45, 2430–2447. [Google Scholar] [CrossRef] [PubMed]
- Ritfeld, G.J.; Kable, J.A.; Holton, J.E.; Coles, C.D. Psychopharmacological treatments in children with fetal alcohol spectrum disorders: A review. Child Psychiatry Hum. Dev. 2022, 53, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Roozen, S.; Ehrhart, F. Fetal alcohol spectrum disorders and the risk of crime. Handb. Clin. Neurol. 2023, 197, 197–204. [Google Scholar]
- Basavarajappa, B.S.; Subbanna, S. Synaptic plasticity abnormalities in fetal alcohol spectrum disorders. Cells 2023, 12, 442. [Google Scholar] [CrossRef] [PubMed]
- Popova, S.; Charness, M.E.; Burd, L.; Crawford, A.; Hoyme, H.E.; Mukherjee, R.A.S.; Riley, E.P.; Elliott, E.J. Fetal alcohol spectrum disorders. Nat. Rev. Dis. Primers. 2023, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.L.; Smith, D.W. Recognition of the fetal alcohol syndrome in early infancy. Lancet 1973, 302, 999–1001. [Google Scholar] [CrossRef]
- Bertrand, J.; Floyd, R.L.; Weber, M.K. Guidelines for identifying and referring persons with fetal alcohol syndrome. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2005, 54, 1–15+CE-1-CE-4. [Google Scholar]
- Mattson, S.N.; Roesch, S.C.; Fagerlund, A.; Autti-Ramo, I.; Jones, K.L.; May, P.A.; Adnams, C.M.; Konovalova, V.; Riley, E.P.; CIFASD. Toward a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol. Clin. Exp. Res. 2010, 34, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Chambers, C.D.; Kalberg, W.O.; Zellner, J.; Feldman, H.; Buckley, D.; Kopald, D.; Hasken, J.M.; Xu, R.; Honerkamp-Smith, G.; et al. Prevalence of fetal alcohol spectrum disorders in 4 US communities. JAMA 2018, 319, 474–482. [Google Scholar] [CrossRef]
- Roussotte, F.F.; Sulik, K.K.; Mattson, S.N.; Riley, E.P.; Jones, K.L.; Adnams, C.M.; May, P.A.; O’Connor, M.J.; Narr, K.L.; Sowell, E.R. Regional brain volume reductions relate to facial dysmorphology and neurocognitive function in fetal alcohol spectrum disorders. Hum. Brain Mapp. 2012, 33, 920–937. [Google Scholar] [CrossRef]
- Archibald, S.L.; Fennema-Notestine, C.; Gamst, A.; Riley, E.P.; Mattson, S.N.; Jernigan, T.L. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev. Med. Child Neurol. 2001, 43, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Sowell, E.R.; Thompson, P.M.; Mattson, S.N.; Tessner, K.D.; Jernigan, T.L.; Riley, E.P.; Toga, A.W. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cereb. Cortex 2002, 12, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, A.; Lebel, C.; Rasmussen, C.; Andrew, G.; Beaulieu, C. Extensive deep gray matter volume reductions in children and adolescents with fetal alcohol spectrum disorders. Alcohol. Clin. Exp. Res. 2011, 35, 1404–1417. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Phillips, O.R.; Kan, E.; Sulik, K.K.; Mattson, S.N.; Riley, E.P.; Jones, K.L.; Adnams, C.M.; May, P.A.; O’Connor, M.J.; et al. Callosal thickness reductions relate to facial dysmorphology in Fetal Alcohol Spectrum Disorders. Alcohol. Clin. Exp. Res. 2012, 36, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Donald, K.A.; Eastman, E.; Howells, F.M.; Adnams, C.; Riley, E.P.; Woods, R.P.; Narr, K.L.; Stein, D.J. Neuroimaging effects of prenatal alcohol exposure on the developing human brain: A magnetic resonance imaging review. Acta Neuropsychiatr. 2015, 27, 251–269. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.V.; Moore, E.M.; Lane, B.; Pohl, K.M.; Riley, E.P.; Pfefferbaum, A. Graded cerebellar lobular volume deficits in adolescents and young adults with fetal alcohol spectrum disorders (FASD). Cereb. Cortex 2020, 30, 4729–4746. [Google Scholar] [CrossRef]
- Coles, C.D.; Platzman, K.A.; Lynch, M.E.; Freides, D. Auditory and visual sustained attention in adolescents prenatally exposed to alcohol. Alcohol. Clin. Exp. Res. 2002, 26, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Mattson, S.N.; Gramling, L.; Riley, E.P.; Delis, D.C.; Jones, K.L. Global-local processing in children prenatally exposed to alcohol. Child Neuropsychol. 1996, 2, 165–175. [Google Scholar] [CrossRef]
- Mattson, S.N.; Riley, E.P.; Gramling, L.; Delis, D.C.; Jones, K.L. Heavy prenatal alcohol exposure with or without physical features of fetal alcohol syndrome leads to IQ deficits. J. Pediatr. 1997, 131, 718–721. [Google Scholar] [CrossRef]
- Schonfeld, A.M.; Mattson, S.N.; Lang, A.R.; Delis, D.C.; Riley, E.P. Verbal and nonverbal fluency in children with heavy prenatal alcohol exposure. J. Stud. Alcohol. 2001, 62, 239–246. [Google Scholar] [CrossRef]
- Connor, P.D.; Sampson, P.D.; Bookstein, F.L.; Barr, H.M.; Streissguth, A.P. Direct and indirect effects of prenatal alcohol damage on executive function. Dev. Neuropsychol. 2000, 18, 331–354. [Google Scholar] [CrossRef] [PubMed]
- Kodituwakku, P.W.; Handmaker, N.S.; Cutler, S.K.; Weathersby, E.K.; Handmaker, S.D. Specific impairments in self-regulation in children exposed to alcohol prenatally. Alcohol. Clin. Exp. Res. 1995, 19, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Warr-Leeper, G.A.; Leeper, H.A., Jr. Fetal alcohol syndrome: A description of oral motor, articulatory, short-term memory, grammatical, and semantic abilities. J. Commun. Disord. 1990, 23, 97–124. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.L.; Agnihotri, S.; Keightley, M. Sensory processing and adaptive behavior deficits of children across the fetal alcohol spectrum disorder continuum. Alcohol. Clin. Exp. Res. 2010, 34, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Connor, P.D.; Sampson, P.D.; Streissguth, A.P.; Bookstein, F.L.; Barr, H.M. Effects of prenatal alcohol exposure on fine motor coordination and balance: A study of two adult samples. Neuropsychologia 2006, 44, 744–751. [Google Scholar] [CrossRef]
- Sathyanesan, A.; Zhou, J.; Scafidi, J.; Heck, D.H.; Sillitoe, R.V.; Gallo, V. Emerging connections between cerebellar development, behavior, and complex brain disorders. Nat. Rev. Neurosci. 2019, 20, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.I.; Tsukahara, N. Cerebrocerebellar communication systems. Physiol. Rev. 1974, 54, 957–1006. [Google Scholar] [CrossRef] [PubMed]
- Strick, P.L.; Dum, R.P.; Fiez, J.A. Cerebellum and nonmotor function. Annu. Rev. Neurosci. 2009, 32, 413–434. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, C.J.; Schmahmann, J.D. Functional topography of the human cerebellum. Handb. Clin. Neurol. 2018, 154, 59–70. [Google Scholar]
- Wang, S.S.; Kloth, A.D.; Badura, A. The cerebellum, sensitive periods, and autism. Neuron 2014, 83, 518–532. [Google Scholar] [CrossRef]
- Hubel, D.H.; Wiesel, T.N. Receptive fields of cells in striate cortex of very young, visually inexperienced kittens. J. Neurophysiol. 1963, 26, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Wells, E.M.; Walsh, K.S.; Khademian, Z.P.; Keating, R.F.; Packer, R.J. The cerebellar mutism syndrome and its relation to cerebellar cognitive function and the cerebellar cognitive affective disorder. Dev. Disabil. Res. Rev. 2008, 14, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Riva, D.; Giorgi, C. The cerebellum contributes to higher functions during development: Evidence from a series of children surgically treated for posterior fossa tumours. Brain 2000, 123 Pt 5, 1051–1061. [Google Scholar] [CrossRef]
- Boltshauser, E. Cerebellum-small brain but large confusion: A review of selected cerebellar malformations and disruptions. Am. J. Med. Genet. A 2004, 126A, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, M.E.; du Plessis, A.J.; Sullivan, N.; Guizard, N.; Zhang, X.; Robertson, R.L.; Limperopoulos, C. Regional cerebellar volumes predict functional outcome in children with cerebellar malformations. Cerebellum 2012, 11, 531–542. [Google Scholar] [CrossRef]
- Limperopoulos, C.; Bassan, H.; Gauvreau, K.; Robertson, R.L., Jr.; Sullivan, N.R.; Benson, C.B.; Avery, L.; Stewart, J.; Soul, J.S.; Ringer, S.A.; et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics 2007, 120, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.M.; Howland, C.P.; Ren, C.; Howland, C.; Vernino, A.; Tsai, P.T. A critical period for development of cerebellar-mediated autism-relevant social behavior. J. Neurosci. 2022, 42, 2804–2823. [Google Scholar] [CrossRef]
- Spoto, G.; Amore, G.; Vetri, L.; Quatrosi, G.; Cafeo, A.; Gitto, E.; Nicotera, A.G.; Di Rosa, G. Cerebellum and Prematurity: A complex interplay between disruptive and dysmaturational events. Front. Syst. Neurosci. 2021, 15, 655164. [Google Scholar] [CrossRef]
- Coviello, C.; Keunen, K.; Kersbergen, K.J.; Groenendaal, F.; Leemans, A.; Peels, B.; Isgum, I.; Viergever, M.A.; de Vries, L.S.; Buonocore, G.; et al. Effects of early nutrition and growth on brain volumes, white matter microstructure, and neurodevelopmental outcome in preterm newborns. Pediatr. Res. 2018, 83, 102–110. [Google Scholar] [CrossRef]
- Hoffman, J.F.; Wright, C.L.; McCarthy, M.M.J. A critical period in Purkinje cell development is mediated by local estradiol synthesis, disrupted by inflammation, and has enduring consequences only for males. J. Neurosci. 2016, 36, 10039–10049. [Google Scholar] [CrossRef]
- Timmann, D.; Brandauer, B.; Hermsdorfer, J.; Ilg, W.; Konczak, J.; Gerwig, M.; Gizewski, E.R.; Schoch, B. Lesion-symptom mapping of the human cerebellum. Cerebellum 2008, 7, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Kipping, J.A.; Tuan, T.A.; Fortier, M.V.; Qiu, A. Asynchronous development of cerebellar, cerebello-cortical, and cortico-cortical functional networks in infancy, childhood, and adulthood. Cereb. Cortex 2017, 27, 5170–5184. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, C.J. Distinct regions of the cerebellum show gray matter decreases in autism, ADHD, and developmental dyslexia. Front. Syst. Neurosci. 2014, 8, 92. [Google Scholar] [CrossRef]
- Cundari, M.; Vestberg, S.; Gustafsson, P.; Gorcenco, S.; Rasmussen, A. Neurocognitive and cerebellar function in ADHD, autism and spinocerebellar ataxia. Front. Syst. Neurosci. 2023, 17, 1168666. [Google Scholar] [CrossRef]
- Limperopoulos, C.; Soul, J.S.; Haidar, H.; Huppi, P.S.; Bassan, H.; Warfield, S.K.; Robertson, R.L.; Moore, M.; Akins, P.; Volpe, J.J.; et al. Impaired trophic interactions between the cerebellum and the cerebrum among preterm infants. Pediatrics 2005, 116, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Mostofsky, S.H.; Powell, S.K.; Simmonds, D.J.; Goldberg, M.C.; Caffo, B.; Pekar, J.J. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain 2009, 132, 2413–2425. [Google Scholar] [CrossRef]
- Kana, R.K.; Maximo, J.O.; Williams, D.L.; Keller, T.A.; Schipul, S.E.; Cherkassky, V.L.; Minshew, N.J.; Just, M.A. Aberrant functioning of the theory-of-mind network in children and adolescents with autism. Mol. Autism 2015, 6, 59. [Google Scholar] [CrossRef]
- Ivanov, I.; Murrough, J.W.; Bansal, R.; Hao, X.; Peterson, B.S. Cerebellar morphology and the effects of stimulant medications in youths with attention deficit-hyperactivity disorder. Neuropsychopharmacology 2014, 39, 718–726. [Google Scholar] [CrossRef]
- Rubia, K.; Halari, R.; Cubillo, A.; Mohammad, A.M.; Brammer, M.; Taylo, R.E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naive children with ADHD during a rewarded continuous performance task. Neuropharmacology 2009, 57, 640–652. [Google Scholar] [CrossRef]
- Wolpert, D.M.; Ghahramani, Z.; Jordan, M.I. An internal model for sensorimotor integration. Science 1995, 269, 1880–1882. [Google Scholar] [CrossRef]
- Todorov, E. Optimality principles in sensorimotor control. Nat. Neurosci. 2004, 7, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Popa, L.S.; Hewitt, A.L.; Ebner, T.J. Predictive and feedback performance errors are signaled in the simple spike discharge of individual Purkinje cells. J. Neurosci. 2012, 32, 15345–15358. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Ishikawa, T.; Lee, J.; Kakei, S. The cerebro-cerebellum as a locus of forward model. Front. Syst. Neurosci. 2020, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Ishikawa, T.; Kakei, S. Neural evidence of the cerebellum as a state predictor. Cerebellum 2019, 18, 349–371. [Google Scholar] [CrossRef] [PubMed]
- Mitoma, H.; Kakei, S.; Tanaka, H.; Manto, M. Morphological and functional principles governing the plasticity reserve in the cerebellum: The cortico-deep cerebellar nuclei loop model. Biology 2023, 12, 1435. [Google Scholar] [CrossRef]
- Tseng, Y.W.; Diedrichsen, J.; Krakauer, J.W.; Shadmehr, R.; Bastian, A.J. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J. Neurophysiol. 2007, 98, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Mitoma, H.; Buffo, A.; Gelfo, F.; Guell, X.; Fucà, E.; Kakei, S.; Lee, J.; Manto, M.; Petrosini, L.; Shaikh, A.G.; et al. Consensus paper. Cerebellar reserve: From cerebellar physiology to cerebellar disorders. Cerebellum 2019, 19, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Schmahmann, J.D. From movement to thought: Anatomic substrates of the cerebellar contribution to cognitive processing. Hum. Brain Mapp. 1996, 4, 174–198. [Google Scholar] [CrossRef]
- Wang, V.Y.; Zoghbi, H.Y. Genetic regulation of cerebellar development. Nat. Rev. Neurosci. 2001, 2, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Ichikawa, R.; Kitamura, K.; Watanabe, M.; Kano, M. Translocation of a “winner” climbing fiber to the Purkinje cell dendrite and subsequent elimination of “losers” from the soma in developing cerebellum. Neuron 2009, 63, 106–118. [Google Scholar] [CrossRef]
- Sugihara, I. Organization and remodeling of the olivocerebellar climbing fiber projection. Cerebellum 2006, 5, 15–22. [Google Scholar] [CrossRef] [PubMed]
- White, J.J.; Sillitoe, R.V. Development of the cerebellum: From gene expression patterns to circuits maps. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Kingdon, D.; Cardoso, C.; McGrath, J.J. Research Review: Executive function deficits in fetal alcohol spectrum disorders and attention-deficit/hyperactivity disorder—A meta-analysis. J. Child Psychol. Psychiatry. 2016, 57, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, X.; Peltier, S.; Hu, X.; Coles, C.D.; Lynch, M.E. Occipital-temporal reduction and sustained visual attention deficit in prenatal alcohol exposed adults. Brain Imaging Behav. 2008, 2, 39–48. [Google Scholar] [CrossRef]
- Fryer, S.L.; Tapert, S.F.; Mattson, S.N.; Paulus, M.P.; Spadoni, A.D.; Riley, E.P. Prenatal alcohol exposure affects frontal-striatal BOLD response during inhibitory control. Alcoholism Clin. Exp. Res. 2007, 31, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Malisza, K.L.; Buss, J.L.; Bolster, R.B.; de Gervai, P.D.; Woods-Frohlich, L.; Summers, R.; Clancy, C.A.; Chudley, A.E.; Longstaffe, S. Comparison of spatial working memory in children with prenatal alcohol exposure and those diagnosed with ADHD; A functional magnetic resonance imaging study. J. Neurodevelop. Disord. 2012, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Kane, C.J.M.; Douglas, J.C.; Rafferty, T.; Johnson, J.W.; Niedzwiedz-Massey, V.M.; Phelan, K.D.; Majewska, A.K.; Drew, P.D. Ethanol modulation of cerebellar neuroinflammation in a postnatal mouse model of fetal alcohol spectrum disorders. J. Neurosci. Res. 2021, 99, 1986–2007. [Google Scholar] [CrossRef]
- Niedzwiedz-Massey, V.M.; Douglas, J.C.; Rafferty, T.; Kane, C.J.M.; Drew, P.D. Ethanol effects on cerebellar myelination in a postnatal mouse model of fetal alcohol spectrum disorders. Alcohol 2021, 96, 43–53. [Google Scholar] [CrossRef]
- Treit, S.; Lebel, C.; Baugh, L.; Rasmussen, C.; Andrew, G.; Beaulieu, C. Longitudinal MRI reveals altered trajectory of brain development during childhood and adolescence in fetal alcohol spectrum disorders. J. Neurosci. 2013, 33, 10098–10109. [Google Scholar] [CrossRef]
- Chatterton, B.J.; Nunes, P.T.; Savage, L.M. The effect of chronic exposure and thiamine deficiency on myelin-related genes in the cortex and the cerebellum. Alcohol. Clin. Exp. Res. 2020, 44, 2481–2493. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitoma, H.; Manto, M.; Shaikh, A.G. Alcohol Toxicity in the Developing Cerebellum. Diagnostics 2024, 14, 1415. https://doi.org/10.3390/diagnostics14131415
Mitoma H, Manto M, Shaikh AG. Alcohol Toxicity in the Developing Cerebellum. Diagnostics. 2024; 14(13):1415. https://doi.org/10.3390/diagnostics14131415
Chicago/Turabian StyleMitoma, Hiroshi, Mario Manto, and Aasef G. Shaikh. 2024. "Alcohol Toxicity in the Developing Cerebellum" Diagnostics 14, no. 13: 1415. https://doi.org/10.3390/diagnostics14131415





