Abstract
Colon cancer is a prevalent and potentially fatal disease that demands early and accurate diagnosis for effective treatment. Traditional diagnostic approaches for colon cancer often face limitations in accuracy and efficiency, leading to challenges in early detection and treatment. In response to these challenges, this paper introduces an innovative method that leverages artificial intelligence, specifically convolutional neural network (CNN) and Fishier Mantis Optimizer, for the automated detection of colon cancer. The utilization of deep learning techniques, specifically CNN, enables the extraction of intricate features from medical imaging data, providing a robust and efficient diagnostic model. Additionally, the Fishier Mantis Optimizer, a bio-inspired optimization algorithm inspired by the hunting behavior of the mantis shrimp, is employed to fine-tune the parameters of the CNN, enhancing its convergence speed and performance. This hybrid approach aims to address the limitations of traditional diagnostic methods by leveraging the strengths of both deep learning and nature-inspired optimization to enhance the accuracy and effectiveness of colon cancer diagnosis. The proposed method was evaluated on a comprehensive dataset comprising colon cancer images, and the results demonstrate its superiority over traditional diagnostic approaches. The CNN–Fishier Mantis Optimizer model exhibited high sensitivity, specificity, and overall accuracy in distinguishing between cancer and non-cancer colon tissues. The integration of bio-inspired optimization algorithms with deep learning techniques not only contributes to the advancement of computer-aided diagnostic tools for colon cancer but also holds promise for enhancing the early detection and diagnosis of this disease, thereby facilitating timely intervention and improved patient prognosis. Various CNN designs, such as GoogLeNet and ResNet-50, were employed to capture features associated with colon diseases. However, inaccuracies were introduced in both feature extraction and data classification due to the abundance of features. To address this issue, feature reduction techniques were implemented using Fishier Mantis Optimizer algorithms, outperforming alternative methods such as Genetic Algorithms and simulated annealing. Encouraging results were obtained in the evaluation of diverse metrics, including sensitivity, specificity, accuracy, and F1-Score, which were found to be 94.87%, 96.19%, 97.65%, and 96.76%, respectively.
1. Introduction
In recent years, colon cancer has emerged as a significant cause of mortality, affecting millions of individuals worldwide [1,2,3]. Lifestyle factors, aging, and genetics are known to contribute to the development of colon cancer, with research establishing a clear link between the consumption of processed meats and alcohol and an increased risk of developing the disease [4,5]. Moreover, studies indicate a higher prevalence of this disease in developed countries, with approximately 65% of cases diagnosed in these regions [6].
However, traditional approaches to colon cancer diagnosis present significant challenges in terms of early identification and accurate diagnosis, making it a prevalent and life-threatening disease [5]. However, traditional approaches to colon cancer diagnosis present significant challenges in terms of early identification and accurate diagnosis, making it a prevalent and life-threatening disease [1,2,3]. To address these challenges and enhance the accuracy and effectiveness of colon cancer diagnosis, advanced technologies such as machine learning and deep learning have been explored in medical image analysis [7,8,9]. Artificial intelligence offers solutions to the limitations of traditional approaches by utilizing techniques such as artificial neural networks (ANNs), support vector machine (SVM), fuzzy methods, expert systems, and metaheuristic methods for disease diagnosis using medical images [10,11,12,13,14,15,16,17].
Additionally, innovative methods for diagnosing and categorizing colon cancer histopathological images are essential to improve the precision of diagnosis and ultimately improve patient outcomes. This revised introduction provides a clearer and more structured overview of the challenges in colon cancer diagnosis and the role of artificial intelligence in addressing these challenges. It eliminates repetitions and organizes the information in a more focused and coherent manner.
In general, progress in medical image analysis shows potential for transforming early detection and diagnosis of colon cancer, ultimately leading to improved outcomes for patients. Researchers are striving to optimize the diagnostic process and enhance the overall management of this life-threatening illness by leveraging state-of-the-art technologies and innovative algorithms. Given the significant global burden of colon cancer, innovative approaches are continuously sought to enhance diagnostic accuracy and treatment efficacy. This research addresses this need by focusing on the following key areas:
- introduction of an innovative method for categorizing colon cancer histopathological images without feature selection using PCA;
- utilization of an intelligent feature selection method with FMO algorithm to enhance the precision of colon cancer diagnosis;
- integration of AI, deep learning, and bio-inspired optimization algorithms for improved early detection and diagnosis of colon cancer;
- focus on streamlining the detection process, improving diagnostic accuracy, and ultimately enhancing patient outcomes;
- potential revolutionization of cancer diagnosis and treatment through cutting-edge technologies in medical imaging analysis.
The study organization provides a brief outline of the content in each section. Section 2 reviews the literature, Section 3 describes the materials and methods, Section 4 and Section 5 presents the results and discussion, and Section 6 provides the conclusion. This overview helps readers understand the structure of the study and anticipate the content of each section.
2. Literature Review
In recent years, colon cancer has claimed numerous lives, making it a significant concern worldwide [18]. Preventive measures, including a healthy lifestyle and regular screening, are essential for reducing the risk of colon cancer [19]. Diagnostic imaging plays a crucial role in identifying various diseases, including Alzheimer’s disease, Multiple Sclerosis, and colorectal carcinoma [20]. With its life-threatening nature, colon cancer requires early detection and accurate diagnosis for effective treatment [21].
Medical imaging modalities such as CT scans and MRI techniques aid in diagnosing colon cancer by detecting abnormal cell growth in the colon [22]. Lifestyle factors, aging, and genetics contribute to the development of colon cancer, with processed meats and alcohol consumption being associated with increased risk [23]. Screening methods like colonoscopy and histopathological screenings are vital for early detection and prevention [24]. Medical imaging, coupled with advancements in machine learning and deep learning, shows promise in improving the accuracy of colon cancer diagnosis [25].
Table 1 summarizes various research studies aimed at improving the diagnosis and prognosis of colorectal cancer through innovative methodologies such as automated algorithms, convolutional neural networks (CNNs), and integration of medical data with artificial intelligence (AI) techniques. Each study outlines its aims, advantages, disadvantages, and results, showcasing diverse approaches to addressing the challenges in colorectal cancer diagnosis and treatment.

Table 1.
Advancements in colorectal cancer diagnosis and prognosis: a comparative study of innovative approaches.
3. Material and Methods
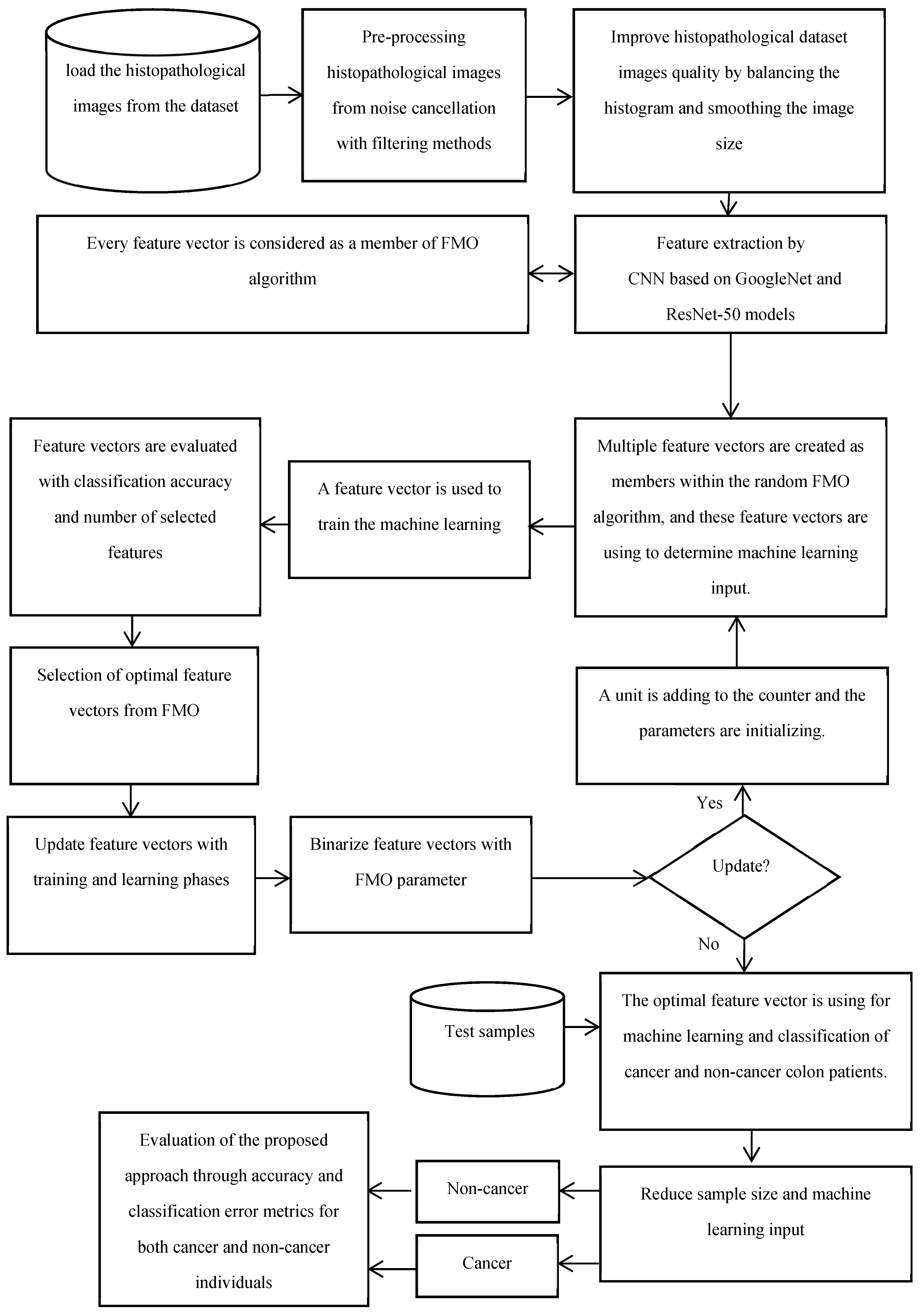
The proposed method for diagnosing both cancer and non-cancer patients is designed to integrate several steps for the analysis of histopathological images. Initially, the method involves gathering and pre-processing sample images from a dataset of colon diseases. The pre-processing step includes noise elimination, adjustment, and image quality enhancement techniques such as histogram balancing. The research employs color images with a light intensity range of 0 to 255 for each channel. The proposed methodology combines the Fishier Mantis Optimizer (FMO) algorithm with convolutional neural networks (CNNs) to enhance the accuracy and reliability of colon cancer diagnosis. This integration aims to improve model performance and interpretability by optimizing feature selection during CNN training. The method utilizes convolutional neural networks based on GoogleNet and ResNet-50 for feature extraction from histopathological images. Textural features of the images are extracted using CNN methods, and essential features for machine learning are calculated. Feature selection is represented as a binary challenge, with a feature vector of n dimensions indicating the presence of n features, where each element is either zero or one. The FMO algorithm is employed for feature selection due to its ability to simulate learning behavior in convolutional algorithms, its superior accuracy compared to other meta-heuristic optimization methods, and its capability to perform global and local searches optimally. The subsequent stage involves training the machine learning algorithm using the optimal feature vector for the classification of cancer and non-cancer histopathological images. The proposed method also includes creating an optimal feature vector and employing machine learning for dimensionality reduction and classification. The final stage involves evaluating the proposed method using testing data. The phases of the proposed method for distinguishing between cancer and non-cancer colon images include collecting histopathological samples related to colon diseases.
- ▪
- The samples are pre-processed to eliminate noise and enhance image quality.
- ▪
- Feature extraction is performed using CNNs based on GoogleNet and ResNet-50.
- ▪
- Textural features of the images are extracted using CNN methods.
- ▪
- Essential features for machine learning are calculated from the images.
- ▪
- Feature selection is represented as a binary challenge with a feature vector of n dimensions.
- ▪
- The FMO algorithm is utilized for feature selection due to its superior accuracy and optimization capabilities.
- ▪
- The machine learning algorithm is trained using the optimal feature vector for classification.
- ▪
- Machine learning is employed for dimensionality reduction and classification of the images.
- ▪
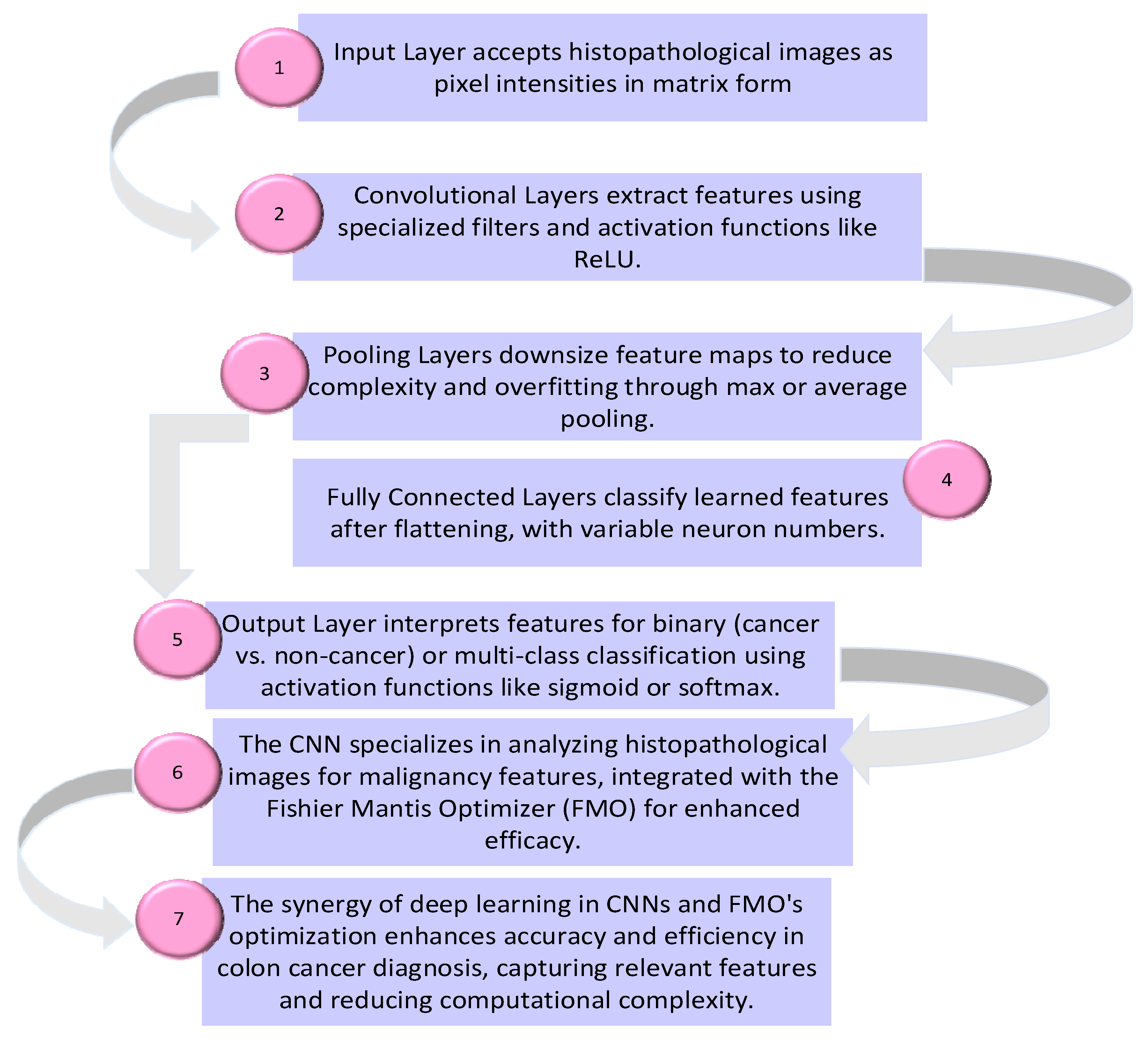
- The proposed method is evaluated using testing data to assess its performance in distinguishing between cancer and non-cancer colon images. Overall, the proposed method integrates traditional data preprocessing techniques with innovative feature selection using the FMO algorithm and CNN training to enhance the accuracy and reliability of colon cancer diagnosis. The proposed methodology’s framework for the diagnosis of patients with colon cancer is shown in Figure 1. The visual representation of the model conceptual framework in Figure 2 illustrates the seamless integration of these components, highlighting the novel approach taken in this research.
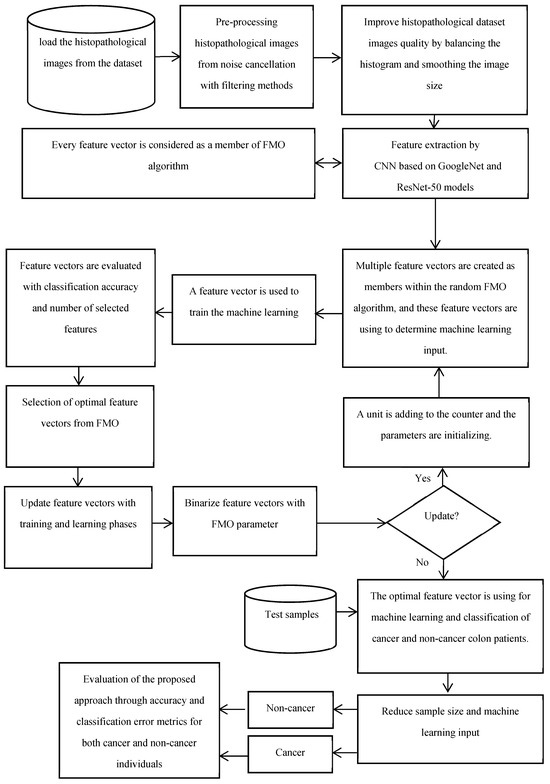 Figure 1. The proposed methodology’s framework for the diagnosis of patients with colon cancer.
Figure 1. The proposed methodology’s framework for the diagnosis of patients with colon cancer.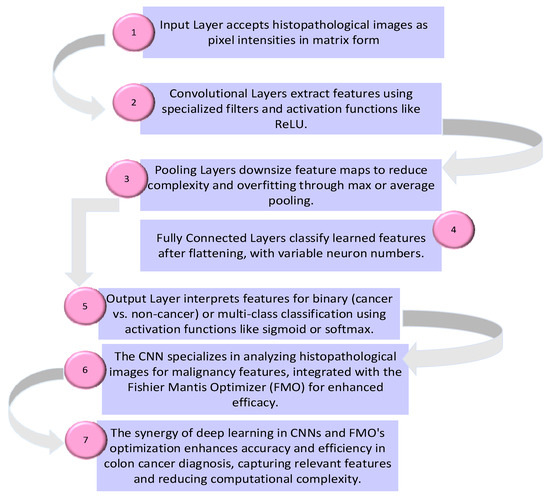 Figure 2. Convolutional neural network for colon cancer diagnosis.
Figure 2. Convolutional neural network for colon cancer diagnosis.
- Fishier Mantis Optimizer Algorithm
The fisher mantis exhibits intelligent hunting behaviors, considering various scenarios and adjusting its position accordingly. It seeks the optimal location for prey or fish. Additionally, the fisher mantis displays uniform behaviors, including preparations for attacking or abandoning the current hunting state.
The proposed method makes use of the FMO algorithm outlined in reference [32]. This algorithm systematically advances through iterations, gradually bringing the mantis closer to its prey. Through this process, the algorithm progressively narrows down the potential scenarios, as illustrated in Equation (1). Here, the parameter “m” signifies the initial states, while “t” represents the states at the current iteration stage.
The feature vector will be used in machine learning for the classification of colon images into cancerous and non-cancerous categories.
The dataset utilized in this study, “Lung and Colon Cancer Histopathological Images,” was sourced from an open-access dataset library available at “https://www.kaggle.com/datasets/andrewmvd/lung-and-colon-cancer-histopathological-images”, accessed on 10 February 2021. It comprises 25,000 histopathology images categorized into five classes. Each image is saved in JPEG format with dimensions of 768 × 768 pixels.
- Augmentation Procedure:
To augment the dataset and increase its diversity, various augmentation techniques were applied to the original images. The augmentation process was implemented using the Augmentor package, which provides a flexible framework for image augmentation. The following augmentation techniques were utilized:
- 1.
- Rotation:
- ▪
- Range: Images were rotated within a range of angles to simulate variations in orientation.
- ▪
- Angle range: [−15°, 15°].
- 2.
- Translation:
- ▪
- Shift: Images were shifted horizontally and vertically to simulate translations.
- ▪
- Shift range: [−20%, 20%] of image width and height.
- 3.
- Scaling:
- ▪
- Scale factor: Images were scaled to simulate changes in size.
- ▪
- Scale range: [0.8, 1.2].
- 4.
- Cropping:
- ▪
- Random cropping: Portions of the images were randomly cropped to simulate variations in composition.
- ▪
- Crop size: Images were cropped to a size of 700 × 700 pixels.
- 5.
- Flipping:
- ▪
- Horizontal flipping: Images were flipped horizontally to simulate mirror reflections.
- ▪
- Vertical flipping: Images were flipped vertically to introduce additional variations.
By applying these augmentation techniques with specified parameters, the dataset was augmented to a total of 25,000 images, ensuring a diverse representation of histopathological features. This augmented dataset was then used for training and evaluating the proposed method for colon cancer detection. The classification task for colon images involved distinguishing between cancerous and non-cancerous classes. The dataset consisted of 25,000 histopathology images divided into five distinct categories, with each category containing 5000 images. It effectively conveys information about the classification task and the dataset related to colon images.
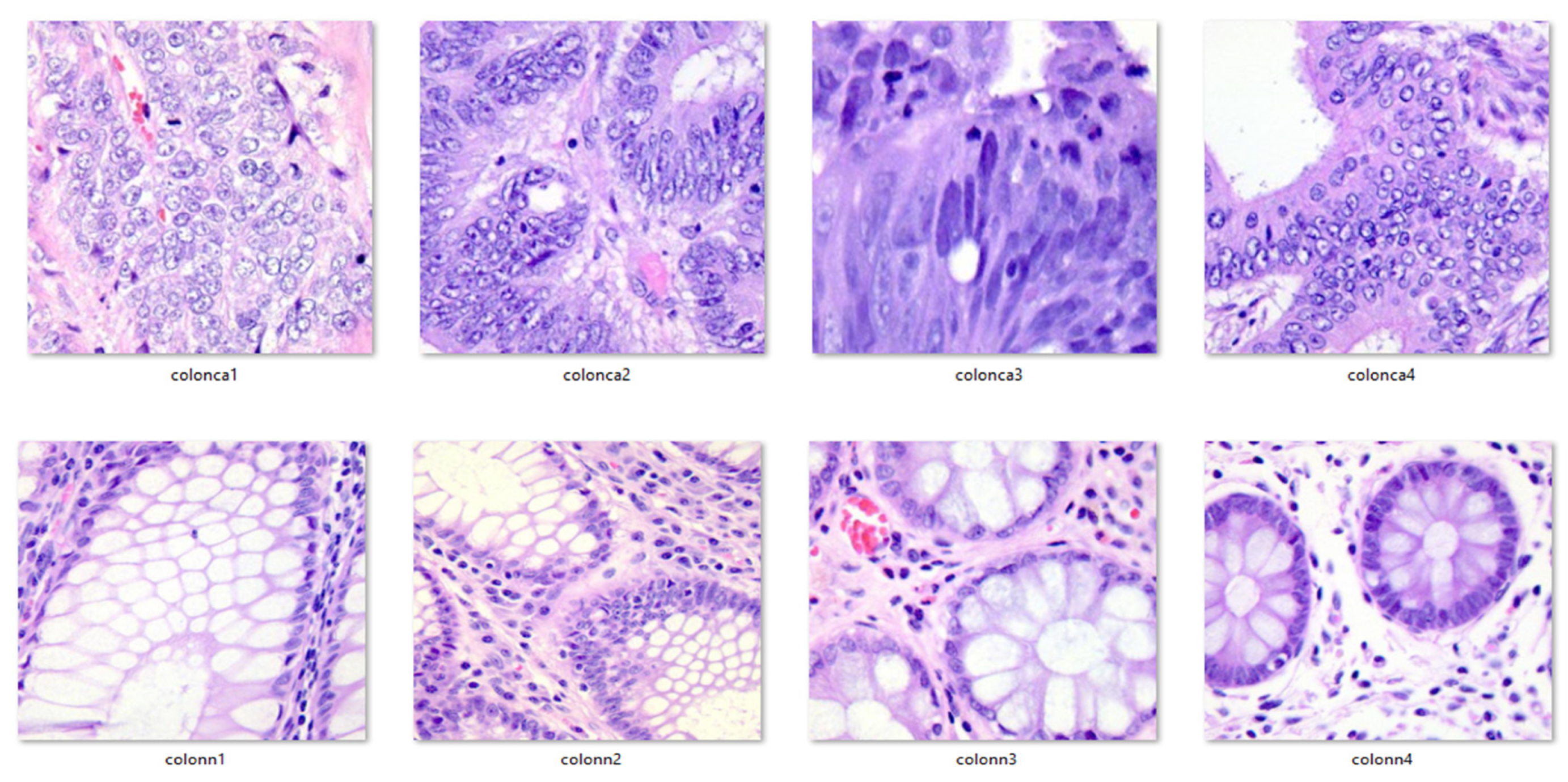
Additionally, for clarity and visualization purposes, six examples of histopathological images from the dataset are provided below. Images prefixed with “colon_n_” indicate healthy colon tissue images, while those prefixed with “colon_ca_” depict images of colon cancer. Refer to Figure 3 for a visual representation of these augmented images.
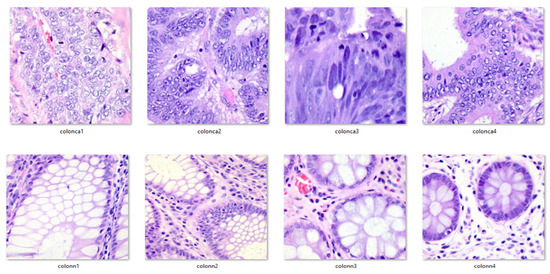
Figure 3.
Exemplar pictures from the dataset.
Histopathological Features and Classifications:
Histopathology entails the examination of tissue samples under a microscope, often obtained through biopsies, where minuscule tissue fragments are extracted and meticulously analyzed by pathologists. This thorough examination is instrumental in identifying both cancerous and pre-cancerous cellular abnormalities. Apart from colon cancer, the colon can be affected by a range of other conditions, underscoring the importance of histopathological analysis in reaching a definitive diagnosis.
4. Results and Discussion
4.1. Classification Using Learnable Classifiers for FMO
To determine the ideal combination of techniques, a thorough investigation has been carried out. An autoencoder method and the FMO algorithm were employed collaboratively on datasets associated with colon disease to isolate and choose the most critical attributes from the input training dataset. The identical datasets used in the first model were categorized using a pre-trained CNN in conjunction with the FMO method. Some important metrics, like accuracy, F1-Score, etc., are applied to assess the effectiveness of methods created from the confusion matrix. For multiclass classification, metrics such as total accuracy, true positive rate, and false positive rate were considered. The fundamental terms used in this analysis include False Positive (FP), True Positive (TP), True Negative (TN), and False Negative (FN), which stand for positive and negative classifications, respectively.
These indicators have been used to calculate accuracy (ACC), sensitivity (True Positive Rate (TPR)), specificity (True Negative Rate (TNR)), precision (positive predictive value (PPV)), negative predictive value (NPV), and F1-Score as follows:
4.2. Using Auto-Encoder with FMO for Colon Disease Dataset
Various scenarios were developed and assessed to validate the effectiveness of the proposed technique and compare different combinations. In the initial stage, a dataset related to colon illness was processed by the autoencoder, along with five different classifier types. The outcomes of the colon illness dataset using the autoencoder and the FMO technique are displayed in Table 2.

Table 2.
Dataset on colon disease auto-encoder using FMO feature selection algorithm.
The most crucial factor in assessing this classification model is accuracy, which is based on the true values of the tested images that were classified. The KNN classifier achieved a higher accuracy rate of 75.35%.
4.3. Pre-Trained CNN with FMO for Colon Disease Dataset
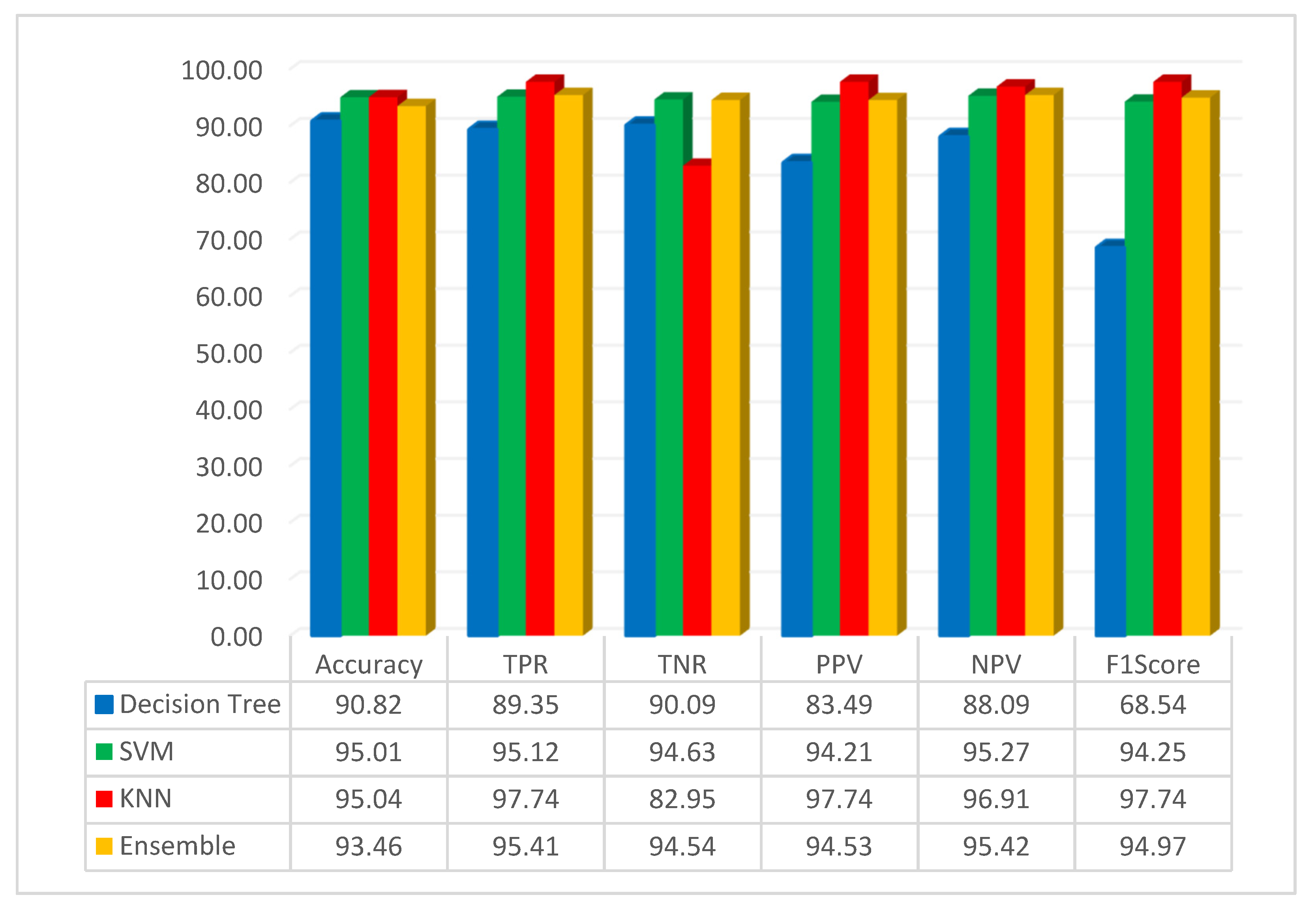
The assessment of pre-trained CNN with FMO was conducted using the dataset related to colon diseases. The simulation outcome, employing the FMO method in conjunction with the pre-trained ResNet-50 network, is presented in Figure 4.
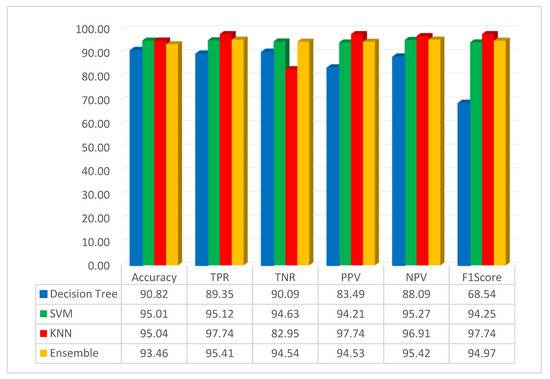
Figure 4.
Result of the simulation based on the ResNet-50 and FMO.
Figure 4 illustrates that the accuracy of the DT, SVM, KNN, and ensemble methods has been achieved at 90.82%, 95.01%, 95.04%, and 93.46%, respectively. The KNN classification method achieved 95.04% accuracy, which was the best result. KNN is a non-parametric supervised learning classifier that is more accurate than other methods such as SVM, decision trees, and ensemble methods. Additionally, in this scenario, the highest accuracy was achieved using the KNN classifier with features that were obtained using the FMO algorithm and a pre-trained ResNet-50 network. Additionally, this study assessed the performance of decision tree, SVM, KNN, and ensemble methods with the F1-Score metric, yielding scores of 68.54%, 94.25%, 97.74%, and 94.97% for these algorithms, respectively. As seen from the F1-Score results, it can be understood that the KNN has higher accuracy than other methods.
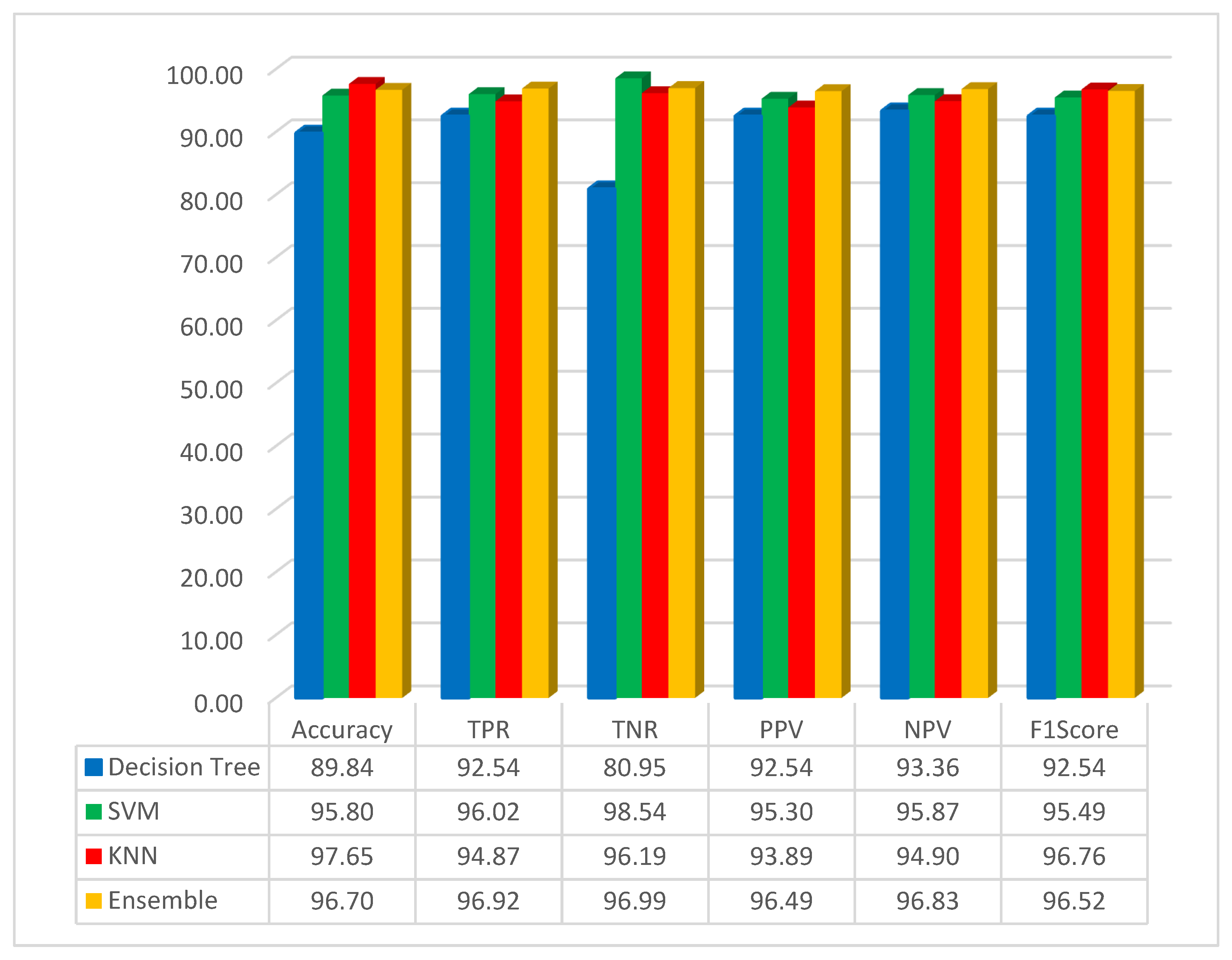
The simulation results, based on the FMO method in conjunction with the pre-trained GoogLeNet network, are depicted in Figure 5.
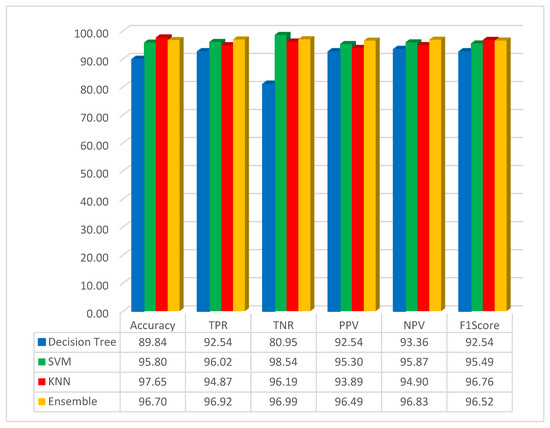
Figure 5.
Simulation result based on the GoogLeNet with FMO.
As it is shown in Figure 5, the accuracy for the decision tree, SVM, KNN, and ensemble methods has been obtained as 89.84%, 95.80%, 97.65%, and 96.70%, respectively. The best result for accuracy is 97.65, obtained by the KNN classifier method. In this scenario, the KNN method has high performance compared with other methods. It can be understood that the classification with KNN and the features obtained by using the FMO and pre-trained network with the GoogLeNet has obtained the highest accuracy. In this study, the F1-Score has been implemented, and the result for the decision tree, SVM, KNN, and ensemble methods has been obtained as 92.54%, 95.49%, 96.76%, and 96.52%, respectively. As seen from the F1-Score result, it can be understood that the KNN has the highest accuracy compared to other methods.
The examination reveals that the sensitivity, specificity, and accuracy indices in the suggested approach are 94.87%, 96.19%, and 97.65%, respectively. The sensitivity index and specificity of the suggested approach outperform the methods examined in [26,33,34,35] in the examination and categorization of colon patients images, as demonstrated in Table 3.

Table 3.
Average sensitivity, specificity, and accuracy comparison between the suggested method and comparable approaches.
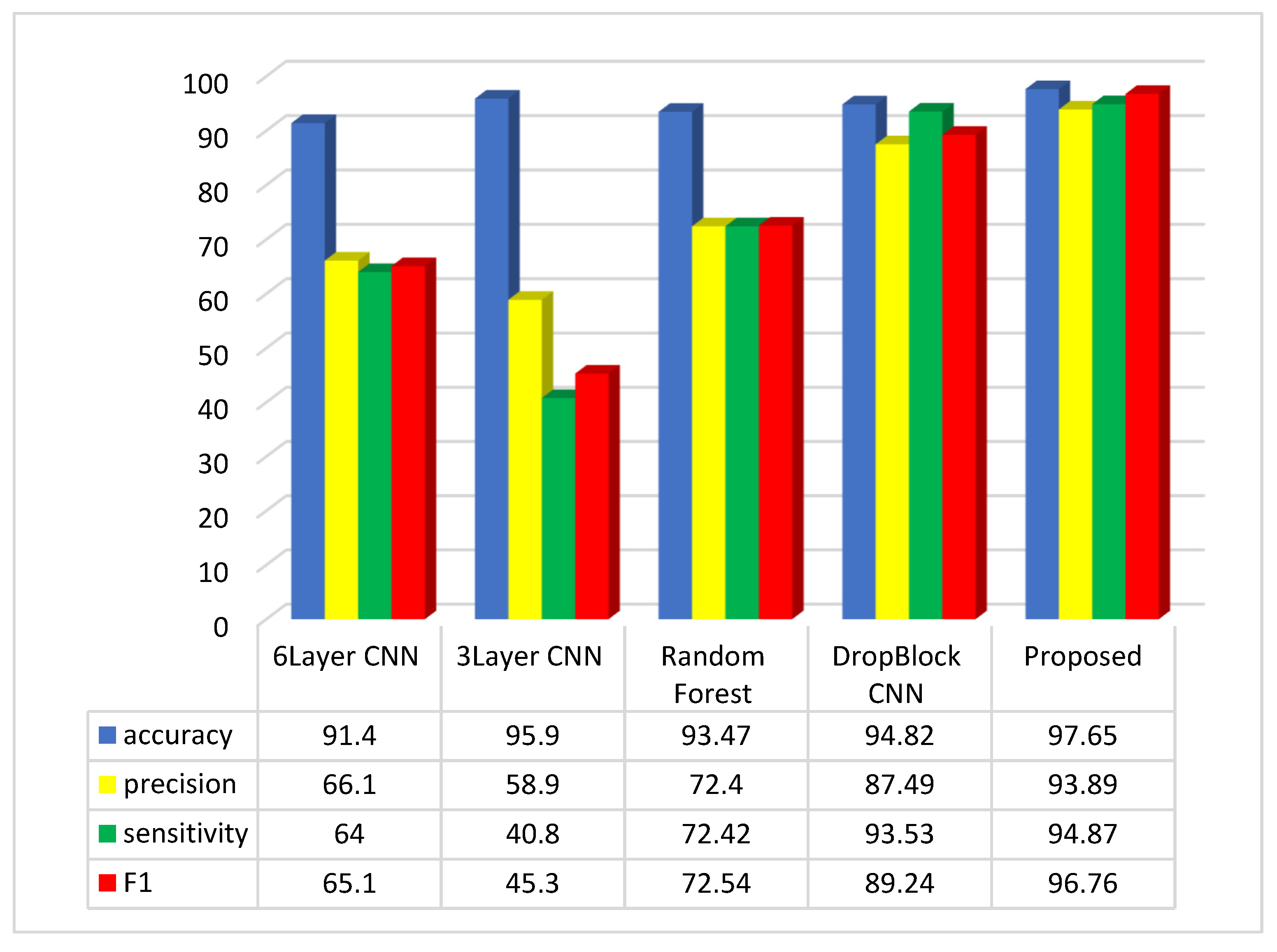
The accuracy score in the method outlined in [35] is recorded as 93.2%. In contrast, the suggested method attains an accuracy score of 97.65%. To conduct a comprehensive evaluation of the suggested approach, the results were compared with various investigations in the field, as illustrated in Figure 6.
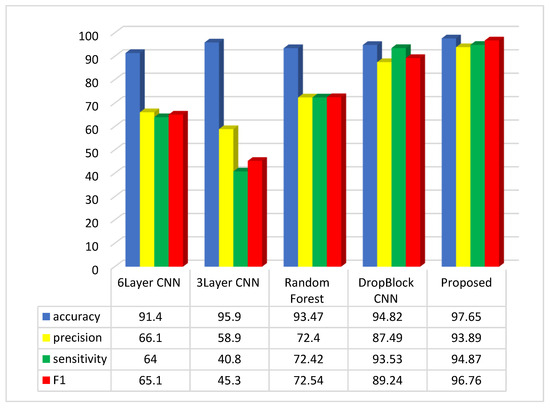
Figure 6.
Comparative analysis of the average of accuracy, sensitivity, precision, and F1-Score in the suggested method and comparable approaches.
The assessment suggests that the suggested approach, relying on the accuracy, precision, sensitivity, and F1-Score metrics, outperforms methods like 6Layer CNN, 3Layer CNN, Random Forest, and CNN DropBlock in colon disease image classification.
The suggested approach exhibits accuracy, precision, sensitivity, and F1-Score values of 97.65%, 93.89%, 94.87%, and 96.76%, respectively, in the colon cancer dataset image classification. Assessments indicate that the three-layer CNN method achieves the top accuracy in image categorization after the suggested approach. Regarding the accuracy metric, the CNN 6Layer approach exhibits the lowest outcome in categorization, with an accuracy of approximately 91.4%. Conversely, the proposed method attains the highest accuracy index in image classification. In terms of the sensitivity index, the suggested approach delivers the best performance, and subsequently, the CNN DropBlock approach technique had the greatest sensitivity index. The CNN 3Layer technique demonstrates the poorest sensitivity index among the compared methods. Among the compared methods, the suggested approach demonstrates the best performance in the F1 index, while the 3Layer CNN method exhibits the poorest performance in this index.
The proposed method was compared with CNN models such as ResNet and GoogleNet to show the influence of the Fishier Mantis Optimizer in the results. Also, in this paper, we used a CNN with three and six layers. These types of CNN based on the FMO give higher accuracy results than CNNs with nine and seven layers.
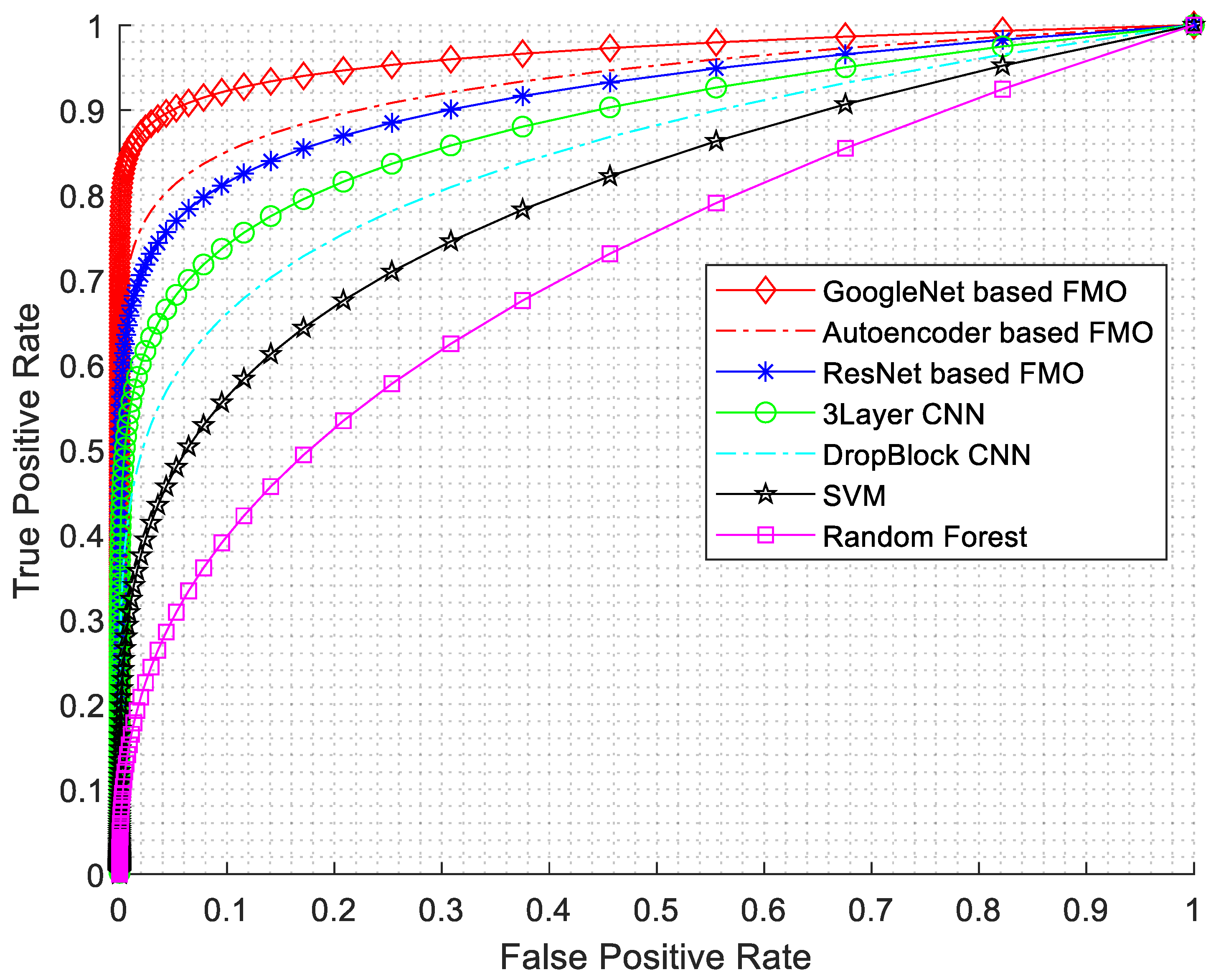
In this research, various evaluation criteria have been employed, encompassing sensitivity, specificity, accuracy, precision, F1-Score, and ROC diagrams. The ROC diagram is presented in Figure 7.
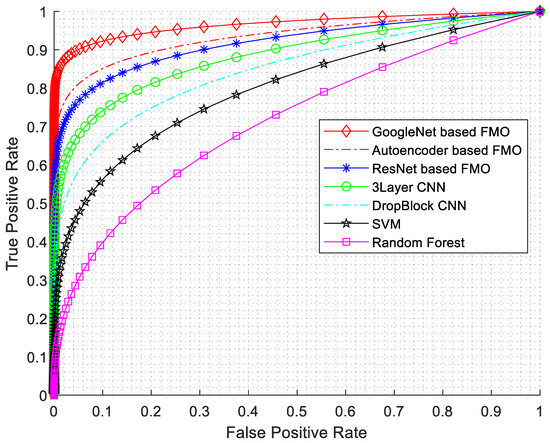
Figure 7.
ROC diagram with AUC numerical results.
As seen from Figure 7, the best results have been obtained for GoogleNet based on the FMO algorithm. The GoogLeNet architecture consists of multiple inception modules stacked together, resulting in a network with remarkable depth and accuracy. Furthermore, the features selected by the FMO algorithm can act as auxiliary classifiers in intermediate layers, thus addressing the vanishing gradient problem.
Table 4 shows how the proposed method compares with other metaheuristic techniques like Genetic Algorithm (GA), Particle Swarm Optimization (PSO), Ant Colony Optimization (ACO), and Gray Wolf Optimization (GWO) algorithms using the same CNN based on the ResNet-50 method in terms of sensitivity, specificity, accuracy, and F1Score index findings. The following represents the mean values of the suggested method for the two classes’ sensitivity, specificity, accuracy, and F1-Score in comparison to alternative approaches.

Table 4.
Comparative results of GA, PSO, ACO, and FMO based on sensitivity, specificity, accuracy, and F1-Scores.
The study’s conclusions indicate that the suggested method has an average sensitivity of 94.87%, specificity of 96.19%, accuracy of 97.65%, and F1score index of 96.76%. After comparing the proposed technique’s sensitivity, specificity, accuracy, and F1-Score to those of several existing metaheuristic methods, it was discovered that the recommended method performed better in the analysis and classification of colon patient photos. The accuracy index for the GA method comes out to be 93.56%. As part of the recommended methodology, this index is currently 97.65%. Table 4 shows that the GA algorithm produces the worst outcomes. Sensitivity, specificity, accuracy, and F1Score results for the GA algorithm were 91.15%, 94.89%, 93.56%, and 93.21%, respectively.
Early stopping was used during fine-tuning to prevent overfitting by halting training when the model’s performance on a validation set stopped improving. The best parameters were determined through a combination of grid search and cross-validation, where various hyperparameter combinations were systematically evaluated, and the set yielding the highest validation accuracy was selected.
4.4. The Advantages of This Study Can Be Summarized as Follows
High accuracy: The use of convolutional neural network (CNN) allows for the extraction of intricate features from medical images, contributing to high accuracy in colon cancer diagnosis.
The integration of the Fishier Mantis Optimizer enhances the optimization process, potentially leading to a more fine-tuned and accurate model.
Automated Diagnosis: The proposed approach provides an automated solution for colon cancer diagnosis, reducing dependence on manual examination and potentially speeding up the diagnostic process.
Advanced Image Analysis: CNNs excel in image recognition tasks, making them well suited for the analysis of medical images. This enables the model to learn complex patterns and structures indicative of colon cancer.
Nature-Inspired Optimization: The Fishier Mantis Optimizer introduces a bio-inspired optimization algorithm, potentially overcoming challenges associated with traditional optimization techniques. This can lead to improved convergence and parameter tuning.
Timely Intervention: The automated and accurate diagnosis facilitated by the proposed approach can contribute to timely intervention and treatment, potentially improving patient outcomes and prognosis.
4.5. The Disadvantages of This Study Can Be Summarized as Follows
Data Dependency: The success of CNNs is often dependent on large and diverse datasets. If the dataset used for training is not representative or lacks diversity, the model may not generalize well to unseen data.
Computational Intensity: Training deep learning models, especially CNNs, can be computationally intensive and time-consuming. This may pose challenges in terms of resource requirements, especially for institutions with limited computational capabilities.
Complex Model Architecture: The complexity of the CNN architecture may lead to difficulties in model interpretation and explainability. Understanding the inner workings of the model may be crucial for gaining trust in medical applications.
Optimization Challenges: While the Fishier Mantis Optimizer introduces a nature-inspired approach to optimization, its performance can be sensitive to the choice of hyperparameters. Finding the optimal configuration may require additional experimentation.
Generalization Issues: The model’s performance on new, unseen data is crucial for its real-world applicability. If the model overfits the training data, it may not perform well on diverse datasets, limiting its generalization capabilities.
In summary, while the proposed approach offers several advantages, it is essential to be aware of potential challenges and limitations, such as data dependencies, computational intensity, model complexity, and ethical considerations, to ensure the responsible development and deployment of the diagnostic.
5. Discussion
While this study demonstrates promising results for CNN-based colon cancer diagnosis, several avenues exist to further enhance model robustness and clinical applicability. Future work should prioritize collecting diverse and representative datasets, particularly those encompassing rare pathologies often underrepresented in training data. Furthermore, exploring advanced computational optimization techniques, such as model pruning or distributed computing frameworks, could address limitations imposed by computational intensity. Finally, rigorous validation on external datasets and in real-world clinical settings is paramount. Collaboration with clinicians and domain experts during this validation phase will be crucial for ensuring model interpretability, addressing ethical considerations, and ultimately building trust in AI-driven diagnostic tools.
6. Conclusions
Colon cancer poses a significant threat to public health, demanding timely and precise diagnosis for effective treatment outcomes. This study introduces a novel approach that amalgamates convolutional neural networks (CNNs) with the Fishier Mantis Optimizer (FMO) for automating the classification of colon cancer. Leveraging deep learning techniques, particularly CNNs, enables the extraction of intricate features from medical imaging data, thereby facilitating the development of a robust and efficient diagnostic model. Inspired by the hunting behavior of mantis shrimps, the FMO algorithm fine-tunes CNN parameters, enhancing model convergence speed and overall performance. This hybrid methodology aims to leverage the strengths of both deep learning and nature-inspired optimization, thereby improving the accuracy and effectiveness of colon cancer diagnosis.
Experimental validation conducted on a comprehensive dataset of colon cancer images showcases the superiority of the proposed method over traditional diagnostic approaches. The CNN-FMO model exhibits remarkable sensitivity, specificity, and overall accuracy in discriminating between cancerous and non-cancerous colon tissues. Notably, FMO-based feature selection outperforms conventional methods like Genetic Algorithms and simulated annealing, resulting in superior performance metrics including sensitivity, specificity, accuracy, and F1-Score.
Furthermore, the seamless integration of established data preprocessing techniques with FMO-based feature selection and CNN training enhances the extraction of critical features from histopathological images. This integration not only improves the model’s ability to differentiate between cancer and non-cancer samples but also enhances interpretability.
The iterative optimization of CNN weights during training with FMO contributes to a more finely tuned and accurate diagnostic model for colon cancer. Addressing challenges associated with feature abundance, the incorporation of FMO algorithms improves both model performance and interpretability.
Overall, the proposed method demonstrates significant potential for enhancing early detection and diagnosis of colon cancer, thereby facilitating timely intervention and improving patient prognosis. By combining deep learning with nature-inspired optimization, this study underscores the promise of innovative approaches in advancing healthcare outcomes.
Author Contributions
Methodology, A.A.A.M. and A.H.; Software, J.R.; Validation, R.R.; Formal analysis, J.M.L.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study because the dataset was obtained from a public repository.
Informed Consent Statement
Patient consent was waived because the dataset was obtained from a public repository.
Data Availability Statement
The data presented in this study are openly available in kaggle repository at https://doi.org/10.48550/arXiv.1912.12142, reference number [LC25000].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R. Gender Disparities: 5 Year Survival Rates of Elderly Colorectal Cancer Patients with Depression. Ph.D. Thesis, Walden University, Minneapolis, MN, USA, 2023. [Google Scholar]
- Masud, M.; Sikder, N.; Al Nahid, A.; Bairagi, A.K.; Alzain, M.A. A Machine Learning Approach to Diagnosing Lung and Colon Cancer Using a Deep Learning-based Classification Framework. Sensors 2021, 21, 748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Z.; Skrzypczynska, K.M.; Fang, Q.; Zhang, W.; O’Brien, S.A.; He, Y.; Wang, L.; Zhang, Q.; Kim, A. Single-Cell Analyses Inform Mechanisms of Myeloid-Targeted Therapies in Colon Cancer. Cell 2020, 181, 442–459. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, Y.; Shen, C.; Jung, H.; Nguyen, D.; Jiang, S.B.; Albuquerque, K.; Jia, X. Semi-Automatic Sigmoid Colon Segmentation in CT for Radiation Therapy Treatment Planning via an Iterative 2.5-D Deep Learning Approach. Med. Image Anal. 2021, 68, 101896. [Google Scholar] [CrossRef] [PubMed]
- Achilli, P.; Crippa, J.; Grass, F.; Mathis, K.L.; D’Angelo, A.D.; Abd El Aziz, M.A.; Day, C.N.; Harmsen, W.S.; Larson, D.W. Survival Impact of Adjuvant Chemotherapy in Patients with Stage IIA Colon Cancer: Analysis of the National Cancer Database. Int. J. Cancer 2021, 148, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Tulum, G.; Osman, O.; Bolat, B.; Dandin, Ö.; Ergin, T.; Cüce, F. Colonic Polyp Classification Using Projection Image and Convolutional Neural Network. In Proceedings of the 2019 Scientific Meeting on Electrical-Electronics & Biomedical Engineering and Computer Science (EBBT), Istanbul, Turkey, 24–26 April 2019; pp. 1–4. [Google Scholar]
- Ben Hamida, A.; Devanne, M.; Weber, J.; Truntzer, C.; Derangère, V.; Ghiringhelli, F.; Forestier, G.; Wemmert, C. Deep Learning for Colon Cancer Histopathological Images Analysis. Comput. Biol. Med. 2021, 136, 104730. [Google Scholar] [CrossRef] [PubMed]
- Talukder, M.A.; Islam, M.M.; Uddin, M.A.; Akhter, A.; Hasan, K.F.; Moni, M.A. Machine Learning-Based Lung and Colon Cancer Detection Using Deep Feature Extraction and Ensemble Learning. Expert Syst. Appl. 2022, 205, 117695. [Google Scholar] [CrossRef]
- Nazari, E.; Aghemiri, M.; Avan, A.; Mehrabian, A.; Tabesh, H. Machine Learning Approaches for Classification of Colorectal Cancer with and without Feature Selection Method on Microarray Data. Gene Rep. 2021, 25, 101419. [Google Scholar] [CrossRef]
- Ananthakrishnan, B.; Shaik, A.; Chakrabarti, S.; Shukla, V.; Paul, D.; Kavitha, M.S. Smart Diagnosis of Adenocarcinoma Using Convolution Neural Networks and Support Vector Machines. Sustainability 2023, 15, 1399. [Google Scholar] [CrossRef]
- Chahal, P.K.; Pandey, S. A Hybrid Weighted Fuzzy Approach for Brain Tumor Segmentation Using MR Images. Neural Comput. Appl. 2023, 35, 23877–23891. [Google Scholar] [CrossRef]
- Kour, H.; Manhas, J.; Sharma, V. Usage and Implementation of Neuro-Fuzzy Systems for Classification and Prediction in the Diagnosis of Different Types of Medical Disorders: A Decade Review. Artif. Intell. Rev. 2020, 53, 4651–4706. [Google Scholar] [CrossRef]
- Al Shalchi, N.F.A.; Rahebi, J. Human Retinal Optic Disc Detection with Grasshopper Optimization Algorithm. Multimed. Tools Appl. 2022, 81, 24937–24955. [Google Scholar] [CrossRef]
- Alshakree, F.; Akbas, A.; Rahebi, J. Human Identification Using Palm Print Images Based on Deep Learning Methods and Gray Wolf Optimization Algorithm. Signal Image Video Process. 2024, 18, 961–973. [Google Scholar] [CrossRef]
- Yaghoubi, E.; Yaghoubi, E.; Khamees, A.; Vakili, A.H. A Systematic Review and Meta-Analysis of Artificial Neural Network, Machine Learning, Deep Learning, and Ensemble Learning Approaches in Field of Geotechnical Engineering. Neural Comput. Appl. 2024, 1–45. [Google Scholar] [CrossRef]
- Yusupov, Z.; Yaghoubi, E.; Yaghoubi, E. Controlling and Tracking the Maximum Active Power Point in a Photovoltaic System Connected to the Grid Using the Fuzzy Neural Controller. In Proceedings of the 2023 14th International Conference on Electrical and Electronics Engineering (ELECO), Bursa, Turkey, 30 November–2 December 2023; pp. 1–5. [Google Scholar]
- Yaghoubi, A.; Khazaei, M.; Avan, A.; Hasanian, S.M.; Soleimanpour, S. The Bacterial Instrument as a Promising Therapy for Colon Cancer. Int. J. Colorectal Dis. 2020, 35, 595–606. [Google Scholar] [CrossRef]
- Kanth, P.; Inadomi, J.M. Screening and Prevention of Colorectal Cancer. BMJ 2021, 374, n1855. [Google Scholar] [CrossRef]
- Gao, F. Integrated Positron Emission Tomography/Magnetic Resonance Imaging in Clinical Diagnosis of Alzheimer’s Disease. Eur. J. Radiol. 2021, 145, 110017. [Google Scholar] [CrossRef]
- Fitzgerald, R.C.; Antoniou, A.C.; Fruk, L.; Rosenfeld, N. The Future of Early Cancer Detection. Nat. Med. 2022, 28, 666–677. [Google Scholar] [CrossRef]
- Tharwat, M.; Sakr, N.A.; El-Sappagh, S.; Soliman, H.; Kwak, K.-S.; Elmogy, M. Colon Cancer Diagnosis Based on Machine Learning and Deep Learning: Modalities and Analysis Techniques. Sensors 2022, 22, 9250. [Google Scholar] [CrossRef]
- Lewandowska, A.; Rudzki, G.; Lewandowski, T.; Stryjkowska-Gora, A.; Rudzki, S. Risk Factors for the Diagnosis of Colorectal Cancer. Cancer Control 2022, 29, 10732748211056692. [Google Scholar] [CrossRef] [PubMed]
- Galeș, L.N.; Păun, M.-A.; Anghel, R.M.; Trifănescu, O.G. Cancer Screening: Present Recommendations, the Development of Multi-Cancer Early Development Tests, and the Prospect of Universal Cancer Screening. Cancers 2024, 16, 1191. [Google Scholar] [CrossRef]
- Alboaneen, D.; Alqarni, R.; Alqahtani, S.; Alrashidi, M.; Alhuda, R.; Alyahyan, E.; Alshammari, T. Predicting Colorectal Cancer Using Machine and Deep Learning Algorithms: Challenges and Opportunities. Big Data Cogn. Comput. 2023, 7, 74. [Google Scholar] [CrossRef]
- Zhang, R.; Zheng, Y.; Mak, T.W.C.; Yu, R.; Wong, S.H.; Lau, J.Y.W.; Poon, C.C.Y. Automatic Detection and Classification of Colorectal Polyps by Transferring Low-Level CNN Features from Nonmedical Domain. IEEE J. Biomed. Health Inform. 2016, 21, 41–47. [Google Scholar] [CrossRef]
- Nisha, J.S.; Gopi, V.P.; Palanisamy, P. Automated Colorectal Polyp Detection Based on Image Enhancement and Dual-Path CNN Architecture. Biomed. Signal Process. Control 2022, 73, 103465. [Google Scholar] [CrossRef]
- Nanni, L.; Fantozzi, C.; Loreggia, A.; Lumini, A. Ensembles of Convolutional Neural Networks and Transformers for Polyp Segmentation. Sensors 2023, 23, 4688. [Google Scholar] [CrossRef]
- Morin, O.; Vallières, M.; Braunstein, S.; Ginart, J.B.; Upadhaya, T.; Woodruff, H.C.; Zwanenburg, A.; Chatterjee, A.; Villanueva-Meyer, J.E.; Valdes, G. An Artificial Intelligence Framework Integrating Longitudinal Electronic Health Records with Real-World Data Enables Continuous Pan-Cancer Prognostication. Nat. Cancer 2021, 2, 709–722. [Google Scholar] [CrossRef]
- Du, Y.; Hu, L.; Wu, G.; Tang, Y.; Cai, X.; Yin, L. Diagnoses in Multiple Types of Cancer Based on Serum Raman Spectroscopy Combined with a Convolutional Neural Network: Gastric Cancer, Colon Cancer, Rectal Cancer, Lung Cancer. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 298, 122743. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Jothilakshmi, S.; Suthir, S. Colorectal Cancer Detection Based on Convolutional Neural Networks (CNN) and Ranking Algorithm. Meas. Sens. 2024, 31, 100976. [Google Scholar] [CrossRef]
- Zhu, H.; Roelands, J.; Ahmed, E.I.; Stouten, I.; Hoorntje, R.; van Vlierberghe, R.L.P.; Ijsselsteijn, M.E.; Lei, X.; de Miranda, N.F.C.C.; Tollenaar, R.A.E.M. Location Matters: Spatial Dynamics of Tumor-Infiltrating T Cell Subsets Is Prognostic in Colon Cancer. Front. Immunol. 2024, 15, 1293618. [Google Scholar] [CrossRef]
- Shin, Y.; Balasingham, I. Comparison of Hand-Craft Feature Based SVM and CNN Based Deep Learning Framework for Automatic Polyp Classification. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju Island, Republic of Korea, 11–15 July 2017; pp. 3277–3280. [Google Scholar]
- Stidham, R.W.; Liu, W.; Bishu, S.; Rice, M.D.; Higgins, P.D.R.; Zhu, J.; Nallamothu, B.K.; Waljee, A.K. Performance of a Deep Learning Model vs. Human Reviewers in Grading Endoscopic Disease Severity of Patients with Ulcerative Colitis. JAMA Netw. Open 2019, 2, e193963. [Google Scholar] [CrossRef]
- Poudel, S.; Kim, Y.J.; Vo, D.M.; Lee, S.-W. Colorectal Disease Classification Using Efficiently Scaled Dilation in Convolutional Neural Network. IEEE Access 2020, 8, 99227–99238. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).