The Role of Magnetic Resonance Imaging in Risk Stratification of Patients with Acute Myocarditis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
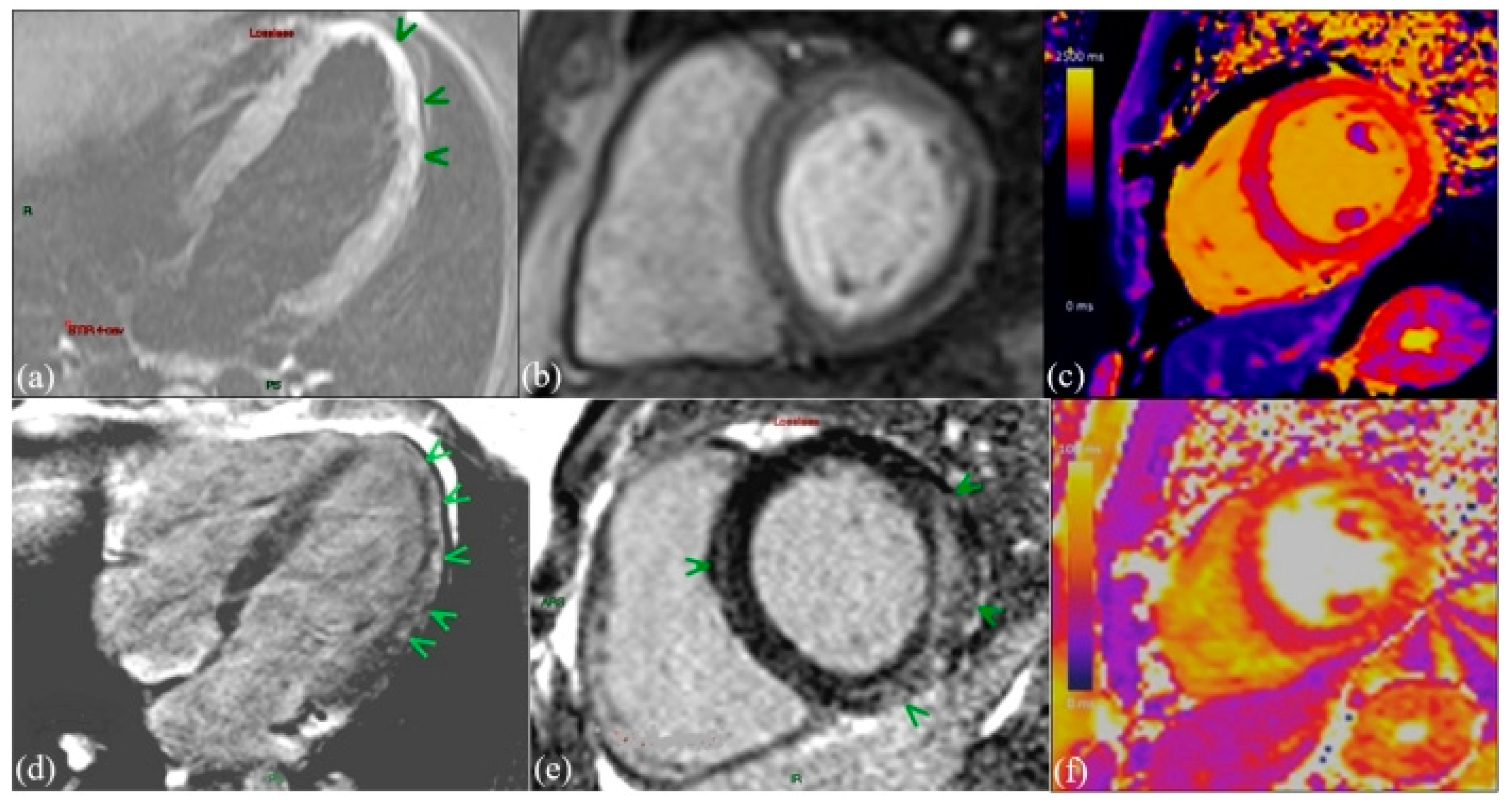
2.2. cMRI
2.3. Clinical Monitoring
2.4. Statistical Analysis
3. Results
3.1. Basic Features
3.2. cMRI Characteristics
3.3. The Ability of Biomarkers to Establish the Diagnosis of Myocardial Fibrosis in AM
3.4. Determination of Independent Predictors of MACE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matsuura, H.; Ichida, F.; Saji, T.; Ogawa, S.; Waki, K.; Kaneko, M.; Tahara, M.; Soga, T.; Ono, Y.; Yasukochi, S. Clinical features of acute and fulminant myocarditis in children—2nd nationwide survey by Japanese Society of pediatric cardiology and cardiac surgery. Circ. J. 2016, 80, 2362–2368. [Google Scholar] [CrossRef] [PubMed]
- Arola, A.; Pikkarainen, E.; Sipilä, J.O.; Pykäri, J.; Rautava, P.; Kytö, V. Occurrence and features of childhood myocarditis: A nationwide study in Finland. J. Am. Heart Assoc. 2017, 6, e005306. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Jabbour, A.; Ismail, T.F.; Guha, K.; Khwaja, J.; Raza, S.; Morarji, K.; Brown, T.D.; Ismail, N.A.; Dweck, M.R.; et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 2013, 309, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Law, Y.M.; Lal, A.K.; Chen, S.; Čiháková, D.; Cooper, L.T., Jr.; Deshpande, S.; Godown, J.; Grosse-Wortmann, L.; Robinson, J.D.; Towbin, J.A. American Heart Association Pediatric Heart Failure and Transplantation Committee of the Council on Lifelong Congenital Heart Disease and Heart Health in the Young and Stroke Council. Diagnosis and management of myocarditis in children: A scientific statement from the American Heart Association. Circulation 2021, 144, e123–e135. [Google Scholar] [PubMed]
- Yao, Y.; Bian, W.; Zhang, H.; Ji, X.; Wang, Z. Quantitative cardiac MRI parameters for assessment of myocarditis in children and adolescents: A systematic review and meta-analysis. Clin. Radiol. 2023, 78, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Putschoegl, A.; Auerbach, S. Diagnosis, evaluation, and treatment of myocarditis in children. Pediatr. Clin. N. Am. 2020, 67, 855–874. [Google Scholar] [CrossRef] [PubMed]
- Baughman, K.L. Diagnosis of myocarditis: Death of Dallas criteria. Circulation 2006, 113, 593–595. [Google Scholar] [CrossRef]
- Ammirati, E.; Kaski, J.P. Resident inflammatory cells in the myocardium of children: On the way to set histologic reference standards to differentiate normal myocardium from myocarditis. Int. J. Cardiol. 2020, 303, 64–65. [Google Scholar] [CrossRef]
- Daly, K.P.; Marshall, A.C.; Vincent, J.A.; Zuckerman, W.A.; Hoffman, T.M.; Canter, C.E.; Blume, E.D.; Bergersen, L. Endomyocardial biopsy and selective coronary angiography are low-risk procedures in pediatric heart transplant recipients: Results of a multicenter experience. J. Heart Lung Transplant. 2012, 31, 398–409. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Non-ischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.M.; Greve, A.M.; Aspelund, T.; Schelbert, E.B.; Cao, J.J.; Danielsen, R.; Þorgeirsson, G.; Sigurðsson, S.; Eiríksdóttir, G.; Harris, T.B.; et al. Prevalence and prognosis of ischaemic and non-ischaemic myocardial fibrosis in older adults. Eur. Heart J. 2019, 40, 529–538. [Google Scholar] [CrossRef] [PubMed]
- De Boer, R.A.; De Keulenaer, G.; Bauersachs, J.; Brutsaert, D.; Cleland, J.G.; Diez, J.; Du, X.J.; Ford, P.; Heinzel, F.R.; Lipson, K.E.; et al. Towards better definition, quantification and treatment of fibrosis in heart failure. A scientific roadmap by the Committee of Translational Research of the Heart Failure Association (HFA) of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 272–285. [Google Scholar] [CrossRef]
- Mileva, N.; Paolisso, P.; Gallinoro, E.; Fabbricatore, D.; Munhoz, D.; Bergamaschi, L.; Belmonte, M.; Panayotov, P.; Pizzi, C.; Barbato, E.; et al. Diagnostic and Prognostic Role of Cardiac Magnetic Resonance in MINOCA: Systematic Review and Meta-Analysis. JACC Cardiovasc. Imaging 2023, 16, 376–389. [Google Scholar] [CrossRef]
- Bergamaschi, L.; Pavon, A.G.; Angeli, F.; Tuttolomondo, D.; Belmonte, M.; Armillotta, M.; Sansonetti, A.; Foà, A.; Paolisso, P.; Baggiano, A.; et al. The Role of Non-Invasive Multimodality Imaging in Chronic Coronary Syndrome: Anatomical and Functional Pathways. Diagnostics 2023, 13, 2083. [Google Scholar] [CrossRef]
- Bergamaschi, L.; Foà, A.; Paolisso, P.; Renzulli, M.; Angeli, F.; Fabrizio, M.; Bartoli, L.; Armillotta, M.; Sansonetti, A.; Amicone, S.; et al. Prognostic Role of Early Cardiac Magnetic Resonance in Myocardial Infarction with Nonobstructive Coronary Arteries. JACC Cardiovasc. Imaging 2024, 17, 149–161. [Google Scholar] [CrossRef]
- Liguori, C.; Tamburrini, S.; Ferrandino, G.; Leboffe, S.; Rosano, N.; Marano, I. Role of CT and MRI in Cardiac Emergencies. Tomography 2022, 8, 1386–1400. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Schelbert, E.B.; Díez, J.; Butler, J. Myocardial Interstitial Fibrosis in Heart Failure: Biological and Translational Perspectives. J. Am. Coll. Cardiol. 2018, 71, 1696–1706. [Google Scholar] [CrossRef] [PubMed]
- Ravassa, S.; Lupón, J.; López, B.; Codina, P.; Domingo, M.; Revuelta-López, E.; Moreno, M.U.; San José, G.; Santiago-Vacas, E.; Cediel, G.; et al. Prediction of Left Ventricular Reverse Remodeling and Outcomes by Circulating Collagen-Derived Peptides. JACC Heart Fail. 2023, 11, 58–72. [Google Scholar] [CrossRef]
- Sharma, U.C.; Pokharel, S.; van Brakel, T.J.; van Berlo, J.H.; Cleutjens, J.P.; Schroen, B.; André, S.; Crijns, H.J.; Gabius, H.J.; Maessen, J.; et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 2004, 110, 3121–3128. [Google Scholar] [CrossRef]
- Lok, D.J.; Van Der Meer, P.; de la Porte, P.W.; Lipsic, E.; Van Wijngaarden, J.; Hillege, H.L.; van Veldhuisen, D.J. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: Data from the DEAL-HF study. Clin. Res. Cardiol. 2010, 99, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Luetkens, J.A.; Doerner, J.; Thomas, D.K.; Dabir, D.; Gieseke, J.; Sprinkart, A.M.; Fimmers, R.; Stehning, C.; Homsi, R.; Schwab, J.O.; et al. Acute myocarditis: Multiparametric cardiac MR imaging. Radiology 2014, 273, 383–392. [Google Scholar] [CrossRef]
- Luetkens, J.A.; Faron, A.; Isaak, A.; Dabir, D.; Kuetting, D.; Feisst, A.; Schmeel, F.C.; Sprinkart, A.M.; Thomas, D. Comparison of original and 2018 Lake Louise criteria for diagnosis of acute myocarditis: Results of a validation cohort. Radiol. Cardiothorac. Imaging 2019, 1, e190010. [Google Scholar] [CrossRef] [PubMed]
- Scully, P.R.; Bastarrika, G.; Moon, J.C.; Treibel, T.A. Myocardial Extracellular Volume Quantification by Cardiovascular Magnetic Resonance and Computed Tomography. Curr. Cardiol. Rep. 2018, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Perfetti, M.; Malatesta, G.; Alvarez, I.; Liga, R.; Barison, A.; Todiere, G.; Eletto, N.; De Caterina, R.; Lombardi, M.; Aquaro, G.D. A fast and effective method to assess myocardial hyperemia in acute myocarditis by magnetic resonance. Int. J. Cardiovasc. Imaging 2014, 30, 629–637. [Google Scholar] [CrossRef]
- Abdel-Aty, H.; Boyé, P.; Zagrosek, A.; Wassmuth, R.; Kumar, A.; Messroghli, D.; Bock, P.; Dietz, R.; Friedrich, M.G.; Schulz-Menger, J. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: Comparison of different approaches. J. Am. Coll. Cardiol. 2005, 45, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Hundley, W.G.; Bluemke, D.A.; Finn, J.P.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Ho, V.B.; Jerosch-Herold, M.; Kramer, C.M.; Manning, W.J.; et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J. Am. Coll. Cardiol. 2010, 55, 2614–2662. [Google Scholar] [CrossRef]
- Aquaro, G.D.; Ghebru Habtemicael, Y.; Camastra, G.; Monti, L.; Dellegrottaglie, S.; Moro, C.; Lanzillo, C.; Scatteia, A.; Di Roma, M.; Pontone, G.; et al. Prognostic Value of Repeating Cardiac Magnetic Resonance in Patients with Acute Myocarditis. J. Am. Coll. Cardiol. 2019, 74, 2439–2448. [Google Scholar] [CrossRef] [PubMed]
- Bohbot, Y.; Garot, J.; Hovasse, T.; Unterseeh, T.; Di Lena, C.; Boukefoussa, W.; Tawa, C.; Renard, C.; Limouzineau, I.; Duhamel, S.; et al. Clinical and Cardiovascular Magnetic Resonance Predictors of Early and Long-Term Clinical Outcome in Acute Myocarditis. Front. Cardiovasc. Med. 2022, 9, 886607. [Google Scholar] [CrossRef]
- Aquaro, G.D.; De Gori, C.; Faggioni, L.; Parisella, M.L.; Cioni, D.; Lencioni, R.; Neri, E. Diagnostic and prognostic role of late gadolinium enhancement in cardiomyopathies. Eur. Heart J. Suppl. 2023, 25 (Suppl. C), C130–C136. [Google Scholar] [CrossRef]
- Shao, X.N.; Jin, Y.N.; Sun, Y.J.; Zhang, W.B.; Cheng, J.L. Evaluation of the correlation between myocardial fibrosis and ejection fraction in dilated cardiomyopathy using magnetic resonance T1 mapping. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12300–12305. [Google Scholar] [PubMed]
- Imazio, M.; Angelico, G.; Andriani, M.; Lobetti-Bodoni, L.; Davini, O.; Giustetto, C.; Rinaldi, M. Prevalence and Prognostic Impact of Septal Late Gadolinium Enhancement in Acute Myocarditis with or without Preserved Left Ventricular Function. Am. J. Cardiol. 2018, 122, 1955–1958. [Google Scholar] [CrossRef] [PubMed]
- Greulich, S.; Seitz, A.; Müller, K.A.L.; Grün, S.; Ong, P.; Ebadi, N.; Kreisselmeier, K.P.; Seizer, P.; Bekeredjian, R.; Zwadlo, C.; et al. Predictors of Mortality in Patients with Biopsy-Proven Viral Myocarditis: 10-Year Outcome Data. J. Am. Heart Assoc. 2020, 9, e015351. [Google Scholar] [CrossRef] [PubMed]
- Gräni, C.; Eichhorn, C.; Bière, L.; Murthy, V.L.; Agarwal, V.; Kaneko, K.; Cuddy, S.; Aghayev, A.; Steigner, M.; Blankstein, R.; et al. Prognostic Value of Cardiac Magnetic Resonance Tissue Characterization in Risk Stratifying Patients with Suspected Myocarditis. J. Am. Coll. Cardiol. 2017, 70, 1964–1976. [Google Scholar] [CrossRef]


| General Data | ||
|---|---|---|
| Age, years | 35 | |
| Male, % | 76.6 | |
| Female, % | 23.3 | |
| BMI, kg/m2 | 26.1 | |
| HR, bpm | 72 | |
| SBP, mm Hg | 125 | |
| Symptoms | All patients (n = 90) | % |
| Chest pain | 82 | 91.1 |
| Dyspnea | 54 | 60 |
| Sweating | 32 | 35.5 |
| Syncope/lipothymia | 8 | 8.8 |
| Palpitations | 23 | 25.5 |
| Electrocardiogram (ECG) | All patients (n = 90) | % |
| ECG changes | 83 | 92 |
| ST elevation | 23 | 25.5 |
| Non-specific ST-T changes | 67 | 74.4 |
| Atrial fibrillation | 6 | 6.6 |
| LAB TEST | Average values | IQR |
| hs-troponin T | 651 ng/L | 123–9300 ng/L |
| hs-CRP | 13.5 ng/mL | 6.2–19.9 ng/mL |
| NT-proBNP | 960 pg/mL | 120–9893 pg/mL |
| Gal-3 | 13.5 ng/mL | 7.5–17.9 ng/mL |
| Initial cMRI (n = 90) | cMRI at 6 Months (n = 90) | p-Value | |
|---|---|---|---|
| Indexed EDV, average (SD), ml/m2 | 82.2 (19.2) | 80.4 (17.1) | NS |
| Indexed ESV, average (SD), ml/m2 | 34.5 (17.4) | 34.4 (13.4) | NS |
| LVEF, average (SD), % | 58.8 (10.2) | 60.1 (10.8) | NS |
| Segmentary kinetic abnormalities, n (%) | 55 (61.1) | 23 (25.5) | <0.001 |
| LV mass, g/m2 (SD) | 86 (19.2) | 84 (17.3) | NS |
| T2 + edema, n (%) | 84 (93.3) | 34 (37.7) | <0.001 |
| LGE +, n (%) | 88 (97.7) | 70 (77.7) | <0.05 |
| -Myocardial localization—septum/lateral/anterior/inferior/ circumferential | 35/12/26/11 /5 | 30/10/22/8/4 | N/A |
| -Myocardial pattern— subepicardial/nodal/midmyocardial | 52-12-24 | 47-08-19 | N/A |
| LV-LGE, g | 19.7 (10.5) | 12.3 (6.7) | <0.05 |
| LV-LGE/LV mass | 26.1 (9.8) | 20.7 (9.9) | <0.05 |
| LGE without edema, n (%) | 0 | 74 (82.2) | N/A |
| Edema without LGE, n (%) | 8 (8.8) | 0 | N/A |
| T1 native mapping, ms | 1123 ± 56 | 1037 ± 24 | <0.01 |
| T1 extended native mapping, n (%) | 87 (96.6) | 53 (58.8) | <0.05 |
| Pericardial collection +, n (%) | 43 (47.7) | 23 (25.5) | <0.001 |
| Unadjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
|---|---|---|---|---|
| Age, years | 1.38 (1.15–1.66) | <0.01 | ||
| BMI, kg/m2 | 1.02 (0.93–1.11) | NS | ||
| Chest pain | 1.03 (0.99-1.81) | <0.01 | 1.00 (0.99–1.01) | NS |
| Dyspnea | 1.04 (0.89–1.34) | <0.01 | 0.98 (0.89–1.18) | NS |
| Clinical heart failure | 6.74 (2.72–17.27) | <0.001 | 3.44 (1.52–9.42) | <0.01 |
| Troponin, ng/L | 0.99 (0.99–1.01) | NS | 1.23 (1.01–1.45) | 0.05 |
| NT-proBNP, pg/mL | 1.42 (0.94–4.59) | 0.001 | 1.01 (0.99–1.02) | NS |
| hs-CRP, ng/mL | 5.79 (0.92–36.4) | <0.01 | 1.06 (0.94–1.14) | NS |
| Gal-3 | 2.67 (1.56–3.77) | <0.001 | 1.23 (1.09–2.11) | <0.01 |
| LV kinetic abnormalities | 0.96 (0.93–0.99) | NS | ||
| ECG changes | 0.99 (0.98–1.00) | <0.05 | 1.00 (0.99–1.01) | NS |
| Myocardial edema+ | 85.8 (6.03–318.74) | 0.001 | 1.70 (1.14–209.3) | <0.001 |
| LV-LGE, g | 1.91 (1.25–2.97) | <0.001 | 1.27 (1.11–1.99) | <0.001 |
| LV-LGE/LV mass | 1.22 (1.18–1.40) | <0.01 | 1.00 (0.84–1.18) | NS |
| Medium-septal LGE pattern | 1.19 (1.09–2.11) | <0.001 | 1.08 (0.92–1.56) | <0.01 |
| Native T1 prolonged | 1.10 (1.03–7.11) | <0.0001 | 0.97 (0.88–3.06) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, A.; Cionca, C.; Agoston, R.; Rusu, F.; Tarcau, B.M.; Negru, A.; Orzan, R.I.; Agoston-Coldea, L. The Role of Magnetic Resonance Imaging in Risk Stratification of Patients with Acute Myocarditis. Diagnostics 2024, 14, 1426. https://doi.org/10.3390/diagnostics14131426
Popa A, Cionca C, Agoston R, Rusu F, Tarcau BM, Negru A, Orzan RI, Agoston-Coldea L. The Role of Magnetic Resonance Imaging in Risk Stratification of Patients with Acute Myocarditis. Diagnostics. 2024; 14(13):1426. https://doi.org/10.3390/diagnostics14131426
Chicago/Turabian StylePopa, Alexandra, Carmen Cionca, Renata Agoston, Flaviu Rusu, Bogdan Mihai Tarcau, Andra Negru, Rares Ilie Orzan, and Lucia Agoston-Coldea. 2024. "The Role of Magnetic Resonance Imaging in Risk Stratification of Patients with Acute Myocarditis" Diagnostics 14, no. 13: 1426. https://doi.org/10.3390/diagnostics14131426





