Anatomical Variants of the Origin of the Coronary Arteries: A Systematic Review and Meta-Analysis of Prevalence
Abstract
1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Electronic Search
2.3. Eligibility Criteria
2.4. Study Selection
2.5. Data Collection Process
2.6. Assessment of the Methodological Quality of the Included Studies
2.7. Statistical Methods
3. Results
3.1. Selection of Articles
3.2. Characteristics of Included Studies
3.3. Description of Variants
3.4. Prevalence and Risk of Bias
3.5. Clinical Considerations
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Al-Dairy, A.; Rezaei, Y.; Pouraliakbar, H.; Mahdavi, M.; Bayati, P.; Gholampour-Dehaki, M. Surgical Repair for Anomalous Origin of the Right Coronary Artery from the Pulmonary Artery. Korean Circ. J. 2017, 47, 144–147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Han, P.L.; Diao, K.Y.; Huang, S.; Gao, Y.; Guo, Y.K.; Yang, Z.G.; Yang, N. Anatomical characteristics of anomalous left coronary artery from the opposite sinus (left-ACAOS) and its clinical relevance: A serial coronary CT angiography study. Int. J. Cardiol. Heart Vasc. 2020, 31, 100649. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Jijeh, A.; Alhuwaymil, R.M.; Alahmari, R.; Alshahrani, R.; Almutairi, R.; Habshan, F.; Shaath, G.A. Long-Term Outcome of the Anomalous Origin of the Left Coronary Artery from the Pulmonary Artery (ALCAPA) in Children after Cardiac Surgery: A Single-Center Experience. Cureus 2020, 12, e11829. [Google Scholar] [CrossRef] [PubMed]
- Jegatheeswaran, A.; Devlin, P.J.; Williams, W.G.; Brothers, J.A.; Jacobs, M.L.; DeCampli, W.M.; Fleishman, C.E.; Kirklin, J.K.; Mertens, L.; Mery, C.M.; et al. Outcomes after anomalous aortic origin of a coronary artery repair: A Congenital Heart Surgeons’ Society Study. J. Thorac. Cardiovasc. Surg. 2020, 160, 757–771.e5. [Google Scholar] [CrossRef] [PubMed]
- Krupiński, M.; Urbańczyk-Zawadzka, M.; Laskowicz, B.; Irzyk, M.; Banyś, R.; Gruszczyńska, K.; Baron, J. Computed tomography in the evaluation of the anomalous origin of the coronary artery: Coexistence with other congenital heart disease in an adult population. Folia Morphol. 2015, 74, 73–77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sidhu, N.S.; Wander, G.S.; Monga, A.; Kaur, A. Incidence, Characteristics and Atherosclerotic Involvement of Coronary Artery Anomalies in Adult Population Undergoing Catheter Coronary Angiography. Cardiol. Res. 2019, 10, 358–368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sohrabi, B.; Habibzadeh, A.; Abbasov, E. The incidence and pattern of coronary artery anomalies in the north-west of iran: A coronary arteriographic study. Korean Circ. J. 2012, 42, 753–760. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramírez, F.; Bitar, P.; Paolinelli, P.; Pérez, D.; Furnaro, F. Congenital anomalies of the Coronary Arteries, study of those with Hemodynamic Importance. Chil. J. Radiol. 2018, 24, 142–150. [Google Scholar] [CrossRef]
- Sayin, M.R.; Akpinar, I.; Karabag, T.; Dogan, S.M.; Aydin, M. Atypical type of dual left anterior descending coronary artery. J. Cardiol. Cases 2013, 8, e39–e41. [Google Scholar] [CrossRef][Green Version]
- Cong, M.; Zhao, H.; Dai, S.; Chen, C.; Xu, X.; Qiu, J.; Qin, S. Transient numerical simulation of the right coronary artery originating from the left sinus and the effect of its acute take-off angle on hemodynamics. Quant. Imaging Med. Surg. 2021, 11, 2062–2075. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kastellanos, S.; Aznaouridis, K.; Vlachopoulos, C.; Tsiamis, E.; Oikonomou, E.; Tousoulis, D. Overview of coronary artery variants, aberrations and anomalies. World J. Cardiol. 2018, 10, 127–140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Z.; Ding, N.; Zhang, J.; Zhu, Y.; Li, Z.; Li, X. Surgical Outcomes for Children with Anomalous Origin of the Left Coronary Artery from the Pulmonary Artery. Pediatr. Cardiol. 2023, 44, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.H.; Itani, Z.; Al-Tannir, M.; Sayegh, S.; Samaha, A. Primary congenital anomalies of the coronary arteries and relation to atherosclerosis: An angiographic study in Lebanon. J. Cardiothorac. Surg. 2009, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; Tomaszewski, K.A.; Walocha, J.A. Methods of Evidence-Based Anatomy: A guide to conducting systematic reviews and meta-analysis of anatomical studies. Ann. Anat. 2016, 205, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, H.; Mery, C.M.; Sami, S.A.; Qureshi, A.M.; Noel, C.V.; Cutitta, K.; Masand, P.; Tejtel, S.K.S.; Wang, Y.; Molossi, S. Decreased Quality of Life in Children with Anomalous Aortic Origin of a Coronary Artery. World J. Pediatr. Congenit. Heart Surg. 2021, 12, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Al-Umairi, R.S.; Al-Kindi, F.; Al-Tai, S. Prevalence and Spectrum of Coronary Anomalies Detected on Coronary Computed Tomography Angiography: A single centre experience in Oman. Sultan Qaboos Univ. Med. J. 2019, 19, e108–e113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akpinar, I.; Sayin, M.R.; Karabag, T.; Gursoy, Y.C.; Kucuk, E.; Kiran, S.; Dogan, S.M.; Aydin, M. Differences in sex, angiographic frequency, and parameters in patients with coronary artery anomalies: Single-center screening of 25,368 patients by coronary angiography. Coron. Artery Dis. 2013, 24, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Dehaki, M.G.; Al-Dairy, A.; Rezaei, Y.; Ghavidel, A.A.; Omrani, G.; Givtaj, N.; Afjehi, R.S.; Tatari, H.; Jalali, A.H.; Mahdavi, M. Mid-term outcomes of surgical repair for anomalous origin of the left coronary artery from the pulmonary artery: In infants, children and adults. Ann. Pediatr. Cardiol. 2017, 10, 137–143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Doan, T.T.; Sachdeva, S.; Bonilla-Ramirez, C.; Reaves-O’Neal, D.L.; Masand, P.; Mery, C.M.; Binsalamah, Z.; Heinle, J.H.; Molossi, S. Ischemia in Anomalous Aortic Origin of a Right Coronary Artery: Large Pediatric Cohort Medium-Term Outcomes. Circ. Cardiovasc. Interv. 2023, 16, e012631. [Google Scholar] [CrossRef] [PubMed]
- Driesen, B.W.; Warmerdam, E.G.; Sieswerda, G.T.; Schoof, P.H.; Meijboom, F.J.; Haas, F.; Stella, P.R.; Kraaijeveld, A.O.; Evens, F.C.M.; Doevendans, P.A.F.M.; et al. Anomalous coronary artery originating from the opposite sinus of Valsalva (ACAOS), fractional flow reserve- and intravascular ultrasound-guided management in adult patients. Catheter. Cardiovasc. Interv. 2018, 92, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Duran, C.; Kantarci, M.; Durur Subasi, I.; Gulbaran, M.; Sevimli, S.; Bayram, E.; Eren, S.; Karaman, A.; Fil, F.; Okur, A. Remarkable anatomic anomalies of coronary arteries and their clinical importance: A multidetector computed tomography angiographic study. J. Comput. Assist. Tomogr. 2006, 30, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, G.; Behr, E.R.; Tanzarella, G.; Papadakis, M.; Malhotra, A.; Dhutia, H.; Miles, C.; Diemberger, I.; Sharma, S.; Sheppard, M.N. Anomalous Coronary Artery Origin and Sudden Cardiac Death: Clinical and Pathological Insights from a National Pathology Registry. JACC Clin. Electrophysiol. 2019, 5, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.; Kondo, T.; Orihara, T.; Sugiyama, J.; Kondo, M.; Kodama, T.; Fukazawa, H.; Nagaoka, H.; Oida, A.; Yamazaki, J.; et al. Prevalence of anomalous origin of coronary artery detected by multi-detector computed tomography at one center. J. Cardiol. 2011, 57, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, M.; Pontailler, M.; Danial, P.; Moreau de Bellaing, A.; Gaudin, R.; du Puy-Montbrun, L.; Murtuza, B.; Haydar, A.; Malekzadeh-Milani, S.; Bonnet, D.; et al. Anomalous aortic origin of coronary arteries: An alternative to the unroofing strategy. Eur. J. Cardiothorac. Surg. 2020, 58, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Graidis, C.; Dimitriadis, D.; Karasavvidis, V.; Dimitriadis, G.; Argyropoulou, E.; Economou, F.; George, D.; Antoniou, A.; Karakostas, G. Prevalence and characteristics of coronary artery anomalies in an adult population undergoing multidetector-row computed tomography for the evaluation of coronary artery disease. BMC Cardiovasc. Disord. 2015, 15, 112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gräni, C.; Benz, D.C.; Schmied, C.; Vontobel, J.; Possner, M.; Clerc, O.F.; Mikulicic, F.; Stehli, J.; Fuchs, T.A.; Pazhenkottil, A.P.; et al. Prevalence and characteristics of coronary artery anomalies detected by coronary computed tomography angiography in 5634 consecutive patients in a single centre in Switzerland. Swiss Med. Wkly. 2016, 146, w14294. [Google Scholar] [CrossRef] [PubMed]
- Gräni, C.; Benz, D.C.; Steffen, D.A.; Clerc, O.F.; Schmied, C.; Possner, M.; Vontobel, J.; Mikulicic, F.; Gebhard, C.; Pazhenkottil, A.P.; et al. Outcome in middle-aged individuals with anomalous origin of the coronary artery from the opposite sinus: A matched cohort study. Eur. Heart J. 2017, 38, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Harikrishnan, S.; Jacob, S.P.; Tharakan, J.; Titus, T.; Kumar, V.K.; Bhat, A.; Sivasankaran, S.; Bimal, F.; Moorthy, K.M.; Kumar, R.P. Congenital coronary anomalies of origin and distribution in adults: A coronary arteriographic study. Indian Heart J. 2002, 54, 271–275. [Google Scholar] [PubMed]
- Jiang, M.X.; Blackstone, E.H.; Karamlou, T.; Ghobrial, J.; Brinza, E.K.; Haupt, M.J.; Pettersson, G.B.; Rajeswaran, J.; Williams, W.G.; Saarel, E.V.; et al. Anomalous Aortic Origin of a Coronary Artery in Adults. Ann. Thorac. Surg. 2021, 112, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.X.; Brinza, E.K.; Ghobrial, J.; Tucker, D.L.; Gupta, S.; Rajeswaran, J.; Karamlou, T. Cleveland Clinic Adult AAOCA Working Group. Coronary artery disease in adults with anomalous aortic origin of a coronary artery. JTCVS Open 2022, 10, 205–221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kashyap, J.R.; Kumar, S.; Reddy, S.; Rao, K.R.; Sehrawat, O.; Kashyap, R.; Kansal, M.; Reddy, H.; Kadiyala, V.; Uppal, L. Prevalence and Pattern of Congenital Coronary Artery Anomalies in Patients Undergoing Coronary Angiography at a Tertiary Care Hospital of Northern India. Cureus 2021, 13, e14399. [Google Scholar] [CrossRef] [PubMed]
- Koppel, C.J.; Jongbloed, M.R.M.; Kiès, P.; Hazekamp, M.G.; Mertens, B.J.A.; Schalij, M.J.; Vliegen, H.W. Coronary anomalies in tetralogy of Fallot—A meta-analysis. Int. J. Cardiol. 2020, 306, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Kudumula, V.; Mehta, C.; Stumper, O.; Desai, T.; Chikermane, A.; Miller, P.; Dhillon, R.; Jones, T.J.; De Giovanni, J.; Brawn, W.J.; et al. Twenty-year outcome of anomalous origin of left coronary artery from pulmonary artery: Management of mitral regurgitation. Ann. Thorac. Surg. 2014, 97, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Li, R.J.; Sun, Z.; Yang, J.; Yang, Y.; Li, Y.J.; Leng, Z.T.; Liu, G.W.; Pu, L.H. Diagnostic Value of Transthoracic Echocardiography in Patients With Anomalous Origin of the Left Coronary Artery from the Pulmonary Artery. Medicine 2016, 95, e3401. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Lang, X.; Zhang, S.; Wang, C.; Jin, Y.; Zhi, A.; Wang, Q. Effectiveness and Safety of Mitral Valve Plasty in Patients with an Anomalous Origin of the Coronary Artery from the Pulmonary Artery. J. Cardiovasc. Dev. Dis. 2023, 10, 75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mainwaring, R.D.; Reddy, V.M.; Reinhartz, O.; Petrossian, E.; MacDonald, M.; Nasirov, T.; Miyake, C.Y.; Hanley, F.L. Anomalous aortic origin of a coronary artery: Medium-term results after surgical repair in 50 patients. Ann. Thorac. Surg. 2011, 92, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Mainwaring, R.D.; Reddy, V.M.; Reinhartz, O.; Petrossian, E.; Punn, R.; Hanley, F.L. Surgical repair of anomalous aortic origin of a coronary artery. Eur. J. Cardiothorac. Surg. 2014, 46, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Michielon, G.; Di Carlo, D.; Brancaccio, G.; Guccione, P.; Mazzera, E.; Toscano, A.; Di Donato, R.M. Anomalous coronary artery origin from the pulmonary artery: Correlation between surgical timing and left ventricular function recovery. Ann. Thorac. Surg. 2003, 76, 581–588, discussion 588. [Google Scholar] [CrossRef] [PubMed]
- Molossi, S.; Agrawal, H.; Mery, C.M.; Krishnamurthy, R.; Masand, P.; Sexson Tejtel, S.K.; Noel, C.V.; Qureshi, A.M.; Jadhav, S.P.; McKenzie, E.D.; et al. Outcomes in Anomalous Aortic Origin of a Coronary Artery Following a Prospective Standardized Approach. Circ. Cardiovasc. Interv. 2020, 13, e008445. [Google Scholar] [CrossRef] [PubMed]
- Mongé, M.C.; Eltayeb, O.; Costello, J.M.; Sarwark, A.E.; Carr, M.R.; Backer, C.L. Aortic Implantation of Anomalous Origin of the Left Coronary Artery from the Pulmonary Artery: Long-Term Outcomes. Ann. Thorac. Surg. 2015, 100, 154–160, discussion 160–161. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K.P.; Morris, G.M.; Al-Najjar, Y.; Clarke, B.; Fath-Ordoubadi, F.; Fraser, D.; Mahadevan, V.; Mamas, M.; El-Omar, M.M. Percutaneous intervention on anomalous circumflex coronary arteries—A single centre experience. Cardiovasc. Revasc. Med. 2012, 13, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Muzaffar, T.; Ahmad Ganie, F.; Gpoal Swamy, S.; Wani, N.U. The surgical outcome of anomalous origin of the left coronary artery from the pulmonary artery. Int. Cardiovasc. Res. J. 2014, 8, 57–60. [Google Scholar] [PubMed] [PubMed Central]
- Nees, S.N.; Flyer, J.N.; Chelliah, A.; Dayton, J.D.; Touchette, L.; Kalfa, D.; Chai, P.J.; Bacha, E.A.; Anderson, B.R. Patients with anomalous aortic origin of the coronary artery remain at risk after surgical repair. J. Thorac. Cardiovasc. Surg. 2018, 155, 2554–2564.e3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ojha, V.; Pandey, N.N.; Kumar, S.; Ramakrishnan, S.; Jagia, P. Anomalous origin of left main coronary artery from pulmonary artery: Patient characteristics and imaging associations on multidetector computed tomography angiography. J. Card. Surg. 2021, 36, 4043–4053. [Google Scholar] [CrossRef] [PubMed]
- Opolski, M.P.; Pregowski, J.; Kruk, M.; Witkowski, A.; Kwiecinska, S.; Lubienska, E.; Demkow, M.; Hryniewiecki, T.; Michalek, P.; Ruzyllo, W.; et al. Prevalence and characteristics of coronary anomalies originating from the opposite sinus of Valsalva in 8522 patients referred for coronary computed tomography angiography. Am. J. Cardiol. 2013, 111, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Osaki, M.; McCrindle, B.W.; Van Arsdell, G.; Dipchand, A.I. Anomalous origin of a coronary artery from the opposite sinus of Valsalva with an interarterial course: Clinical profile and approach to management in the pediatric population. Pediatr. Cardiol. 2008, 29, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Ouali, S.; Neffeti, E.; Sendid, K.; Elghoul, K.; Remedi, F.; Boughzela, E. Congenital anomalous aortic origins of the coronary arteries in adults: A Tunisian coronary arteriography study. Arch. Cardiovasc. Dis. 2009, 102, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Paratz, E.D.; van Heusden, A.; Zentner, D.; Morgan, N.; Smith, K.; Ball, J.; Thompson, T.; James, P.; Connell, V.; Pflaumer, A.; et al. Prevalence of Coronary Artery Anomalies in Young and Middle-Aged Sudden Cardiac Death Victims (from a Prospective State-Wide Registry). Am. J. Cardiol. 2022, 175, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.; Bannerjee, S.K.; Akhter, N.; Anam, K.; Rahman, M.; Subedi, B.; Haque, S.S. Clinical and angiographic profile of congenital anomalous origin of coronary arteries. Mymensingh. Med. J. 2012, 21, 49–54. [Google Scholar] [PubMed]
- Rodríguez, Z.I.; Murillo, L.E.; Mendoza, A.; Talledo, S. Prevalence of coronary anomalies detected by computed tomography at the National Cardiovascular Institute—INCOR. Peruv. Arch. Cardiol. Cardiovasc. Surg. 2022, 3, 153–161. [Google Scholar] [CrossRef]
- Romeih, S.; Kaoud, A.; Shaaban, M.; Elzoghaby, M.; Abdelfattah, M.; Hashem, M.; Sayed, S.; Gibreel, M.; Elmozy, W. Coronary artery anomalies in tetralogy of Fallot patients evaluated by multi slice computed tomography; myocardial bridge is not a rare finding. Medicine 2021, 100, e24325. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, R.; Froehner, S.; Brunn, J.; Wagner, M.; Brunner, H.; Cherevatyy, O.; Gietzen, F.; Christopoulos, G.; Kerber, S.; Fellner, F. Congenital anomalies of the coronary arteries: Imaging with contrast-enhanced, multidetector computed tomography. Eur. Radiol. 2005, 15, 1110–1121. [Google Scholar] [CrossRef] [PubMed]
- Smettei, O.A.; Sayed, S.; Abazid, R.M. The prevalence of coronary artery anomalies in Qassim Province detected by cardiac computed tomography angiography. J. Saudi Heart Assoc. 2017, 29, 84–89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sivri, N.; Aktoz, M.; Yalta, K.; Ozcelik, F.; Altun, A. A retrospective study of angiographic ally determined anomalous coronary arteries in 12,844 subjects in Thrace region of Turkey. Hippokratia 2012, 16, 246–249. [Google Scholar] [PubMed] [PubMed Central]
- Temel, M.T.; Coşkun, M.E.; Başpınar, O.; Demiryürek, A.T. Prevalence and characteristics of coronary artery anomalies in children with congenital heart disease diagnosed with coronary angiography. Turk Kardiyol. Dern. Ars. 2017, 45, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Yuan, Y.; Lu, H.; Xu, L.; Yang, W.X.; Mu, C.W.; Liu, H.B.; Chen, J.; Dou, K.F.; Tang, Y.D.; et al. Analysis of anomalous origin of coronary arteries by coronary angiography in Chinese patients with coronary artery disease. Int. J. Cardiovasc. Imaging 2018, 34, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Tuncer, C.; Batyraliev, T.; Yilmaz, R.; Gokce, M.; Eryonucu, B.; Koroglu, S. Origin and distribution anomalies of the left anterior descending artery in 70,850 adult patients: Multicenter data collection. Catheter Cardiovasc. Interv. 2006, 68, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Türkoğlu, S.; Ünlü, S.; Taçoy, G.A.; Özdemir, M. Right Coronary Artery Originating from the Left: Do Not Miss the Diagnosis! Cardiol. Res. Pract. 2018, 2018, 1210791. [Google Scholar] [CrossRef] [PubMed]
- Türkvatan, A.; Güray, Y.; Altınsoy, D. Multidetector computed tomography imaging of coronary artery anomalies. Cardiol. Young 2013, 23, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Ugalde, H.; Ramírez, A.; Ugalde, D.; Farías, E.; Silva, A.M. Nacimiento anómalo de las arterias coronarias en 10.000 pacientes adultos sometidos a coronariografía [Coronary artery origin anomalies. Analysis of 10.000 coronary angiographies]. Rev. Med. Chil. 2010, 138, 7–14. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Vinnakota, A.; Stewart, R.D.; Najm, H.; Blackstone, E.H.; Ghobrial, J.; Pettersson, G.B. Anomalous Aortic Origin of the Coronary Arteries: A Novel Unroofing Technique in an Adult Cohort. Ann. Thorac. Surg. 2019, 107, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.L.; Tao, H.K.; Ma, L.; Cui, Y.Q.; Zou, M.H.; Li, J.R.; Li, F.X.; Li, J.; Zhang, X.; Chen, X.X. Pre-operative evaluation and mid-term outcomes of anomalous origin of the left coronary artery from the pulmonary artery based on left ventricular ejection fraction. Front. Cardiovasc. Med. 2022, 9, 961491. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhu, Y.; Zhu, X.; Tang, L.; Xu, Y. Anomalous coronary arteries: Depiction at dual-source computed tomographic coronary angiography. J. Thorac. Cardiovasc. Surg. 2012, 143, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Yakut, K.; Tokel, N.K.; Ozkan, M.; Varan, B.; Erdogan, I.; Aslamaci, M.S. Diagnosis and treatment of abnormal left coronary artery originating from the pulmonary artery: A single-center experience. Anatol. J. Cardiol. 2019, 22, 325–331. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, S.; Zeng, M.S.; Zhang, Z.Y.; Ling, Z.Q.; Ma, J.Y.; Chen, G. Sixty-four-multi-detector computed tomography diagnosis of coronary artery anomalies in 66 patients. Chin. Med. J. 2010, 123, 838–842. [Google Scholar] [PubMed]
- Yousif, N.; Shahin, M.; Lüscher, T.F.; Obeid, S. Gender Differences in Types, Frequency, Clinical Manifestations and Atherosclerotic Burden of Coronary Artery Anomalies. Rev. Recent. Clin. Trials 2019, 14, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ren, Q.; Chen, T.; Qiu, H.; Wen, S.; Zhuang, J.; Liu, X. Outcome of surgical repair of anomalous left coronary artery from the pulmonary artery in a single-center experience. Hell. J. Cardiol. 2023, 73, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, Q.S.; Wang, X.F.; Sun, J.; Yu, L.W.; Ding, M.; Li, Y.G. Diagnostic value of echocardiography on detecting the various types of anomalous origin of the left coronary artery from the pulmonary artery. J. Thorac. Dis. 2020, 12, 319–328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yildiz, A.; Okcun, B.; Peker, T.; Arslan, C.; Olcay, A.; Bulent Vatan, M. Prevalence of coronary artery anomalies in 12,457 adult patients who underwent coronary angiography. Clin. Cardiol. 2010, 33, E60–E64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, L.J.; Yang, G.F.; Huang, W.; Zhou, C.S.; Chen, P.; Lu, G.M. Incidence of anomalous origin of coronary artery in 1879 Chinese adults on dual-source CT angiography. Neth. Heart J. 2010, 18, 466–470. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Zheng, J.Y.; Han, L.; Ding, W.H.; Jin, M.; Zhang, G.Z.; Xiao, Y.Y.; Luo, Y.; Cheng, P.; Meng, X.; Zhao, Q.M. Clinical features and long-term prognosis of patients with anomalous origin of the left coronary artery from the pulmonary artery. Chin. Med. J. 2010, 123, 2888–2894. [Google Scholar] [PubMed]
- Angelini, P.; Uribe, C.; Monge, J.; Tobis, J.M.; Elayda, M.A.; Willerson, J.T. Origin of the right coronary artery from the opposite sinus of Valsalva in adults: Characterization by intravascular ultrasonography at baseline and after stent angioplasty. Catheter Cardiovasc. Interv. 2015, 86, 199–208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ben Ali, W.; Metton, O.; Roubertie, F.; Pouard, P.; Sidi, D.; Raisky, O.; Vouhé, P.R. Anomalous origin of the left coronary artery from the pulmonary artery: Late results with special attention to the mitral valve. Eur. J. Cardiothorac. Surg. 2009, 36, 244–248, discussion 248–249. [Google Scholar] [CrossRef] [PubMed]
- Bibevski, S.; Ruzmetov, M.; Turner, I.I.; Scholl, F.G. Anomalous Aortic Origin of Right Coronary Artery: Outcomes of Surgical and Nonsurgical Treatment. Ann. Thorac. Surg. 2022, 114, 2338–2345. [Google Scholar] [CrossRef] [PubMed]
- Catanoso, A.; Rizzini, A.L.; Cacucci, M.; Valentini, P.; Inama, G. Coronary angioplasty of anomalous coronary arteries. G. Ital. Cardiol. 2010, 11 (Suppl. S1), 72S–77S. (In Italian) [Google Scholar] [PubMed]
- Cheezum, M.K.; Ghoshhajra, B.; Bittencourt, M.S.; Hulten, E.A.; Bhatt, A.; Mousavi, N.; Shah, N.R.; Valente, A.M.; Rybicki, F.J.; Steigner, M.; et al. Anomalous origin of the coronary artery arising from the opposite sinus: Prevalence and outcomes in patients undergoing coronary CTA. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 224–235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, Z.; Wang, X.; Duan, Y.; Wu, L.; Wu, D.; Liang, C.; Liu, C.; Xu, Z. Detection of coronary artery anomalies by dual-source CT coronary angiography. Clin Radiol. 2010, 65, 815–822. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzi, F.; Valentini, F.; Pistoresi, S.; Frascaro, F.; Piu, P.; Cavigli, L.; Valente, S.; Focardi, M.; Cameli, M.; Bonifazi, M.; et al. Causes of sudden cardiac death in young athletes and non-athletes: Systematic review and meta-analysis: Sudden cardiac death in the young. Trends Cardiovasc. Med. 2022, 32, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.N.; Bhambri, K.; Verma, M.; Jagia, P.; Kothari, S.S.; Saxena, A. Anomalies of coronary arteries in tetralogy of Fallot: Evaluation on multidetector CT angiography using dual-source scanner. J. Card. Surg. 2021, 36, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Ponzoni, M.; Frigo, A.C.; Padalino, M.A. Surgery for Anomalous Aortic Origin of a Coronary Artery (AAOCA) in Children and Adolescents: A Meta-Analysis. World J. Pediatr. Congenit. Heart Surg. 2022, 13, 485–494. [Google Scholar] [CrossRef] [PubMed]


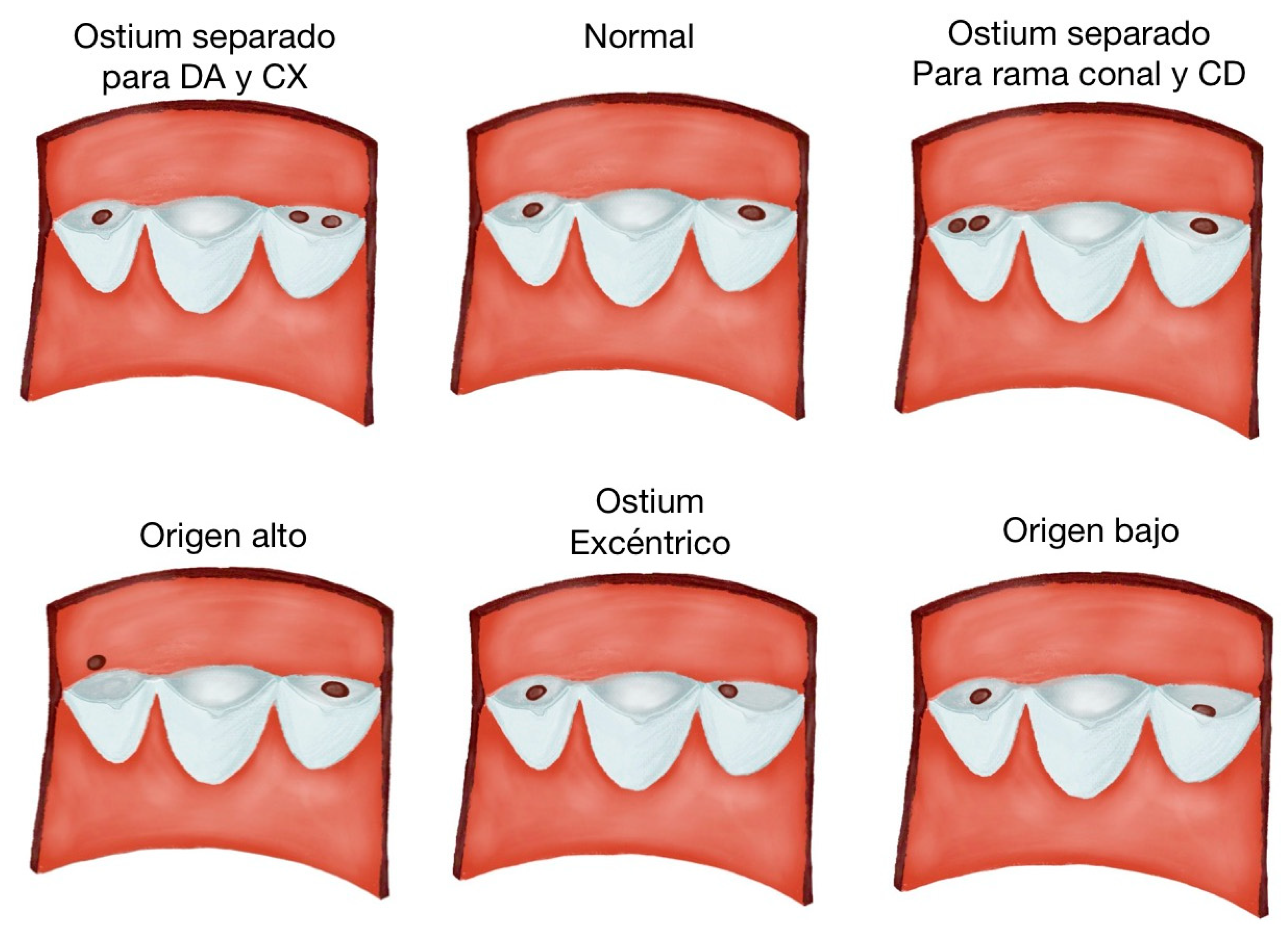
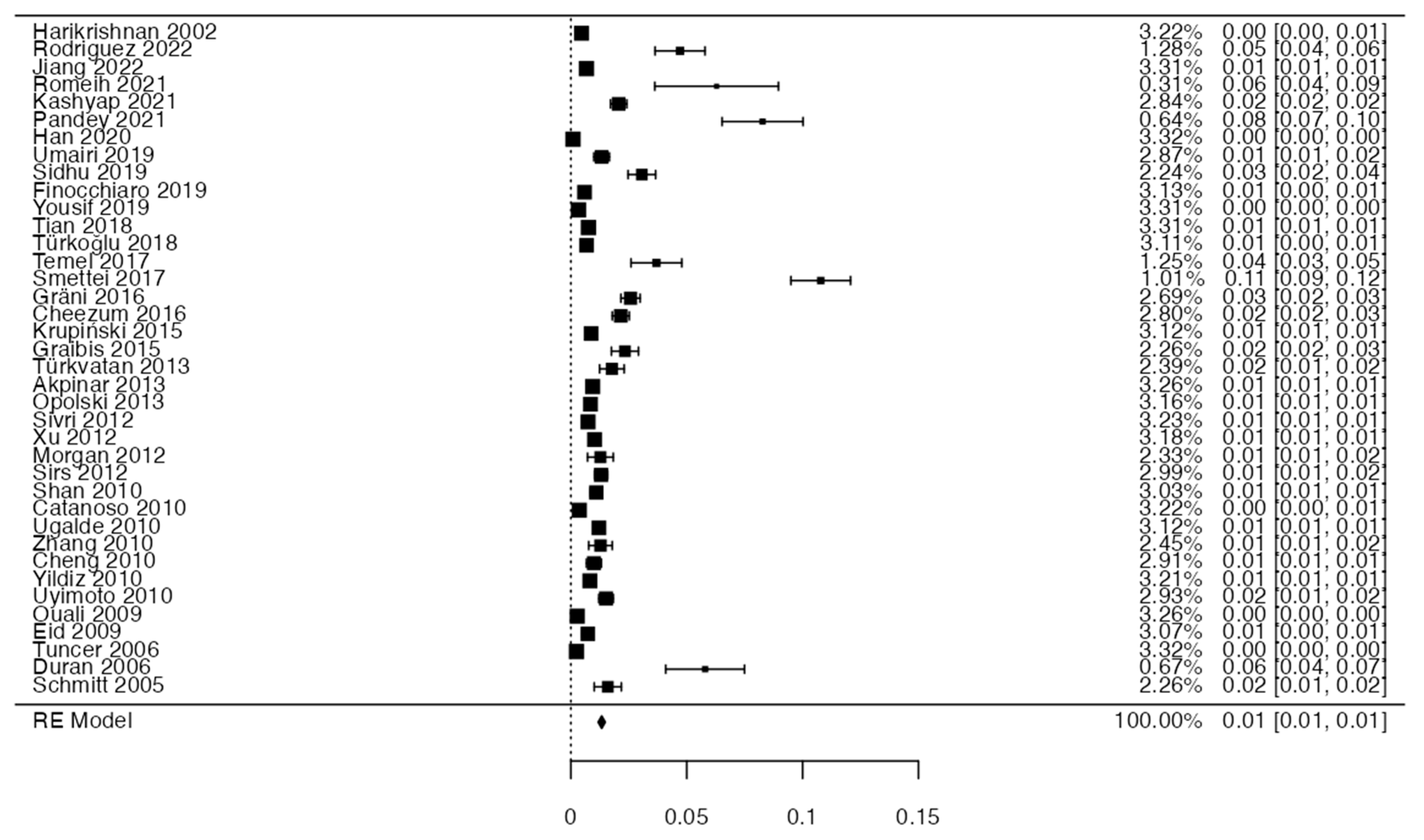
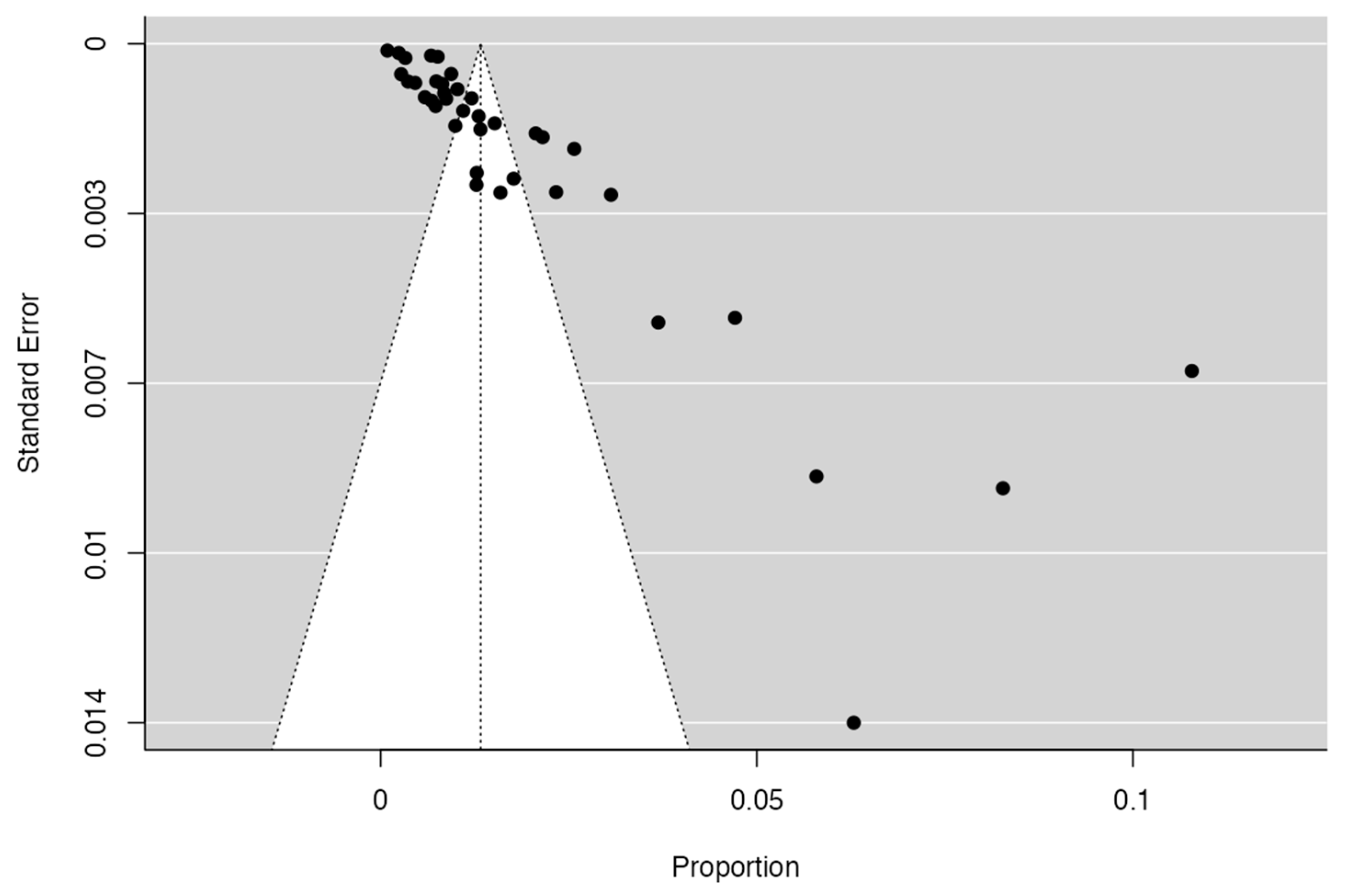
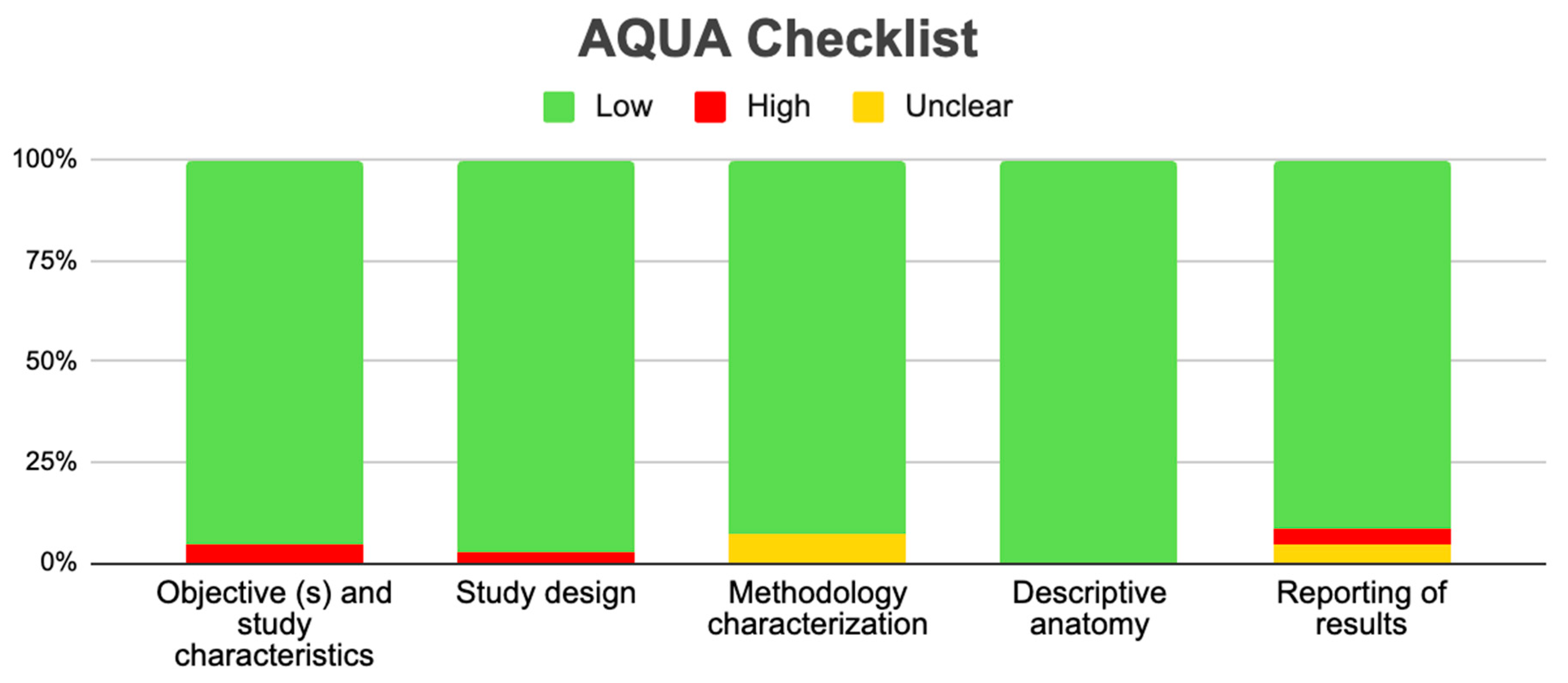
| Author | N of Subjects and Diagnostic Methods | Variant | Prevalence | Age | Location | Male or Female | Clinical Considerations |
|---|---|---|---|---|---|---|---|
| Harikrishnan et al., 2002 | 7400 coronary angiography | Coronary anomalies | 34 cases. The most common anomaly was the origin separation of the left anterior descending artery (LAD) and left circumflex artery (LCx) | 50 | Does not report | 22 men and 12 women | Does not report |
| Michielon et al., 2003 | 31 electrocardiogram and echocardiogram | Origin anomalous of the coronary artery from the PA | 31 anomalous origin left coronary arteries (n = 28), right (n = 2), both (n = 1) of pulmonary artery (PA) | Range, 1.1–203 months | Rome | 16 men and 15 women | Does not report |
| Schmitt et al., 2005 | 1758 TC multidetector (TCMD) | Congenital anomalies of coronary arteries | 28 were found, where 12 anomalies were not hemodynamic and affected only coronary origins (n = 10) or the path of peripheral vessels. | 37–83 | Germany | Does not report | Chest pain |
| Durán et al., 2006 | 725 coronary angiography | Anatomical anomalies of coronary arteries | 42 ectopic origin of the LAD (n = 1, 0.13%), absence of left coronary artery (LCA), principal coronary artery (n = 4, 0.52%) | 33–78 | Afghanistán | 497 patients with 228 women | Chest pain |
| Tuncer et al., 2006 | 70,850 coronary angiography | Origin anomalies and distribution of LAD | 171 | 18–84 | Turkey | 99 men and 72 women | Does not report |
| Osaki et al., 2008 | 35 donors | Origin anomalies of the coronary artery opposite to the sinus of Valsalva. | 35 were found. 13 left principal coronary arteries (TCI) intra-arterial (42%) and 18 right coronary arteries (RCA) intra-arterial (58%) | From Newborn to 16 years old | Canada | 25 men and 6 women | Does not report |
| Eid et al., 2009 | 4650 angiographies | Anomalous aortic origin of the coronary arteries | 34 were found. The anomalous LCx was the most common (19 of 34 patients), while the second most common abnormality was the anomalous origin of coronary right (CR) (9 of 34 patients) | 30–85 years old | Lebanon | 26 men and 8 women | Chest pain, palpitations, and dyspnea or exertional angina |
| Ben Ali et al., 2009 | 62 surgery | LCA from the PA | 62 boys with coronary artery (CA) from PA | 10 days–11 years old | France | Does not report | Congestive heart failure |
| Ouali et al., 2009 | 7330 diagnostic coronary angiography patients | Coronary artery anomaly | 20 were found. The RCA was the most frequently affected vessel (n = 10). The isolated anomalous CX was the second most common anomaly (n = 6). | 21–72 years old | Túnez | 13 men and 7 women | Myocardial infarction |
| Fujimoto et al., 2010 | 5869 multidetector computed tomography | Coronary artery anomaly origin | 89 | 65.9 | Japan | 3.186 men and 2.683 women | Associated with sudden death and ischemic heart disease |
| Yildiz et al., 2010 | 12,457 coronary angiography | Coronary artery abnormalities | 112 were found. The most common anomaly was the separation of the origins of the LAD and LCx arteries from the left sinus of Valsalva (LSV) (63.4%). | 22–79 years | Turkey | 70 men and 42 women | Arrhythmias, syncope, myocardial infarction, or sudden death |
| Zheng et al., 2010 | 23 echocardiogram, angiography, computed tomography (CT), or confirmed during cardiac surgery. | Anomalous origin of the LCA from the PA (ALCAPA) | 23 | 2.5 months–65 years old | China | 13 men and 10 women | Does not report |
| Cheng et al., 2010 | 3625 coronary angiography DSC | Coronary artery abnormalíties | 36 were found; 11 anomalies of the RCA; 5 anomalies of the LCA; 10 anomalies of the LCx; 2 single coronary artery | 15–76 years old | China | 20 men and 16 women | Chest pain during exercise |
| Zhang et al., 2010 | 1879 angiography for TC, dual source | Anomalous origin of the coronary artery (OCA) | 24 were found; 15 patients had an anomalous origin of artery CR (12 of the breast CI, 3 high takeoff), and 8 patients had an anomalous origin of artery coronary left (CL). | 25–91 years old | China | 1017 men and 864 women | Chest pain during exercise or difficulty breathing |
| Ugalde et al., 2010 | 10,000 coronary angiography | Anomalous OCA | 121 were found. The most common anomaly was the origin of the RCA from the left coronary sinus (LCS) in 75%, followed by the origin of the right circumflex artery (RCx) in 20%. | 58 years old | Chili | 70% men | Does not report |
| Catanoso et al., 2010 | 6300 coronary angiography | Coronary artery anomalous | 23 | 35–79 years old | Rome | 20 men and 3 women | Angina and ventricular arrhythmias |
| Yang et al., 2010 | 6014 computed tomography | coronary artery abnormalities | 66 | 4–82 years old | China | 5 men and 21 women | Chest pain, dyspnea, palpitations, myocardial infarction, or various arrhythmias. |
| Mainwaring et al., 2011 | 50 surgical repair | Anomalous aortic OCAs | 50 were found; 31 had the right coronary artery originating in the LSV, 17 had the LCA originating in the right sinus, and 2 had a single eccentric coronary ostium. | 5 days–7 days | USA | 36 men and 14 women | Chest pain, syncope or near syncope, myocardial infarction, or heart failure |
| Rahman et al., 2012 | 24 patients | Anomalous aortic OCAs | 21 | Bangladesh | Doe not specify | Associated with angina pectoris, arrhythmias, sudden death, myocardial infarction, syncope, and congestive heart failure | |
| Sohrabi et al., 2020 | 6065 catheterization cardiac | Abnormalities in the coronary arteries | 79 were found. The most common abnormality was separate ostia of the LAD and the LCx, which was found in 42 patients (53.16%). | 34–84 years old | Irán | 58 men and 21 women | Chest pain |
| Morgan et al., 2012 | 1570 Percutaneous intervention | Anomalous circumflex coronary arteries | 20 were found. In 9 cases the circumflex arose from the left coronary cusp, in 7 cases it arose from the right coronary cusp, and in the remaining 4 cases it arose from the proximal RCA | Does not report | England | Does not report | Chest pain, ECG changes, and troponin elevation |
| Xu et al., 2012 | 12,145 DSCT-CA dual-source computed tomography coronary angiography | Coronary artery anomalies | 124. An anomalous origin of the LCx from the right sinus of Valsalva (RSV) or the RCA was described in 17 patients. An anomalous origin of the left main artery from the RSV was described in 1 patient. | 5–86 years old | China | 80 men and 44 women | Chest pain, syncope, or dyspnea on exertion |
| Sivri et al., 2012 | 12,844 coronary angiography | Coronary artery anomalous | 95 were found. The LCx of the RSV or the RCA are the most prevalent (46 of 95 patients), and the second most common anomaly is the anomalous aortic origin of the RCA (32 of 95 patients). | Does not report | Tukey | 69 men and 26 women | Does not report |
| Opolski et al., 2013 | 8522 coronary computed tomography angiography | Coronary anomalies originating from the opposite sinus of Valsalva | 72 were found. Right-sided origins of the LCA (n = 11), the LAD (n = 9), the LCx (n = 33), and the left origin of the RCA (n = 20). | 12–93 years old | Poland | 37 men and 35 women | Atypical chest pain and anginal pain |
| Akpinar et al., 2013 | 25,368 coronary angiography | Coronary anomalies | 238 were found. The most common was a LAD circumflex artery originating from the separated ostium (0.29%). The second most common anomaly was an RCA originating from the left sinus of Valsalva (LSV) (0.23%) | 30–82 years old | Turkey | 92 women and 14 men | Coronary pain, angina, and chest pain |
| Türkvatan et al., 2013 | 2375 multidetector computed tomography | Coronary arteries anomalies | 42 | 18–82 years old | Turkey | 24 men and 18 women | Chest pain |
| Mainwaring et al., 2014 | 76 stress tests, stress echocardiography, and myocardial perfusion studies | Anomalous aortic OCA | 65 were found; 38 were anomalous RCAs and 17 were anomalous LCAs | 15–47 years old | USA | 55 men and 21 women | Chest pain and syncope |
| Muzaffar et al., 2014 | 53 | ALCAPA | 53 | 4 months | India | 29 men and 24 women | Does not report |
| Kudumula et al., 2014 | 25 | ALCAPA | 25 | Does not report | England | 7 men and 18 women | Arrhythmias, chest pain, heart failure, and sudden death. |
| Graidis et al., 2015 | 2572 64-slice MDCT coronary angiography | Congenital coronary anomalies | 60 were found. In 16 patients, an anomaly in the left main coronary artery (LMCA) and both in 2 patients. A separate origin of the (LAD and CX) from the (LSV) was found in 15 patients. In 9 patients the RCA arose from the opposite sinus of Valsalva with a separate ostium. An abnormal origin of LCx was found in 6 patients. | 29–80 years old | Greece | 83.3% men | Chest pain |
| Angelini et al., 2015 | 67 baseline intravascular ultrasound | Origin of the RCA from the opposite sinus of Valsalva | 67 | 12–73 years old | USA | 67% men | Chest pain and dyspnea |
| Mongé et al., 2015 | 36 transthoracic echocardiography (TTE) and cardiac catheterization | Anomalous origin of the LCA from the PA | 36 were found. The anomalous coronary artery arose from the main PA in 3 patients, the right PA in 2, and from the junction of the right and main PAs in 2. | From 14 months to 18 years old | USA | 18 men and 18 women | Does not report |
| Krupiński et al., 2015 | 7115 patients undergoing cardiac CT | Anomalous OCA | 62 were found. Anomalous aortic and pulmonary OCA affected 59 (0.83%) and 3 (0.04%) cases, respectively. | 17–80 years old | Poland | 34 men | Does not report |
| Gräni et al., 2016 | 5634 coronary computed tomography angiography (CCTA) | Anomalies of the coronary artery | 145 were found; 49 patients showed malignant CAAs, and the remaining 96 patients (66.2%) were classified as having benign variants. | Does not report | Switzerland | Does not report | Does not report |
| Agrawal et al., 2016 | 61 | Anomalous aortic OCA | 53 | 8–18 years old | USA | Does not report | Does not report |
| Cheezum et al., 2017 | 5991 coronary CT angiography | Anomalous OCA from the opposite sinus (ACAOS) | 129 patients with ≥1 ACAOS vessel with the following course subtypes: pre-pulmonic, subpulmonic, inter-arterial, retro aortic, and retrocardiac | 5–83 years old | USA | 68% men | Does not report |
| Li et al., 2016 | 22 transthoracic echocardiography | ALCAPA | 22 | 12.9 ± 19.5 years old | Does not report | 9 men and 13 women | Does not report |
| Grani et al., 2017 | 68 coronary computed tomography angiography (CCTA) | ACAOS | 68 | 56 years old | Switzerland | 73% men | Associated with adverse cardiac events in young people |
| Smettei et al., 2017 | 2235 computed tomography angiography (CTA) | Coronary artery abnormalities | 241 | 24–77 years old | USA | 166 men and 75 women | Syncope, chest pain, and sudden cardiac death |
| Temel et al., 2017 | 1138 catheterization and angiography | Anomalous of the coronary artery | 42 were found. It was determined that 38 were anomalies of origin, 2 were anomalies of the intrinsic coronary artery anatomy, and 2 were anomalies of the coronary termination. | Does not report | Turkey | 20 men and 22 women | Does not report |
| Dehaki et al., 2017 | 21 | ALCAPA | 21 | 22 days–51 years old | Iran | 12 men and 9 women | Chest pain, dyspnea, and palpitations |
| Vinnakota et al., 2018 | 40 Transthoracic echocardiography, cardiac catheterization, computed tomography angiography, or cardiac magnetic resonance imaging | Anomalous aortic OCAs | 40 were found. The coronary anomaly was from right to left in 35 patients, left to right in 4, and left coronary from the noncoronary sinus in 1 patient. | 19–67 years old | USA | 23 men and 17 women | Association with sudden cardiac death and symptoms similar to ischemia or arrhythmias |
| Nees et al., 2018 | 60 | anomalous aorta of a coronary artery | 60 were found; 30 with FTAA and 30 with ARCA | From 4 months to 68 years old | USA | 38 men and 22 women | Chest pain and difficulty breathing |
| Driesen et al., 2018 | 30 cardiac catheterization | Anomalous coronary artery originating from the opposite sinus of Valsalva | 25 | 36–64 years old | Does not report | 19 men and 11 women | Chest pain |
| Türkoğlu et al., 2018 | 5165 coronary angiograms. | RCA originating from the left | 35 were found (16 ACD originating from LCS, 13 LCx from the right coronaries (RCs) or ACD and 6 others). The most common form was RCA originating from LCS. Furthermore, we identified 5 cases of ACD originating in the LCS | 17–90 years old | Does not report | 24 men and 11 women | Does not report |
| Tian et al., 2018 | 110,158 coronary angiograms. | Anomalous OCAs | 835 were found. The incidences of anomalous origin of the RCA, of the LCA, of both the RCA and the LCA, of the artery | Does not report | China | Does not report | Chest pain, dyspnea, palpitations, ventricular fibrillation, myocardial infarction, and even syncope, and sudden cardiac death |
| Yousif et al., 2019 | 39,577 angiographic studies | Anomalous of the coronary arteries | 130 were found. The most prevalent anomaly overall was the LCx to RCA/sinus, which was present in n = 47 (36.2%). | Does not report | Switzerland | 40 men and 90 women | Does not report |
| Finocchiaro et al., 2019 | 5100 sudden cardiac deaths | Anomalous OCA | 30 were found. Anomalous ICA arising from the right sinus of Valsalva (ALCA) (n = 11) and anomalous ICA arising from the left sinus of Valsalva (ARCA) (n = 11) were the most common | 16 years old | London | 23 men and 7 women | Syncope and sudden cardiac death |
| Yakut et al., 2019 | 33 electrocardiogram (ECG), telecardiogram | ALCAPA | 33 | 6–12 months | Turkey | 11 men and 22 women | The most commonly presenting signs and symptoms were dyspnea, tachypnea, diaphoresis, prolonged feeding time, and developmental delay. |
| Sidhu et al., 2019 | 3233 coronary catheter angiograms | Coronary artery anomalies (CAAs) | 99 were found. Split right coronary artery (RCA) was the most common coronary anomaly and was observed in 27 patients. Dual LADs were the second most common anomaly and were observed in 22 cases. | 20–86 years old | India | 74.75% men and 25.25% women | Acute coronary syndrome, stable IHD with exertional angina or exertional dyspnea, atypical chest pain with electrocardiographic (ECG) or echocardiographic changes, heart failure, or LV dysfunction |
| Al-Umairi et al., 2019 | 4445 coronary computed tomography angiography | Coronary abnormalities | 59 | 12–80 years old | Sultanato de Omán | Does not report | Chest pain |
| Han et al., 2020 | 48,719 CT coronary angiography | left ACAOS | 44 were found. The right sinus of Valsalva (RSV) was the most common origin (36/46, 78.26%). | Does not report | China | Does not report | Chest pain, palpitations, shortness of breath, and arrhythmia |
| Yu et al., 2020 | 30 echocardiographic examination | ALCAPA | 24 ALCAPA | From 1 month to 51 years old | China | 18 men and 12 women | Does not report |
| Ismail et al., 2020 | 29 echocardiographic | ALCAPA | 29 | Does not report | Saudi Arabia | 15 men and 14 women | Does not report |
| Molosi et al., 2020 | 209 | Anomalous aortic OCAy | 163 were found; 116 anomalous right AC, 25 anomalous left AC, 17 single AC, and 5 anomalous circumflex AC | ≤20 years old | USA | 104 men and 53 women | Cardiac arrest/shock |
| Gaillard et al., 2020 | 61 anomalous aortic origin of a coronary artery | Anomalous aortic OCA | 61 were found; 40 had right AAOCA and 21 had left AAOCA | Does not report | France | 73.8% men | Chest pain |
| Bibevski et al., 2021 | 86 Echocardiograms | Anomalous origin of the RCA | 86 | 16 years old | USA | 52 men and 34 women | The presence of the variant can cause sudden death; in addition, given early diagnosis, surgical correction is suggested. |
| Pandey et al., 2021 | 955 multidetector CT angiography using dual-source scanner | Coronary artery abnormalities | 79 | 2–5 years old | India | 690 mn and 265 women | Does not report |
| Jiang et al., 2021 | 645 anomalous origin of a coronary artery | Anomalous OCA | 167 were found. The RCA in 57% (96 of 167), the LCA in 23% (39 of 167), the LAD in 2% (4 of 167), LCx. in 16% (26 of 167), and multiple coronaries in 1% (2 of 167) | 18 years and over | USA | 91 men and 76 women | Chest pain and dyspnea |
| Kashyap et al., 2021 | 6258 coronary angiograms | Congenital coronary artery anomalies | 129 were found. Anomalous origin and course of the coronary arteries were the most frequently observed anomalies (81 cases), followed by intrinsic anomalies of the coronary artery system in (44 cases). | 32–81 years old | India | 87 men and 42 women | Angina, dyspnea, syncope, acute coronary syndrome, heart failure, ventricular arrhythmias, and sudden cardiac death (SCD) |
| Cong et al., 2021 | 26 CTA images | RCA originating from the left sinus | 26 | 62 years old | CHINA | 17 men and 9 women | Dizziness and chest tightness. |
| Romeih et al., 2021 | 318 scanned with 128 dual-source multi-slice SOMATOM scanners (Siemens, Erlangen, Germany) | Anomalous OCA | 20 | From 1 month to 46 years old | Egypt | 175 men and 143 women | Does not report |
| Ojha et al., 2021 | 21 arteriography | ALCAPA | 21 | From 2 months to 54 years old | India | 8 men, 4 women, and 9 children | Gradually progressive dyspnea and growth retardation in children, asymptomatic adults |
| Jiang et al., 2022 | 118,167 patients from our cardiac catheterization database | Anomalous aortic OCA | 793 | Does not report | USA | Does not report | Does not report |
| Rodríguez et al., 2022 | 1486 computed tomography scanner | Anomalous coronary (AC) | 70 were found. The coronary artery of the opposite coronary sinus was the most common (48.6%), with the RCA being the main anomalous artery (31%) and the main course being the interarterial (31%). | From 3 months to 90 years | Peru | 4.3% women | Typical chest pain, dyspnea, palpitations, syncope, and electrocardiographic abnormalities |
| Wang et al., 2022 | 89 | ALCAPA | 89 | Does not report | China | Does not report | Does not report |
| Xia et al., 2022 | 51 | Anomalous origin of the LCA from the PA | 51 | 12 months | China | 22 men and 29 women | Does not report |
| Doan et al., 2023 | 220 computed tomography | Anomalous aortic origin of the RCA | 220 | <21 months | USA | 60% men | Chest pain and exertional syncope |
| Lv et al., 2023 | 65 | Anomalous OCA from the PA | 65 | <3 months | China | Does not report | Does not report |
| Yu et al., 2023 | 136 | ALCAPA | 136 | 1–53 years old | China | 88 women and 48 men | Difficulty breathing and cough. |
| Author | Total, n | Prevalence |
|---|---|---|
| Harikrishnan et al., 2002 | 7400 | 34 |
| Rodríguez et al., 2022 | 1486 | 70 |
| Jiang et al., 2022 | 118,167 | 793 |
| Romeih et al., 2021 | 318 | 20 |
| Kashyap et al., 2021 | 6.258 | 129 |
| Pandey et al., 2021 | 955 | 79 |
| Han et al., 2020 | 48.719 | 44 |
| Al-Umairi et al., 2019 | 4.445 | 59 |
| Sidhu et al., 2019 | 3.233 | 99 |
| Finocchiaro et al., 2019 | 5.100 | 30 |
| Yousif et al., 2019 | 39.577 | 130 |
| Tian et al., 2018 | 110.158 | 835 |
| Türkoğlu et al., 2018 | 5.165 | 35 |
| Temel et al., 2017 | 1.138 | 42 |
| Smettei et al., 2017 | 2235 | 241 |
| Gräni et al., 2016 | 5.634 | 145 |
| Cheezum et al., 2017 | 5991 | 129 |
| Krupińsk et al., 2015 | 7.115 | 62 |
| Graidis et al., 2015 | 2572 | 60 |
| Türkvatan et al., 2013 | 2.375 | 42 |
| Akpinar et al., 2013 | 25.368 | 238 |
| Opolski et al., 2013 | 8.522 | 72 |
| Sivri et al., 2012 | 12.844 | 95 |
| Xu et al., 2012 | 12.145 | 124 |
| Morgan et al., 2012 | 1570 | 20 |
| Rahman et al., 2012 | 6.065 | 79 |
| Yang et al., 2010 | 6.014 | 66 |
| Catanoso et al., 2010 | 6.300 | 23 |
| Ugalde et al., 2010 | 10.000 | 121 |
| Zhang et al., 2010 | 1.879 | 24 |
| Cheng et al., 2010 | 3625 | 36 |
| Yildiz et al., 2010 | 12.457 | 112 |
| Fujimoto et al., 2010 | 5.869 | 89 |
| Ouali et al., 2009 | 7.330 | 20 |
| Eid et al., 2009 | 4.650 | 34 |
| Tuncer et al., 2006 | 70.850 | 171 |
| Durán et al., 2006 | 725 | 42 |
| Schmitt et al., 2005 | 1758 | 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuenzalida, J.J.V.; Becerra-Rodriguez, E.S.; Quivira Muñoz, A.S.; Baez Flores, B.; Escalona Manzo, C.; Orellana-Donoso, M.; Nova-Baeza, P.; Suazo-Santibañez, A.; Bruna-Mejias, A.; Sanchis-Gimeno, J.; et al. Anatomical Variants of the Origin of the Coronary Arteries: A Systematic Review and Meta-Analysis of Prevalence. Diagnostics 2024, 14, 1458. https://doi.org/10.3390/diagnostics14131458
Fuenzalida JJV, Becerra-Rodriguez ES, Quivira Muñoz AS, Baez Flores B, Escalona Manzo C, Orellana-Donoso M, Nova-Baeza P, Suazo-Santibañez A, Bruna-Mejias A, Sanchis-Gimeno J, et al. Anatomical Variants of the Origin of the Coronary Arteries: A Systematic Review and Meta-Analysis of Prevalence. Diagnostics. 2024; 14(13):1458. https://doi.org/10.3390/diagnostics14131458
Chicago/Turabian StyleFuenzalida, Juan José Valenzuela, Emelyn Sofia Becerra-Rodriguez, Alonso Sebastián Quivira Muñoz, Belén Baez Flores, Catalina Escalona Manzo, Mathias Orellana-Donoso, Pablo Nova-Baeza, Alejandra Suazo-Santibañez, Alejandro Bruna-Mejias, Juan Sanchis-Gimeno, and et al. 2024. "Anatomical Variants of the Origin of the Coronary Arteries: A Systematic Review and Meta-Analysis of Prevalence" Diagnostics 14, no. 13: 1458. https://doi.org/10.3390/diagnostics14131458
APA StyleFuenzalida, J. J. V., Becerra-Rodriguez, E. S., Quivira Muñoz, A. S., Baez Flores, B., Escalona Manzo, C., Orellana-Donoso, M., Nova-Baeza, P., Suazo-Santibañez, A., Bruna-Mejias, A., Sanchis-Gimeno, J., Gutiérrez-Espinoza, H., & Granite, G. (2024). Anatomical Variants of the Origin of the Coronary Arteries: A Systematic Review and Meta-Analysis of Prevalence. Diagnostics, 14(13), 1458. https://doi.org/10.3390/diagnostics14131458






