New Insights on Using Oral Semaglutide versus Dapagliflozin in Patients with Type 2 Diabetes and Metabolic Dysfunction-Associated Steatotic Liver Disease
Abstract
1. Introduction
2. Materials and Methods
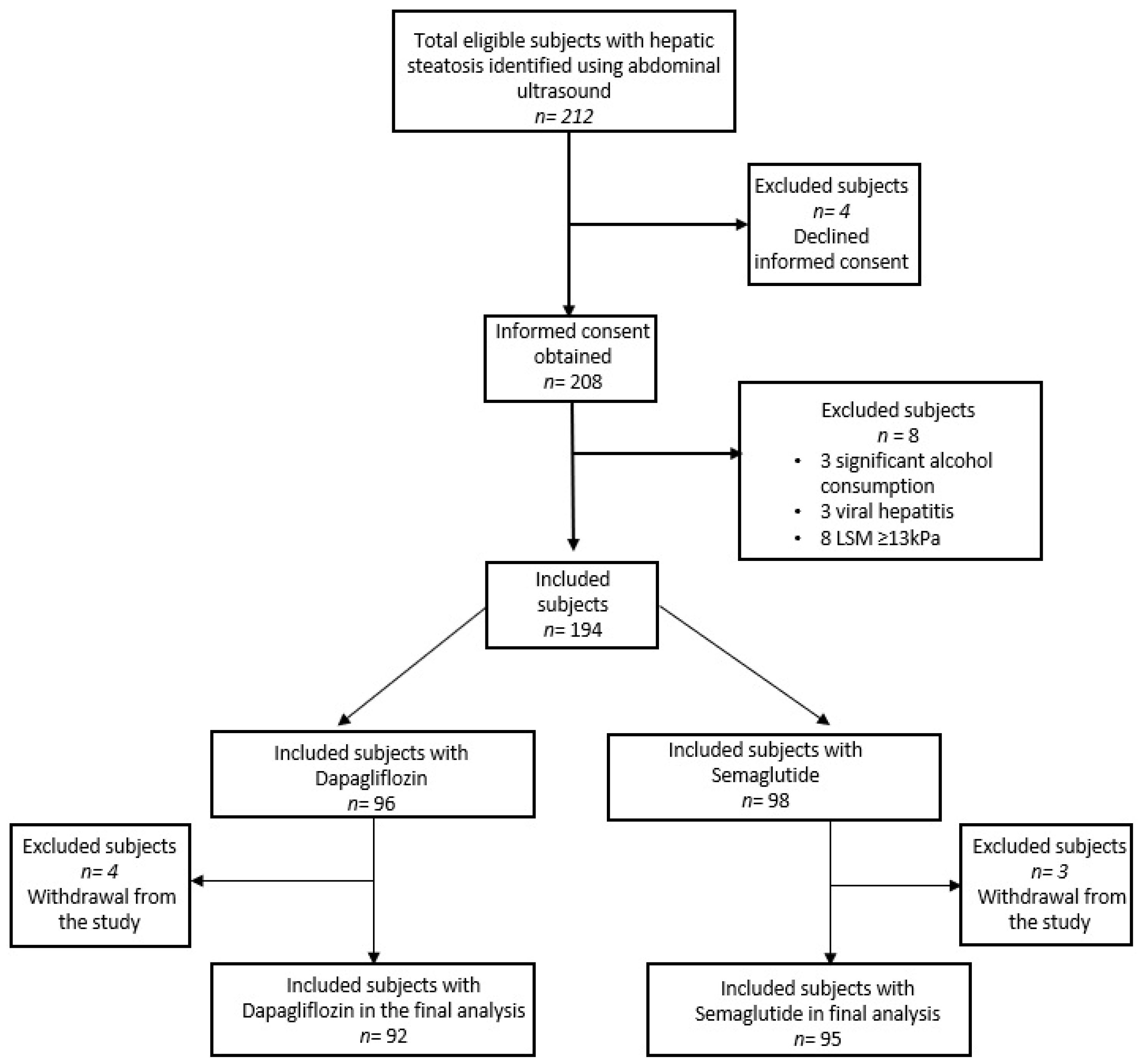
2.1. Patients
2.2. Study Procedures and Clinical Examination
2.3. Biological Tests and VCTE Examinations
2.4. Non-Invasive Tests for the Evaluation of Liver Fibrosis
2.5. Anthropometric of Body Composition
2.6. Study Outcomes and Statistics
3. Results
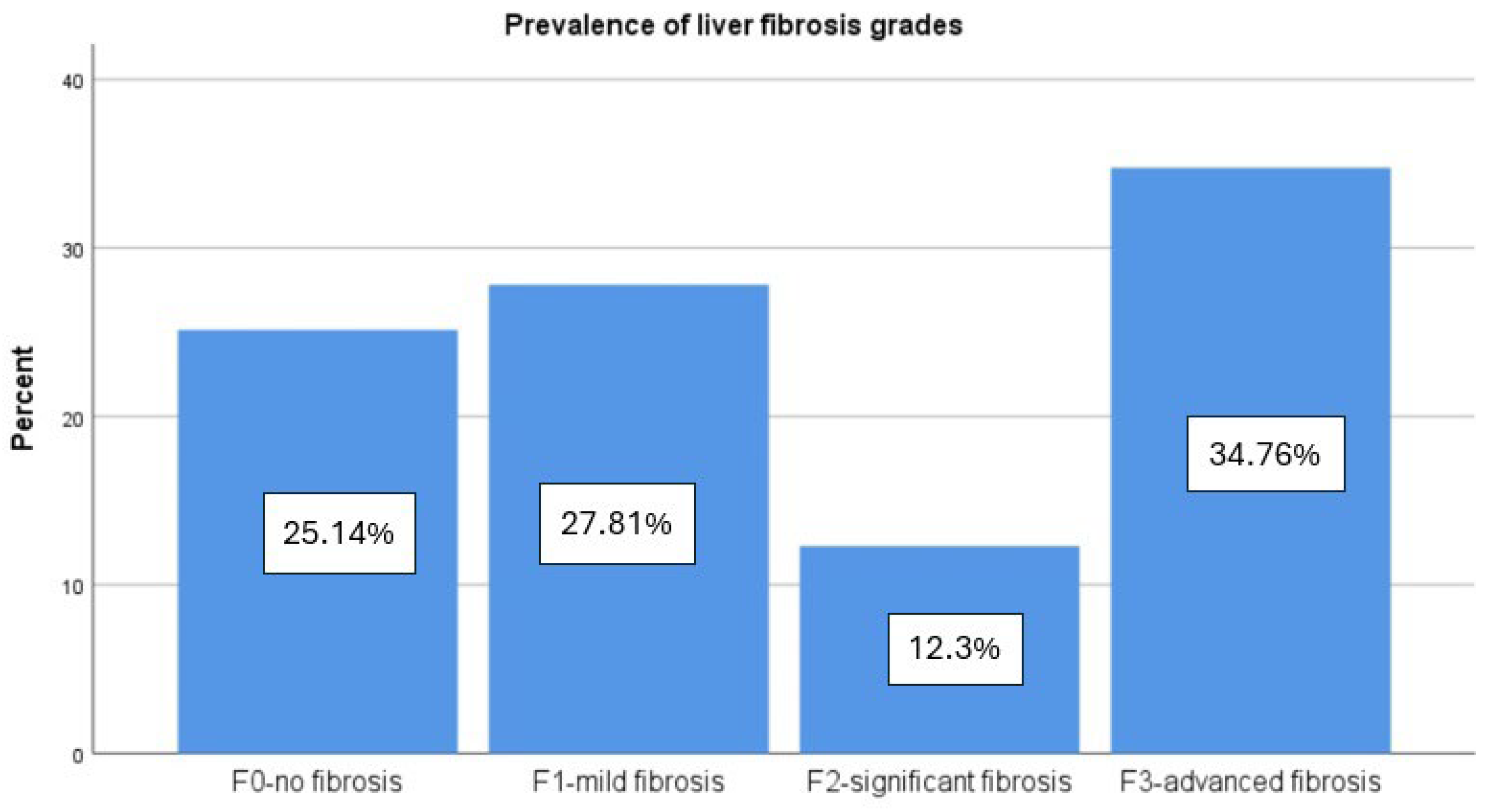
3.1. Patient Characteristics
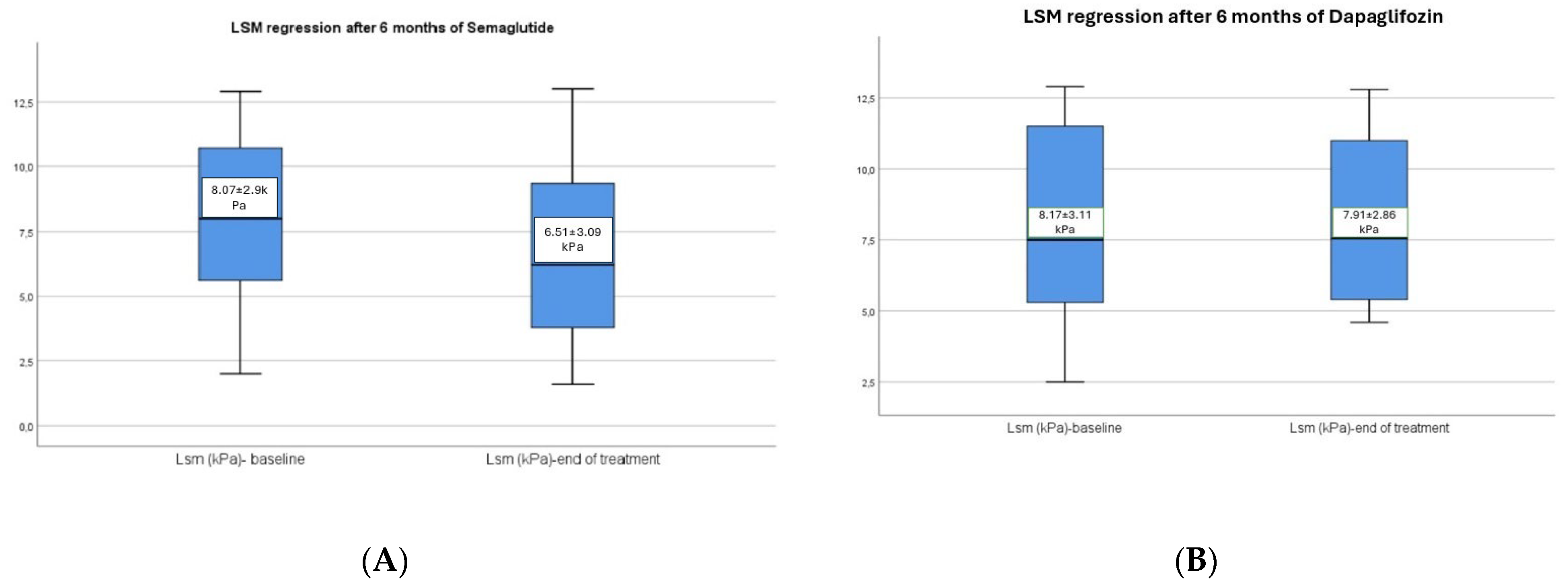
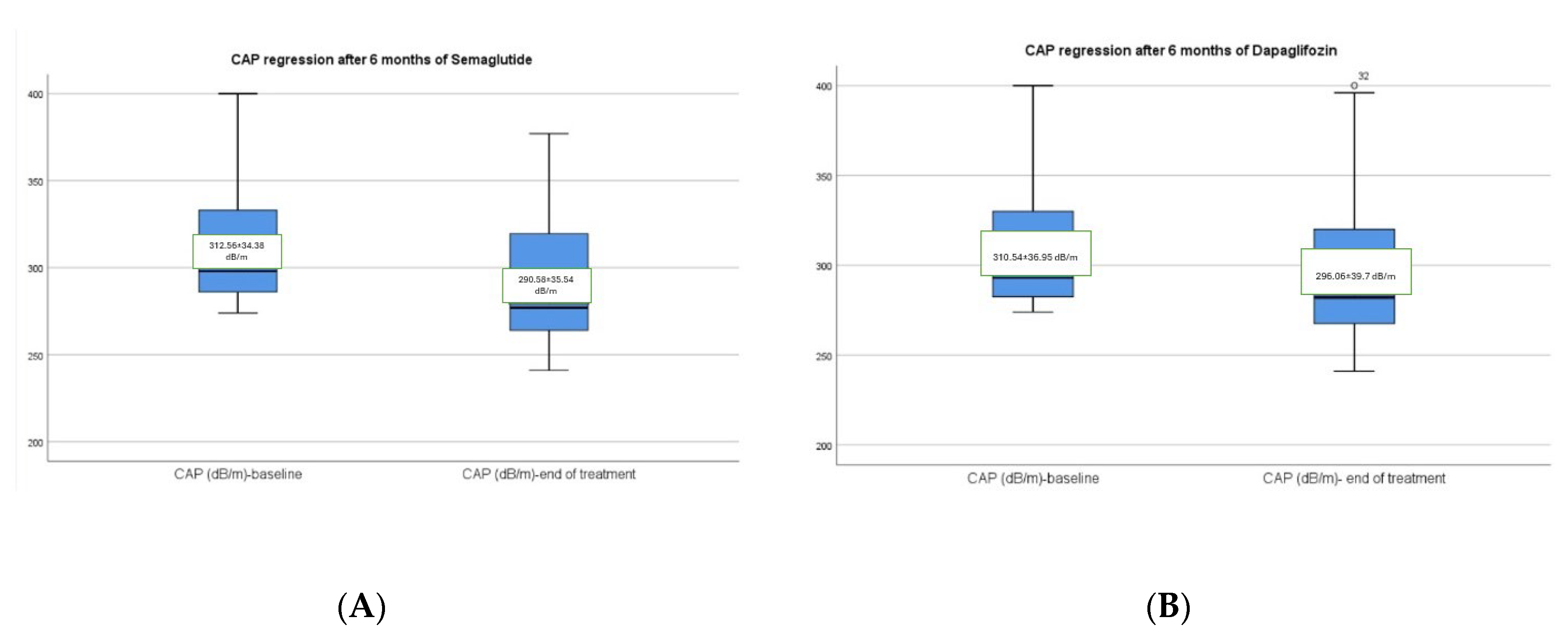
3.2. Hepatic Changes Associated with Semaglutide and Dapagliflozin Treatment
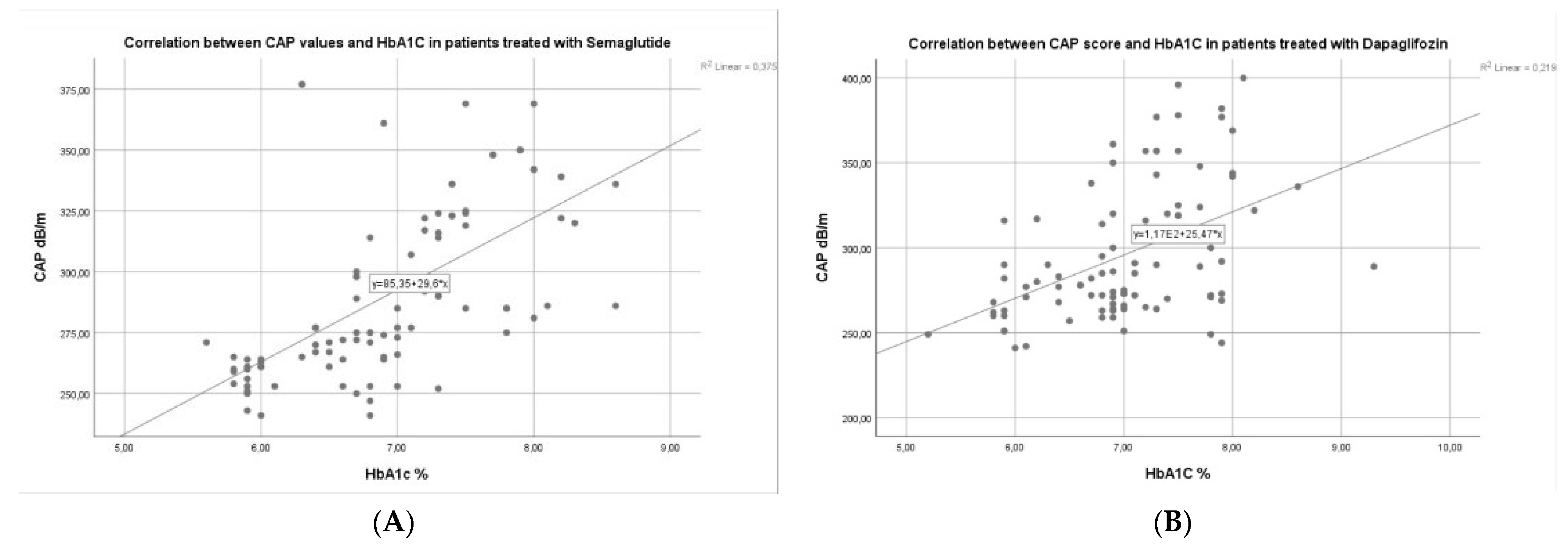
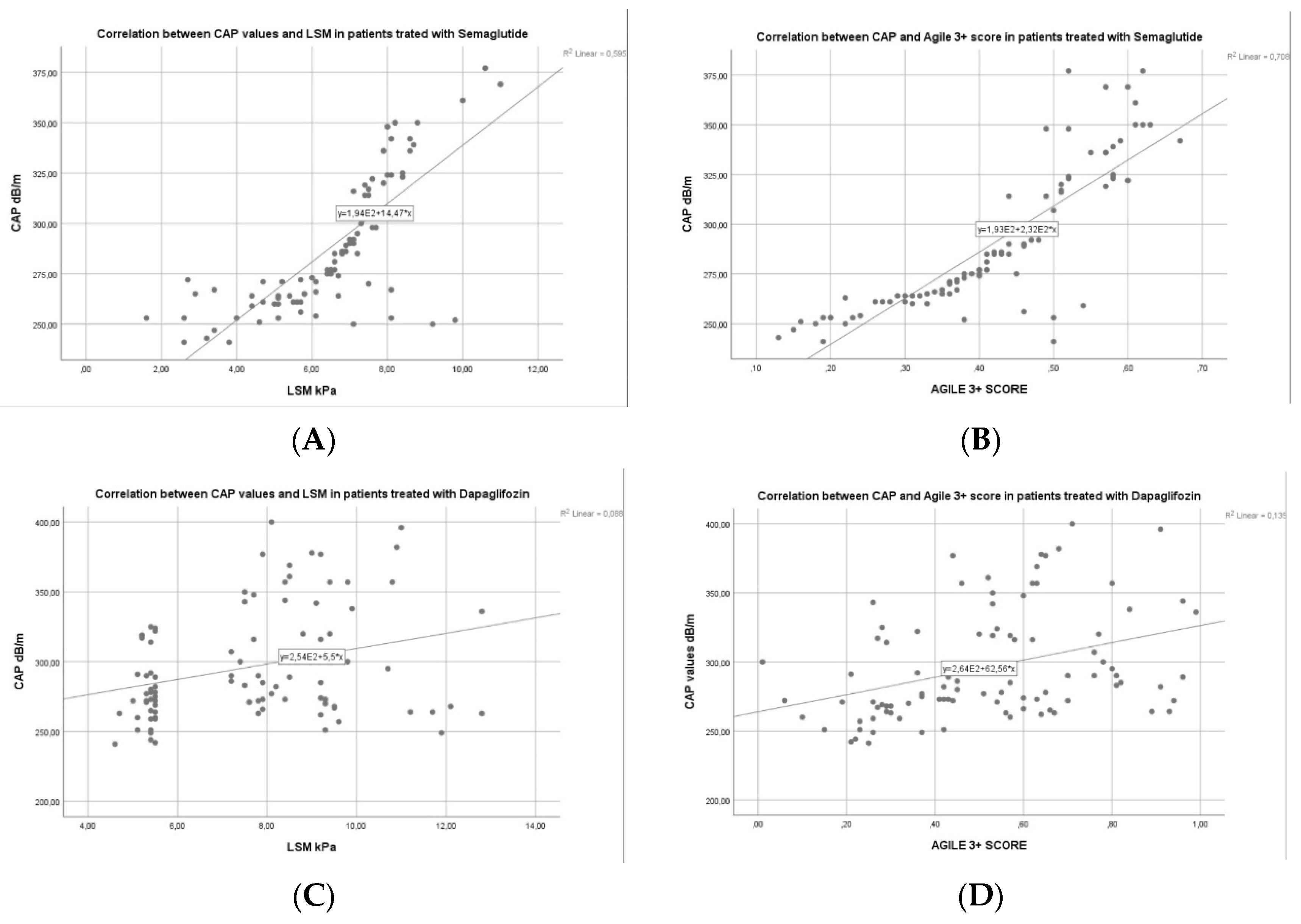
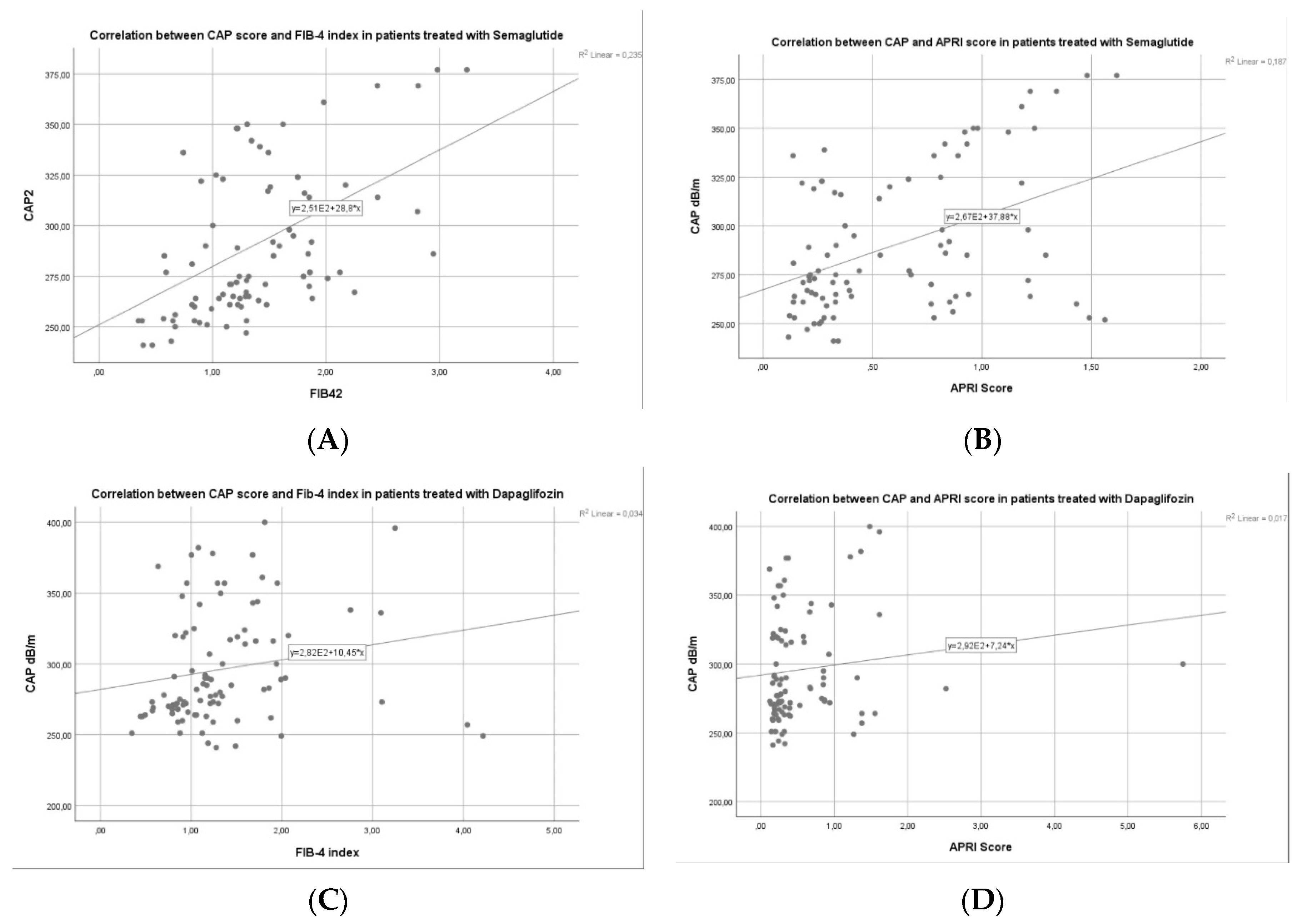
3.3. Factors Associated with Liver Steatosis Response for Patients with Semaglutide vs. Dapagliflozin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Zummo, F.P.; Cullen, K.S.; Honkanen-Scott, M.; Shaw, J.A.M.; Lovat, P.E.; Arden, C. Glucagon-Like Peptide 1 Protects Pancreatic β-Cells from Death by Increasing Autophagic Flux and Restoring Lysosomal Function. Diabetes 2017, 66, 1272–1285. [Google Scholar] [CrossRef] [PubMed]
- Cornu, M.; Yang, J.Y.; Jaccard, E.; Poussin, C.; Widmann, C.; Thorens, B. Glucagon-like peptide-1 protects beta-cells against apoptosis by increasing the activity of an IGF-2/IGF-1 receptor autocrine loop. Diabetes 2009, 58, 1816–1825. [Google Scholar] [CrossRef]
- Khound, R.; Taher, J.; Baker, C.; Adeli, K.; Su, Q. GLP-1 Elicits an Intrinsic Gut-Liver Metabolic Signal to Ameliorate Diet-Induced VLDL Overproduction and Insulin Resistance. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2252–2259. [Google Scholar] [CrossRef]
- Jin, T.; Weng, J. Hepatic functions of GLP-1 and its based drugs: Current disputes and perspectives. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E620–E627. [Google Scholar] [CrossRef] [PubMed]
- Turton, M.D.; O’Shea, D.; Gunn, I.; Beak, S.A.; Edwards, C.M.; Meeran, K.; Choi, S.J.; Taylor, G.M.; Heath, M.M.; Lambert, P.D.; et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996, 379, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Katsurada, K.; Yada, T. Neural effects of gut- and brain-derived glucagon-like peptide-1 and its receptor agonist. J. Diabetes Investig. 2016, 7 (Suppl. S1), 64–69. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Ahmed, F.; Mara, K.C.; Addissie, B.D.; Allen, A.M.; Gores, G.J.; Roberts, L.R. Diabetes is Associated with Increased Risk of Hepatocellular Carcinoma in Patients with Cirrhosis from Nonalcoholic Fatty Liver Disease. Hepatology 2020, 71, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef]
- Alexopoulos, A.S.; Crowley, M.J.; Wang, Y.; Moylan, C.A.; Guy, C.D.; Henao, R.; Piercy, D.L.; Seymour, K.A.; Sudan, R.; Portenier, D.D.; et al. Glycemic Control Predicts Severity of Hepatocyte Ballooning and Hepatic Fibrosis in Nonalcoholic Fatty Liver Disease. Hepatology 2021, 74, 1220–1233. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Ratziu, V.; Harrison, S.A.; NASH Clinical Trial Design International Working Group. Expert Panel Review to Compare FDA and EMA Guidance on Drug Development and Endpoints in Nonalcoholic Steatohepatitis. Gastroenterology 2022, 162, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Kochanowska, A.; Rusztyn, P.; Szczerkowska, K.; Surma, S.; Gąsecka, A.; Jaguszewski, M.J.; Szarpak, Ł.; Filipiak, K.J. Sodium-Glucose Cotransporter 2 Inhibitors to Decrease the Uric Acid Concentration-A Novel Mechanism of Action. J. Cardiovasc. Dev. Dis. 2023, 10, 268. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration (FDA). FDA Approves New Treatment for a Type of Heart Failure. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-type-heart-failure (accessed on 10 September 2023).
- US Food and Drug Administration (FDA). FDA Approves Treatment for Chronic Kidney Disease. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-treatment-chronic-kidney-disease (accessed on 15 December 2022).
- Kim, J.W.; Lee, Y.J.; You, Y.H.; Moon, M.K.; Yoon, K.H.; Ahn, Y.B.; Ko, S.H. Effect of sodium-glucose cotransporter 2 inhibitor, empagliflozin, and α-glucosidase inhibitor, voglibose, on hepatic steatosis in an animal model of type 2 diabetes. J. Cell Biochem. 2019, 120, 8534–8546. [Google Scholar] [CrossRef] [PubMed]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef] [PubMed]
- Pirmoazen, A.M.; Khurana, A.; El Kaffas, A.; Kamaya, A. Quantitative ultrasound approaches for diagnosis and monitoring hepatic steatosis in nonalcoholic fatty liver disease. Theranostics 2020, 10, 4277–4289. [Google Scholar] [CrossRef]
- Wong, V.W.; Chu, W.C.; Wong, G.L.; Chan, R.S.; Chim, A.M.; Ong, A.; Yeung, D.K.; Yiu, K.K.; Chu, S.H.; Woo, J.; et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: A population study using proton-magnetic resonance spectroscopy and transient elastography. Gut 2012, 61, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Pimpin, L.; Cortez-Pinto, H.; Negro, F.; Corbould, E.; Lazarus, J.V.; Webber, L.; Sheron, N.; EASL HEPAHEALTH Steering Committee. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J. Hepatol. 2018, 69, 718–735. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight. Available online: http://www.who.int/mediacentre/factssheets/fs311/en/ (accessed on 15 June 2023).
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Semmler, G.; Wernly, B.; Datz, C. What’s in a name? New nomenclature for steatotic liver disease—To be or not to be? J. Hepatol. 2024, 80, e56–e58. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Tang, Y.; Zhu, W.; Huang, P.; Lian, P.; Ran, J.; Huang, X. Sodium-glucose cotransporter protein-2 inhibitors and glucagon-like peptide-1 receptor agonists versus thiazolidinediones for non-alcoholic fatty liver disease: A network meta-analysis. Acta Diabetol. 2022, 59, 519–533. [Google Scholar] [CrossRef]
- Lingvay, I.; Catarig, A.M.; Frias, J.P.; Kumar, H.; Lausvig, N.L.; le Roux, C.W.; Thielke, D.; Viljoen, A.; McCrimmon, R.J. Efficacy and safety of once-weekly semaglutide versus daily canagliflozin as add-on to metformin in patients with type 2 diabetes (SUSTAIN 8): A double-blind, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Atsukawa, M.; Tsubota, A.; Ono, H.; Kawano, T.; Yoshida, Y.; Okubo, T.; Hayama, K.; Nakagawa-Iwashita, A.; Itokawa, N.; et al. Efficacy and safety of oral semaglutide in patients with non-alcoholic fatty liver disease complicated by type 2 diabetes mellitus: A pilot study. JGH Open 2022, 6, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, W.R.; Kim, H.J.; Therneau, T.M. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology 2013, 57, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-A.; Lee, H.C.; Choe, J.; Kim, M.-J.; Lee, M.J.; Chang, H.-S.; Bae, I.Y.; Kim, H.-K.; An, J.; Shim, J.H.; et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J. Hepatol. 2018, 68, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Gameil, M.A.; Rozaik, S.E.; Elsebaie, A.; Marzouk, R. Influence of Liraglutide, Dulaglutide versus Conventional Treatment on Fatty Liver Index and Fibrosis-4 Score in Egyptian Patients with Type 2 Diabetes Mellitus and Non-Alcoholic Fatty Liver Disease. Med. J. Viral Hepat. 2020, 5, 25–32. [Google Scholar] [CrossRef]
- Flint, A.; Andersen, G.; Hockings, P.; Johansson, L.; Morsing, A.; Sundby Palle, M.; Vogl, T.; Loomba, R.; Plum-Mörschel, L. Randomised clinical trial: Semaglutide versus placebo reduced liver steatosis but not liver stiffness in subjects with non-alcoholic fatty liver disease assessed by magnetic resonance imaging. Aliment. Pharmacol. Ther. 2021, 54, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.-S.; Harrison, S.A. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef]
- Zhu, K.; Kakkar, R.; Chahal, D.; Yoshida, E.M.; Hussaini, T. Efficacy and safety of semaglutide in non-alcoholic fatty liver disease. World J. Gastroenterol. 2023, 29, 5327–5338. [Google Scholar] [CrossRef]
- Kumar, J.; Memon, R.S.; Shahid, I.; Rizwan, T.; Zaman, M.; Menezes, R.G.; Kumar, S.; Siddiqi, T.J.; Usman, M.S. Antidiabetic drugs and non-alcoholic fatty liver disease: A systematic review, meta-analysis and evidence map. Dig. Liver Dis. 2021, 53, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Mittag-Roussou, V.; Wagenpfeil, S.; Lammert, F.; Stokes, C.S. Noninvasive monitoring of liver fat during treatment with GLP-1 analogues and SGLT-2 inhibitors in a real-world setting. Endocrinol. Diabetes Metab. 2020, 3, e00131. [Google Scholar] [CrossRef] [PubMed]

| Semaglutide Group | Dapagliflozin Group | |||||
|---|---|---|---|---|---|---|
| Baseline (n = 95) | 6 mo (n = 95) | p | Baseline (n = 92) | 6 mo (n = 92) | p | |
| Sex F, n (%) | 52 (54.7) | 50 (54.3) | ||||
| Age (y) | 56 ± 9.85 | 61.47 ± 11.22 | ||||
| Hypertension, n (%) | 67 (70.5) | 64 (69.6) | ||||
| Dyslipidemia, n (%) | 53 (55.8) | 47 (51.1) | ||||
| Metabolic syndrome, n (%) | 79 (83.15) | 73 (79.3) | ||||
| Smoking status, n (%) | 24 (25.3) | 29 (31.5) | ||||
| BMI (kg/m2) | 29.49 ± 5.21 | 27.32 ± 4.61 | <0.001 | 29.57 ± 5.47 | 27.86 ± 4.85 | 0.005 |
| Waist circumference (cm) | 98.34 ± 9.8 | 94.8 ± 8.91 | <0.001 | 97.72 ± 9.66 | 95.13 ± 4.42 | 0.008 |
| WtHr | 0.57 ± 0.07 | 0.51 ± 0.04 | <0.001 | 0.59 ± 0.09 | 0.54 ± 0.04 | 0.007 |
| Normal weight, n (%) | 17 (17.9) | 24 (25.3) | 0.016 | 16 (17.4) | 24 (26.1) | 0.009 |
| Overweight, n (%) | 32 (33.7) | 42 (44.2) | 0.006 | 30 (32.6) | 33 (35.8) | 0.072 |
| Obesity, n (%) | 46 (48.4) | 29 (30.5) | <0.001 | 46 (50) | 35 (38.1) | 0.011 |
| Biological parameters | ||||||
| HGB (g/dL) | 13.48 ± 1.69 | 13.27 ± 1.62 | 0.452 | 13.27 ± 1.67 | 13.06 ± 1.63 | 0.106 |
| Platelet count (g/L) | 216.01 ± 65.48 | 218.95 ± 67.01 | 0.236 | 225.68 ± 69.09 | 228.98 ± 71.22 | 0.291 |
| CRP (mg/dL) | 0.72 ± 0.31 | 0.7 ± 0.29 | 0.185 | 0.76 ± 0.33 | 0.73 ± 0.28 | 0.162 |
| Ferritin (ng/mL) | 149.63 ± 57.94 | 144.01 ± 56.2 | 0.264 | 137.16 ± 54.82 | 131.08 ± 52.16 | 0.314 |
| Bilirubin (mg/dL) | 0.82 ± 0.44 | 0.84 ± 0.45 | 0.322 | 0.88 ± 0.47 | 0.85 ± 0.43 | 0.106 |
| Total proteins (g/dL) | 7.63 ± 0.69 | 7.85 ± 0.72 | 0.081 | 7.63 ± 0.68 | 7.83 ± 0.71 | 0.161 |
| Albumin (g/dL) | 4.73 ± 0.55 | 4.79 ± 0.63 | 0.076 | 4.71 ± 0.56 | 4.77 ± 0.6 | 0.320 |
| BUN (mg/dL) | 40.94 ± 23.26 | 37.03 ± 22.84 | 0.075 | 38.32 ± 20.01 | 37.75 ± 19.66 | 0.370 |
| Creatinine (mg/dL) Serum uric acid (mg/dL) | 0.83 ± 0.3 6.46 ± 0.75 | 0.72 ± 0.27 6.41 ± 0.73 | 0.038 0.102 | 0.81 ± 0.26 6.59 ± 0.8 | 0.71 ± 0.24 6.48 ± 0.76 | 0.034 0.088 |
| ALT (IU/L) AST (IU/L) | 57.18 ± 26.78 55.8 ± 25.32 | 33.18 ± 12.8 35.28 ± 13.65 | <0.001 <0.001 | 54.06 ± 24.82 56.7 ±26.1 | 38.16 ± 14.6 39.11 ±15.2 | <0.001 <0.001 |
| Fasting glucose (mg/dL) | 147.46 ± 41.07 | 112.52 ± 36.23 | <0.001 | 137.96 ± 40.78 | 108.44 ± 33.81 | <0.001 |
| HbA1c (%) | 8.11 ± 0.84 | 6.94 ± 0.74 | <0.001 | 7.75 ± 0.81 | 7.01 ± 0.72 | 0.010 |
| Cholesterol (mg/dL) Triglycerides (mg/dL) | 221.24 ± 60.96 189.14 ± 58.46 | 219.69 ± 60.02 175.89 ± 56.32 | 0.143 0.032 | 211.38 ± 57.51 174.7 ± 56.24 | 208.96 ± 56.68 168.54 ± 55.36 | 0.211 0.062 |
| LDL-C (mg/dL) | 154.29 ± 46.7 | 142.26 ± 43.38 | 0.027 | 141.57 ± 41.96 | 139.95 ± 41.02 | 0.195 |
| HDL-C (mg/dL) | 45.55 ± 9.53 | 47.38 ± 9.71 | 0.090 | 45.29 ± 9.46 | 47.11 ± 9.84 | 0.093 |
| LF non-invasive markers | ||||||
| FIB-4 index | 1.79 ± 1.12 | 1.36 ± 0.89 | 0.004 | 1.62 ± 0.95 | 1.34 ± 0.79 | 0.036 |
| NFS-score APRI score Agile 3+ score | 0.54 ± 1.01 0.58 ± 0.2 0.48 ± 0.18 | 0.42 ± 0.96 0.46 ± 0.17 0.38 ± 0.12 | 0.029 <0.001 <0.001 | 0.52 ± 1.04 0.56 ± 0.21 0.44 ± 0.16 | 0.46 ± 0.97 0.5 ± 0.21 0.4 ± 0.15 | 0.046 0.037 0.061 |
| Semaglutide Group | Dapagliflozin Group | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| Parameter | Β | p | Β | p | β | p | β | p |
| BMI at baseline | 0.496 | <0.001 | 0.491 | 0.009 | 0.423 | 0.006 | 0.415 | 0.017 |
| WC at baseline | 0.356 | 0.008 | 0.347 | 0.023 | 0.322 | 0.034 | 0.318 | 0.079 |
| WtHr at baseline | 0.254 | 0.012 | 0.248 | 0.047 | 0.241 | 0.022 | 0.239 | 0.094 |
| CAP at baseline | 0.474 | 0.004 | −0.468 | 0.014 | −0.438 | 0.008 | −0.0429 | 0.022 |
| Glucose at baseline | 0.321 | 0.012 | 0.313 | 0.028 | 0.315 | 0.013 | 0.306 | 0.031 |
| HbA1c at baseline | 0.298 | 0.007 | 0.292 | 0.016 | 0.272 | 0.021 | 0.269 | 0.054 |
| TG at baseline | 0.277 | 0.006 | 0.269 | 0.015 | 0.268 | 0.088 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stratina, E.; Stanciu, C.; Nastasa, R.; Zenovia, S.; Stafie, R.; Rotaru, A.; Cuciureanu, T.; Muzica, C.; Sfarti, C.; Girleanu, I.; et al. New Insights on Using Oral Semaglutide versus Dapagliflozin in Patients with Type 2 Diabetes and Metabolic Dysfunction-Associated Steatotic Liver Disease. Diagnostics 2024, 14, 1475. https://doi.org/10.3390/diagnostics14141475
Stratina E, Stanciu C, Nastasa R, Zenovia S, Stafie R, Rotaru A, Cuciureanu T, Muzica C, Sfarti C, Girleanu I, et al. New Insights on Using Oral Semaglutide versus Dapagliflozin in Patients with Type 2 Diabetes and Metabolic Dysfunction-Associated Steatotic Liver Disease. Diagnostics. 2024; 14(14):1475. https://doi.org/10.3390/diagnostics14141475
Chicago/Turabian StyleStratina, Ermina, Carol Stanciu, Robert Nastasa, Sebastian Zenovia, Remus Stafie, Adrian Rotaru, Tudor Cuciureanu, Cristina Muzica, Catalin Sfarti, Irina Girleanu, and et al. 2024. "New Insights on Using Oral Semaglutide versus Dapagliflozin in Patients with Type 2 Diabetes and Metabolic Dysfunction-Associated Steatotic Liver Disease" Diagnostics 14, no. 14: 1475. https://doi.org/10.3390/diagnostics14141475
APA StyleStratina, E., Stanciu, C., Nastasa, R., Zenovia, S., Stafie, R., Rotaru, A., Cuciureanu, T., Muzica, C., Sfarti, C., Girleanu, I., Minea, H., Petrea, O., Huiban, L., Chiriac, S., Singeap, A.-M., Vlad, O., Cojocariu, C., & Trifan, A. (2024). New Insights on Using Oral Semaglutide versus Dapagliflozin in Patients with Type 2 Diabetes and Metabolic Dysfunction-Associated Steatotic Liver Disease. Diagnostics, 14(14), 1475. https://doi.org/10.3390/diagnostics14141475










