Role of the Stress Index in Predicting Mortality among Patients with Traumatic Femoral Fractures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Enrollment of Patients and Research Design
2.2. Statistical Analysis
3. Results
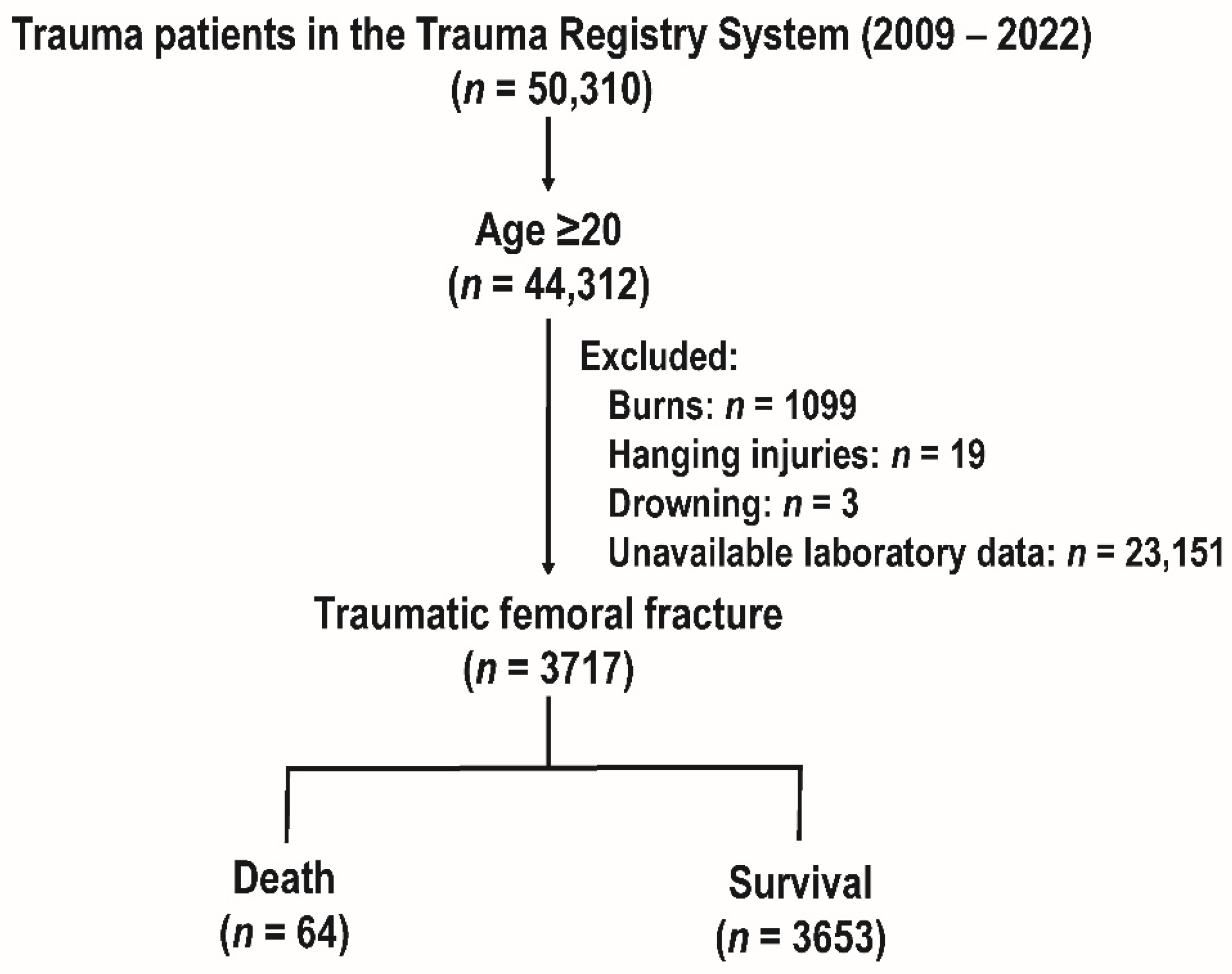
3.1. The Study Cohort and Patient Enrollment
3.2. Patient Characteristics
3.3. The SI’s Mortality Prediction Accuracy
3.4. Comparative Evaluation of the Group of Patients Split by the Optimal Cut-Off SI Value
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weiss, R.J.; Montgomery, S.M.; Al Dabbagh, Z.; Jansson, K.A. National data of 6409 Swedish inpatients with femoral shaft fractures: Stable incidence between 1998 and 2004. Injury 2009, 40, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Riswanda, N.; Dwi, A.; Abdul, A.; Sulis, B. The Characteristic of Patients with Femoral Fracture in Department of Orthopaedic and Traumatology RSUD Dr. Soetomo Surabaya 2013–2016. J. Orthop. Traumatol. Surabaya 2017, 6, 1–11. [Google Scholar] [CrossRef]
- Su, Y.; Chen, W.; Zhang, Q.; Li, B.; Li, Z.; Guo, M.; Pan, J.; Zhang, Y. An irreducible variant of femoral neck fracture: A minimally traumatic reduction technique. Injury 2011, 42, 140–145. [Google Scholar] [CrossRef]
- Anandasivam, N.S.; Russo, G.S.; Fischer, J.M.; Samuel, A.M.; Ondeck, N.T.; Swallow, M.S.; Chung, S.H.; Bohl, D.D.; Grauer, J.N. Analysis of Bony and Internal Organ Injuries Associated with 26,357 Adult Femoral Shaft Fractures and Their Impact on Mortality. Orthopedics 2017, 40, e506–e512. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.S.; Jordan, G.B.; Bilaniuk, J.W.; Benfante, A.; Kong, K.; Rolandelli, R.H.; Curran, T.; Nemeth, Z.H. The impact of BMI on morbidity and mortality after femoral fractures. Eur. J. Trauma Emerg. Surg. 2022, 48, 2441–2447. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.E.; Rau, C.S.; Tsai, Y.C.; Hsu, S.Y.; Hsieh, H.Y.; Hsieh, C.H. Risk factors and complications contributing to mortality in elderly patients with fall-induced femoral fracture: A cross-sectional analysis based on trauma registry data of 2407 patients. Int. J. Surg. 2019, 66, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Jennison, T.; Divekar, M. Geriatric distal femoral fractures: A retrospective study of 30 day mortality. Injury 2019, 50, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Nyholm, A.M.; Gromov, K.; Palm, H.; Brix, M.; Kallemose, T.; Troelsen, A.; the Danish Fracture Database Collaborators. Time to Surgery Is Associated with Thirty-Day and Ninety-Day Mortality After Proximal Femoral Fracture: A Retrospective Observational Study on Prospectively Collected Data from the Danish Fracture Database Collaborators. JBJS 2015, 97, 1333–1339. [Google Scholar] [CrossRef]
- Eakins, J. Blood glucose control in the trauma patient. J. Diabetes Sci. Technol. 2009, 3, 1373–1376. [Google Scholar] [CrossRef]
- Peffer, J.; McLaughlin, C. The correlation of early hyperglycemia with outcomes in adult trauma patients: A systematic review. J. Spec. Oper. Med. 2013, 13, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Laird, A.M.; Miller, P.R.; Kilgo, P.D.; Meredith, J.W.; Chang, M.C. Relationship of early hyperglycemia to mortality in trauma patients. J. Trauma 2004, 56, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.E.; Kauffmann, R.M.; Zuckerman, S.L.; Obremskey, W.T.; May, A.K. Relationship of hyperglycemia and surgical-site infection in orthopaedic surgery. J. Bone Jt. Surg. Am. 2012, 94, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Ay, B.; Parolia, K.; Liddell, R.S.; Qiu, Y.; Grasselli, G.; Cooper, D.M.L.; Davies, J.E. Hyperglycemia compromises Rat Cortical Bone by Increasing Osteocyte Lacunar Density and Decreasing Vascular Canal Volume. Commun. Biol. 2020, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Boyuk, F. The Predictor Potential Role of the Glucose to Potassium Ratio in the Diagnostic Differentiation of Massive and Non-Massive Pulmonary Embolism. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221076146. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.M.; Paik, J.H.; Kim, S.Y.; Hong, D.Y. Association of Plasma Glucose to Potassium Ratio and Mortality After Aneurysmal Subarachnoid Hemorrhage. Front. Neurol. 2021, 12, 661689. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ma, X.; Zhou, X.; Wang, Y. The association between serum glucose to potassium ratio on admission and short-term mortality in ischemic stroke patients. Sci. Rep. 2022, 12, 8233. [Google Scholar] [CrossRef] [PubMed]
- Marini, J.I.; Sein, M.E. The Role of the Glucose Potassium Ratio in the Management of Traumatic Brain Injury. Korean J. Neurotrauma 2023, 19, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Satoh, A.; Nakamura, H.; Kobayashi, S.; Miyata, A.; Matsutani, M. Management of severe subarachnoid hemorrhage; significance of assessment of both neurological and systemic insults at acute stage. Acta Neurochir. Suppl. 2005, 94, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Shibata, A.; Matano, F.; Saito, N.; Fujiki, Y.; Matsumoto, H.; Mizunari, T.; Morita, A. Serum Glucose-to-Potassium Ratio as a Prognostic Predictor for Severe Traumatic Brain Injury. J. Nippon. Med. Sch. 2021, 88, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Doi, T.; Abe, T.; Takeuchi, I. Trauma severity associated with stress index in emergency settings: An observational prediction-and-validation study. Acute Med. Surg. 2020, 7, e493. [Google Scholar] [CrossRef] [PubMed]
- Godinjak, A.; Iglica, A.; Burekovic, A.; Jusufovic, S.; Ajanovic, A.; Tancica, I.; Kukuljac, A. Hyperglycemia in Critically Ill Patients: Management and Prognosis. Med. Arch. 2015, 69, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Vedantam, D.; Poman, D.S.; Motwani, L.; Asif, N.; Patel, A.; Anne, K.K. Stress-Induced Hyperglycemia: Consequences and Management. Cureus 2022, 14, e26714. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.W.; Huang, C.Y.; Liu, H.T.; Chen, Y.C.; Hsieh, C.H. Stress-Induced and Diabetic Hyperglycemia Associated with Higher Mortality among Intensive Care Unit Trauma Patients: Cross-Sectional Analysis of the Propensity Score-Matched Population. Int. J. Environ. Res. Public Health 2018, 15, 992. [Google Scholar] [CrossRef] [PubMed]
- Rau, C.S.; Wu, S.C.; Chen, Y.C.; Chien, P.C.; Hsieh, H.Y.; Kuo, P.J.; Hsieh, C.H. Higher Mortality in Trauma Patients Is Associated with Stress-Induced Hyperglycemia, but Not Diabetic Hyperglycemia: A Cross-Sectional Analysis Based on a Propensity-Score Matching Approach. Int. J. Environ. Res. Public Health 2017, 14, 1161. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, C.-S.; Shen, L.-J.; Lv, Q.-W.; Xu, Q.-C. Usefulness of serum glucose and potassium ratio as a predictor for 30-day death among patients with severe traumatic brain injury. Clin. Chim. Acta 2020, 506, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Alamri, F.F.; Almarghalani, D.A.; Alraddadi, E.A.; Alharbi, A.; Algarni, H.S.; Mulla, O.M.; Alhazmi, A.M.; Alotaibi, T.A.; Beheiry, D.H.; Alsubaie, A.S.; et al. The utility of serum glucose potassium ratio as a predictive factor for haemorrhagic transformation, stroke recurrence, and mortality among ischemic stroke patients. Saudi Pharm. J. 2024, 32, 102082. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Liu, H.T.; Hsieh, T.M.; Huang, C.Y.; Chou, S.E.; Su, W.T.; Li, C.; Hsu, S.Y.; Hsieh, C.H. Change of neutrophil-to-monocyte ratio to stratify the mortality risk of adult patients with trauma in the intensive care units. Formos. J. Surg. 2022, 55, 177–183. [Google Scholar] [CrossRef]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and its associated cutoff point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Chen, Y.C.; Chien, T.H.; Chang, H.Y.; Chen, Y.H.; Chien, C.Y.; Huang, T.S. Impact of comorbidities on the prognoses of trauma patients: Analysis of a hospital-based trauma registry database. PLoS ONE 2018, 13, e0194749. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.; Booker, J.; Addison, A.; Egglestone, R.; Dushianthan, A. Predictors of mortality for major trauma patients in intensive care: A retrospective cohort study [version 1; peer review: 2 approved with reservations]. F1000Research 2023, 12, 974. [Google Scholar] [CrossRef]
- Rau, C.S.; Wu, S.C.; Chen, Y.C.; Chien, P.C.; Hsieh, H.Y.; Kuo, P.J.; Hsieh, C.H. Mortality Rate Associated with Admission Hyperglycemia in Traumatic Femoral Fracture Patients Is Greater Than Non-Diabetic Normoglycemic Patients but Not Diabetic Normoglycemic Patients. Int. J. Environ. Res. Public Health 2017, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Vanek, V.W.; Seballos, R.M.; Chong, D.; Bourguet, C.C. Serum potassium concentrations in trauma patients. South. Med. J. 1994, 87, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Morell, V.; Lundgren, E.; Gillott, A. Predicting severity of trauma by admission white blood cell count, serum potassium level, and arterial pH. South. Med. J. 1993, 86, 658–659. [Google Scholar] [CrossRef] [PubMed]
- Ookuma, T.; Miyasho, K.; Kashitani, N.; Beika, N.; Ishibashi, N.; Yamashita, T.; Ujike, Y. The clinical relevance of plasma potassium abnormalities on admission in trauma patients: A retrospective observational study. J. Intensive Care 2015, 3, 37. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.T.; Cao, X.Y.; Gu, R.; Zhao, J.X.; Zhang, Y. Incidence and clinical significance of abnormalities in potassium, sodium and calcium levels in elderly patients with hip fractures during the perioperative period. Ann. Ital. Chir. 2020, 91, 187–191. [Google Scholar]
- Mosfeldt, M.; Pedersen, O.B.; Riis, T.; Worm, H.O.; Mark, S.; Jørgensen, H.L.; Duus, B.R.; Lauritzen, J.B. Value of routine blood tests for prediction of mortality risk in hip fracture patients. Acta Orthop. 2012, 83, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.L.; Wang, L.H.; Dai, C.Q.; Shentu, G.J.; Xu, G.H. Risk Factors and Outcomes for Preoperative Asymptomatic Pulmonary Embolism in Patients Aged 60 Years and Over with Hip Fracture. Orthop. Surg. 2021, 13, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Uramatsu, M.; Nakajima, S.; Yoshida, K.I. Lethal ventricular tachycardia triggered after femoral fracture repair in an obese man with insulin-resistant diabetes. Int. J. Legal. Med. 2016, 130, 1587–1591. [Google Scholar] [CrossRef] [PubMed]
- Turan, E.; Şahin, A. Role of glucose/potassium ratio and shock index in predicting mortality in patients with isolated thoracoabdominal blunt trauma. Turk. J. Trauma Emerg. Surg. 2022, 28, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Vanitallie, T.B. Stress: A risk factor for serious illness. Metabolism 2002, 51, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Del Rey, A.; Besedovsky, H.O. Metabolic and neuroendocrine effects of pro-inflammatory cytokines. Eur. J. Clin. Investig. 1992, 22 (Suppl. S1), 10–15. [Google Scholar]
- Beal, A.L.; Deuser, W.E.; Beilman, G.J. A Role for Epinephrine in Post-Traumatic Hypokalemia. Shock 2007, 27, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Beal, A.L.; Scheltema, K.E.; Beilman, G.J.; Deuser, W.E. Hypokalemia Following Trauma. Shock 2002, 18, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Safavi, M.; Honarmand, A.; Mehrizi, M.K.; Dastjerdi, M.S.; Ardestani, M.E. Hypokalemia at the Time of Admission to the Intensive Care Unit (ICU) Increases the Need for Mechanical Ventilation and Time of Ventilation in Critically Ill Trauma Patients. Adv. Biomed. Res. 2017, 6, 50. [Google Scholar] [CrossRef]
- Fujiki, Y.; Matano, F.; Mizunari, T.; Murai, Y.; Tateyama, K.; Koketsu, K.; Kubota, A.; Kobayashi, S.; Yokota, H.; Morita, A. Serum glucose/potassium ratio as a clinical risk factor for aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2018, 129, 870–875. [Google Scholar] [CrossRef] [PubMed]

| Variables | Death n = 64 | Survival n = 3653 | OR (95%CI) | p |
|---|---|---|---|---|
| Sex | 0.272 | |||
| Male, n (%) | 31 (48.4) | 1520 (41.6) | 1.32 (0.80–2.16) | |
| Female, n (%) | 33 (51.6) | 2133 (58.4) | 0.76 (0.46–1.24) | |
| Age, years (mean ± SD) | 66.6 ± 18.7 | 68.3 ± 18.7 | - | 0.491 |
| Stress Index | 53.1 ± 28.0 | 41.6 ± 17.6 | - | <0.001 |
| Sugar (mg/dL) | 199.3 ± 102.8 | 159.0 ± 67.3 | - | <0.001 |
| Potassium (mEq/L) | 3.9 ± 1.0 | 3.9 ± 0.6 | - | 0.818 |
| Comorbidities | ||||
| CVA, n (%) | 2 (3.1) | 371 (10.2) | 0.29 (0.07–1.17) | 0.063 |
| HTN, n (%) | 34 (53.1) | 1843 (50.5) | 1.11 (0.68–1.83) | 0.672 |
| CAD, n (%) | 7 (10.9) | 271 (7.4) | 1.53 (0.69–3.39) | 0.289 |
| CHF, n (%) | 1 (1.6) | 62 (1.7) | 0.92 (0.13–6.74) | 0.934 |
| DM, n (%) | 23 (35.9) | 1009 (27.6) | 1.47 (0.88–2.46) | 0.141 |
| ESRD, n (%) | 3 (4.7) | 131 (3.6) | 1.32 (0.41–4.27) | 0.639 |
| GCS, median (IQR) | 11 (6–15) | 15 (15–15) | - | <0.001 |
| 3–8, n (%) | 23 (35.9) | 59 (1.6) | 34.17 (19.29–60.53) | <0.001 |
| 9–12, n (%) | 4 (6.2) | 110 (3.0) | 2.15 (0.77–6.01) | 0.136 |
| 13–15, n (%) | 37 (57.8) | 3484 (95.4) | 0.07 (0.04–0.11) | <0.001 |
| ISS, median (IQR) | 21 (9–34) | 10 (9–9) | - | <0.001 |
| 1–15, n (%) | 33 (51.6) | 3355 (91.8) | 0.10 (0.06–0.16) | <0.001 |
| 16–24, n (%) | 6 (9.4) | 138 (3.8) | 2.64 (1.12–6.21) | 0.021 |
| ≥25, n (%) | 25 (39.1) | 160 (4.4) | 13.99 (8.27–23.69) | <0.001 |
| Hospital stay (days) | 16.2 ± 20.7 | 10.1 ± 9.3 | - | <0.001 |
| SI | ||||
|---|---|---|---|---|
| Variables | ≥49.7 n = 811 | <49.7 n = 2906 | OR (95%CI) | p |
| Sex | 0.014 | |||
| Male, n (%) | 308 (38.0) | 1243 (42.8) | 0.82 (0.70–0.96) | |
| Female, n (%) | 503 (62.0) | 1663 (57.2) | 1.22 (1.04–1.43) | |
| Age, years (mean ± SD) | 68.8 ± 16.4 | 68.1 ± 19.3 | - | 0.295 |
| Comorbidities | ||||
| CVA, n (%) | 86 (10.6) | 287 (9.9) | 1.08 (0.84–1.40) | 0.542 |
| HTN, n (%) | 460 (56.7) | 1417 (48.8) | 1.38 (1.18–1.61) | <0.001 |
| CAD, n (%) | 63 (7.8) | 215 (7.4) | 1.05 (0.79–1.41) | 0.723 |
| CHF, n (%) | 13 (1.6) | 50 (1.7) | 0.93 (0.50–1.72) | 0.819 |
| DM, n (%) | 475 (58.6) | 557 (19.2) | 5.96 (5.04–7.05) | <0.001 |
| ESRD, n (%) | 29 (3.6) | 105 (3.6) | 0.99 (0.65–1.50) | 0.960 |
| GCS, median (IQR) | 15 (15–15) | 15 (15–15) | - | <0.001 |
| 3–8, n (%) | 44 (5.4) | 38 (1.3) | 4.33 (2.79–6.73) | <0.001 |
| 9–12, n (%) | 34 (4.2) | 80 (2.8) | 1.55 (1.03–2.33) | 0.036 |
| 13–15, n (%) | 733 (90.4) | 2788 (95.9) | 0.40 (0.30–0.54) | <0.001 |
| ISS, median (IQR) | 9 (9–9) | 9 (9–9) | - | <0.001 |
| 1–15, n (%) | 679 (83.7) | 2709 (93.2) | 0.37 (0.30–0.47) | <0.001 |
| 16–24, n (%) | 50 (6.2) | 94 (3.2) | 1.97 (1.38–2.80) | <0.001 |
| ≥25, n (%) | 82 (10.1) | 103 (3.5) | 3.06 (2.27–4.14) | <0.001 |
| Hospital stay (days) | 12.8 ± 13.6 | 9.5 ± 8.0 | - | <0.001 |
| Mortality, n (%) | 34 (4.2) | 30 (1.0) | 4.20 (2.55–6.90) | <0.001 |
| AOR of mortality * | - | - | 2.05 (1.14–3.67) | 0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-Y.; Chou, S.-E.; Huang, C.-Y.; Tsai, C.-H.; Hsu, S.-Y.; Hsieh, C.-H. Role of the Stress Index in Predicting Mortality among Patients with Traumatic Femoral Fractures. Diagnostics 2024, 14, 1508. https://doi.org/10.3390/diagnostics14141508
Huang C-Y, Chou S-E, Huang C-Y, Tsai C-H, Hsu S-Y, Hsieh C-H. Role of the Stress Index in Predicting Mortality among Patients with Traumatic Femoral Fractures. Diagnostics. 2024; 14(14):1508. https://doi.org/10.3390/diagnostics14141508
Chicago/Turabian StyleHuang, Ching-Ya, Sheng-En Chou, Chun-Ying Huang, Ching-Hua Tsai, Shiun-Yuan Hsu, and Ching-Hua Hsieh. 2024. "Role of the Stress Index in Predicting Mortality among Patients with Traumatic Femoral Fractures" Diagnostics 14, no. 14: 1508. https://doi.org/10.3390/diagnostics14141508
APA StyleHuang, C.-Y., Chou, S.-E., Huang, C.-Y., Tsai, C.-H., Hsu, S.-Y., & Hsieh, C.-H. (2024). Role of the Stress Index in Predicting Mortality among Patients with Traumatic Femoral Fractures. Diagnostics, 14(14), 1508. https://doi.org/10.3390/diagnostics14141508






