Perineural Invasion in Cervical Cancer: A Hidden Trail for Metastasis
Abstract
1. Introduction
2. Evolution of Surgical Classifications for Cervical Cancer
3. Anatomic Mapping of Uterine Innervation
4. Clinical Studies of Cervical Cancer PNI
5. Preoperative Assessment Methods
5.1. Biopsy
5.2. Imaging Methods
5.3. Predictive Nomogram
6. Mechanisms of PNI in Cervical Cancer
6.1. “Defects” of the Peripheral Nerve Sheaths
6.2. Neurotrophic Factors
6.3. Chemokines
6.4. Schwann Cells
6.5. Mucin
7. PNI in Other Gynecological Cancers
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TME | tumor microenvironment |
| PNI | perineural invasion |
| NSRH | nerve-sparing radical hysterectomy |
| RH | radical hysterectomy |
| SHP | superior hypogastric plexus |
| IHP | inferior hypogastric plexus |
| TH | tyrosine hydroxylase |
| NPY | neuropeptide Y |
| VIP | vasointestinal peptide |
| OS | overall survival |
| DFS | disease-free survival |
| PFS | progression-free survival |
| H&E | hematoxylin and eosin |
| IHC | immunohistochemistry |
| SCs | Schwann cells |
| AUC | area under the receiver operating characteristic curve |
| NTs | neurotrophins |
| CNTFs | ciliary neurotrophic factors |
| GDNFs | glial cell-derived neurotrophic factors |
| NGF | nerve growth factor |
| BDNF | brain-derived neurotrophic factor |
| NT-3 | neurotrophin 3 |
| NT-4/5 | neurotrophin 4/5 |
| NT-6 | neurotrophin 6 |
| EMT | Epithelial–mesenchymal transition |
| MMPs | matrix metalloproteinases |
| SP | substance P |
| CGRP | calcitonin gene-related peptide |
| GFAP | glial fibrillary acidic protein |
| PACAP | pituitary adenylate cyclase-activating polypeptide |
| NMB | neuromedin B |
| MUC1 | membrane-bound mucin 1 |
| MAG | myelin-associated glycoprotein |
References
- Jiang, S.H.; Hu, L.P.; Wang, X.; Li, J.; Zhang, Z.G. Neurotransmitters: Emerging targets in cancer. Oncogene 2020, 39, 503–515. [Google Scholar] [CrossRef]
- Reavis, H.D.; Chen, H.I.; Drapkin, R. Tumor Innervation: Cancer Has Some Nerve. Trends Cancer 2020, 6, 1059–1067. [Google Scholar] [CrossRef]
- Le, T.T.; Oudin, M.J. Understanding and modeling nerve-cancer interactions. Dis. Model. Mech. 2023, 16, dmm049729. [Google Scholar] [CrossRef]
- Ferdoushi, A.; Griffin, N.; Marsland, M.; Xu, X.; Faulkner, S.; Gao, F.; Liu, H.; King, S.J.; Denham, J.W.; van Helden, D.F.; et al. Tumor innervation and clinical outcome in pancreatic cancer. Sci. Rep. 2021, 11, 7390. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Gao, T.; Ju, J.; Zhang, Y.; Ni, Q.; Li, Y.; Zhao, Z.; Chai, J.; Yang, X.; Sun, M. Sympathetic innervation contributes to perineural invasion of salivary adenoid cystic carcinoma via the beta2-adrenergic receptor. OncoTargets Ther. 2019, 12, 1475–1495. [Google Scholar] [CrossRef]
- Kamiya, A.; Hayama, Y.; Kato, S.; Shimomura, A.; Shimomura, T.; Irie, K.; Kaneko, R.; Yanagawa, Y.; Kobayashi, K.; Ochiya, T. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat. Neurosci. 2019, 22, 1289–1305. [Google Scholar] [CrossRef]
- Zhao, C.M.; Hayakawa, Y.; Kodama, Y.; Muthupalani, S.; Westphalen, C.B.; Andersen, G.T.; Flatberg, A.; Johannessen, H.; Friedman, R.A.; Renz, B.W.; et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 2014, 6, 250ra115. [Google Scholar] [CrossRef] [PubMed]
- March, B.; Faulkner, S.; Jobling, P.; Steigler, A.; Blatt, A.; Denham, J.; Hondermarck, H. Tumour innervation and neurosignalling in prostate cancer. Nat. Rev. Urol. 2020, 17, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.K.; Armaiz-Pena, G.N.; Nagaraja, A.S.; Sadaoui, N.C.; Ortiz, T.; Dood, R.; Ozcan, M.; Herder, D.M.; Haemmerle, M.; Gharpure, K.M.; et al. Sustained Adrenergic Signaling Promotes Intratumoral Innervation through BDNF Induction. Cancer Res. 2018, 78, 3233–3242. [Google Scholar] [CrossRef]
- Liebig, C.; Ayala, G.; Wilks, J.A.; Berger, D.H.; Albo, D. Perineural invasion in cancer: A review of the literature. Cancer 2009, 115, 3379–3391. [Google Scholar] [CrossRef]
- Jiang, S.H.; Zhang, X.X.; Hu, L.P.; Wang, X.; Li, Q.; Zhang, X.L.; Li, J.; Gu, J.R.; Zhang, Z.G. Systemic Regulation of Cancer Development by Neuro-Endocrine-Immune Signaling Network at Multiple Levels. Front. Cell Dev. Biol. 2020, 8, 586757. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Krishnan, A. Neural invasion: A scenic trail for the nervous tumor and hidden therapeutic opportunity. Am. J. Cancer Res. 2020, 10, 2258–2270. [Google Scholar] [PubMed]
- Selvaggi, F.; Melchiorre, E.; Casari, I.; Cinalli, S.; Cinalli, M.; Aceto, G.M.; Cotellese, R.; Garajova, I.; Falasca, M. Perineural Invasion in Pancreatic Ductal Adenocarcinoma: From Molecules towards Drugs of Clinical Relevance. Cancers 2022, 14, 5793. [Google Scholar] [CrossRef] [PubMed]
- Bapat, A.A.; Hostetter, G.; Von Hoff, D.D.; Han, H. Perineural invasion and associated pain in pancreatic cancer. Nat. Rev. Cancer 2011, 11, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, G.N.; Shi, Y.; Cui, L.; Leng, X.F.; Huang, J.M. Perineural invasion in cervical cancer: Pay attention to the indications of nerve-sparing radical hysterectomy. Ann. Transl. Med. 2019, 7, 203. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.C.; Meinel, A.; Fischer, U.; Bilek, K.; Hentschel, B. Perineural invasion in carcinoma of the cervix uteri--prognostic impact. J. Cancer Res. Clin. Oncol. 2010, 136, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Gynecologic Oncology Group, Chinese Obstetricians and Gynecologists Association; Li, B.; Xiang, Y. Chinese expert consensus on nerve-sparing radical hysterectomy for cervical cancer. Zhonghua Zhong Liu Za Zhi 2021, 43, 736–742. [Google Scholar] [CrossRef]
- Swailes, A.L.; Gockley, A.; Phaeton, R.; Kesterson, J.P. The Wertheim hysterectomy: Development, modifications, and impact in the present day. Gynecol. Oncol. 2017, 145, 3–8. [Google Scholar] [CrossRef]
- Piver, M.S.; Rutledge, F.; Smith, J.P. Five classes of extended hysterectomy for women with cervical cancer. Obstet. Gynecol. 1974, 44, 265–272. [Google Scholar] [CrossRef]
- Querleu, D.; Morrow, C.P. Classification of radical hysterectomy. Lancet Oncol. 2008, 9, 297–303. [Google Scholar] [CrossRef]
- Koh, W.J.; Greer, B.E.; Abu-Rustum, N.R.; Apte, S.M.; Campos, S.M.; Cho, K.R.; Chu, C.; Cohn, D.; Crispens, M.A.; Dorigo, O.; et al. Cervical Cancer, Version 2.2015. J. Natl. Compr. Cancer Netw. 2015, 13, 395–404; quiz 404. [Google Scholar] [CrossRef] [PubMed]
- Querleu, D.; Cibula, D.; Abu-Rustum, N.R. 2017 Update on the Querleu-Morrow Classification of Radical Hysterectomy. Ann. Surg. Oncol. 2017, 24, 3406–3412. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Abu-Rustum, N.R.; Benedetti-Panici, P.; Kohler, C.; Raspagliesi, F.; Querleu, D.; Morrow, C.P. New classification system of radical hysterectomy: Emphasis on a three-dimensional anatomic template for parametrial resection. Gynecol. Oncol. 2011, 122, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Rossetti, D.O.; Ditto, A.; Signorelli, M.; Martinelli, F.; Mosca, L.; Scaffa, C.; Leone Roberti Maggiore, U.; Chiappa, V.; Sabatucci, I.; et al. Nerve-Sparing Approach Improves Outcomes of Patients Undergoing Minimally Invasive Radical Hysterectomy: A Systematic Review and Meta-Analysis. J. Minim. Invasive Gynecol. 2018, 25, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Yao, D.S.; Pan, X.W.; Ou, T.Y. Clinical efficacy and safety of nerve-sparing radical hysterectomy for cervical cancer: A systematic review and meta-analysis. PLoS ONE 2014, 9, e94116. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Kamoi, S.; Ikeda, M.; Yamada, T.; Yoneyama, K.; Takeshita, T. Effectiveness and Long-term Outcomes of Nerve-Sparing Radical Hysterectomy for Cervical Cancer. J. Nippon. Med. Sch. 2021, 88, 386–397. [Google Scholar] [CrossRef] [PubMed]
- van Gent, M.D.; Romijn, L.M.; van Santen, K.E.; Trimbos, J.B.; de Kroon, C.D. Nerve-sparing radical hysterectomy versus conventional radical hysterectomy in early-stage cervical cancer. A systematic review and meta-analysis of survival and quality of life. Maturitas 2016, 94, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Rob, L.; Halaska, M.; Robova, H. Nerve-sparing and individually tailored surgery for cervical cancer. Lancet Oncol. 2010, 11, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Tate, S.; Nishikimi, K.; Shozu, M. Bladder function after modified posterior exenteration for primary gynecological cancer. Gynecol. Oncol. 2013, 129, 229–233. [Google Scholar] [CrossRef]
- Raspagliesi, F.; Ditto, A.; Fontanelli, R.; Solima, E.; Hanozet, F.; Zanaboni, F.; Kusamura, S. Nerve-sparing radical hysterectomy: A surgical technique for preserving the autonomic hypogastric nerve. Gynecol. Oncol. 2004, 93, 307–314. [Google Scholar] [CrossRef]
- Kosmas, I.P.; Malvasi, A.; Vergara, D.; Mynbaev, O.A.; Sparic, R.; Tinelli, A. Adrenergic and Cholinergic Uterine Innervation and the Impact on Reproduction in Aged Women. Curr. Pharm. Des. 2020, 26, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Pinsard, M.; Mouchet, N.; Dion, L.; Bessede, T.; Bertrand, M.; Darai, E.; Bellaud, P.; Loget, P.; Mazaud-Guittot, S.; Morandi, X.; et al. Anatomic and functional mapping of human uterine innervation. Fertil. Steril. 2022, 117, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Di Tommaso, S.; Cavallotti, C.; Malvasi, A.; Vergara, D.; Rizzello, A.; De Nuccio, F.; Tinelli, A. A Qualitative and Quantitative Study of the Innervation of the Human Non Pregnant Uterus. Curr. Protein Pept. Sci. 2017, 18, 140–148. [Google Scholar] [CrossRef]
- Cui, L.; Shi, Y.; Zhang, G.N. Perineural invasion as a prognostic factor for cervical cancer: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2015, 292, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Liu, Q.; Yang, X.; Chen, L.; Yu, J.; Qi, X.; Wang, Y. Perineural invasion as a prognostic risk factor in patients with early cervical cancer. Oncol. Lett. 2019, 17, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Tu, H.; Liu, L.; Huang, H.; Feng, Y.; Liu, J. Perineural Invasion Should Be Regarded as an Intermediate-Risk Factor for Recurrence in Surgically Treated Cervical Cancer: A Propensity Score Matching Study. Dis. Markers 2021, 2021, 1375123. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, G.; Yang, Y.; Cui, L.; Jia, S.; Shi, Y.; Song, S.; Xu, S. Perineural invasion in early-stage cervical cancer and its relevance following surgery. Oncol. Lett. 2018, 15, 6555–6561. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.W.; Wang, H.; Zheng, H.; Chen, J.; Shi, R.X. Survival Impacts of Perineural Invasion on Patients Under Different Radical Hysterectomies Due to Early Cervical Cancer. Front. Oncol. 2022, 12, 889862. [Google Scholar] [CrossRef]
- Chen, X.; Duan, H.; Zhao, H.; He, F.; Yin, L.; Liu, Y.; Wang, X.; Chen, C. Perineural invasion in cervical cancer: A multicenter retrospective study. Eur. J. Surg. Oncol. 2024, 50, 108313. [Google Scholar] [CrossRef]
- Wei, Y.S.; Yao, D.S.; Long, Y. Evaluation of the association between perineural invasion and clinical and histopathological features of cervical cancer. Mol. Clin. Oncol. 2016, 5, 307–311. [Google Scholar] [CrossRef]
- Skret-Magierlo, J.E.; Soja, P.J.; Skret, A.; Kruczek, A.; Kaznowska, E.; Wicherek, L. Perineural space invasion in cervical cancer (FIGO IB1-IIB) accompanied by high-risk factors for recurrence. J. Cancer Res. Ther. 2014, 10, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Meinel, A.; Fischer, U.; Bilek, K.; Hentschel, B.; Horn, L.C. Morphological parameters associated with perineural invasion (PNI) in carcinoma of the cervix uteri. Int. J. Surg. Pathol. 2011, 19, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Elsahwi, K.S.; Barber, E.; Illuzzi, J.; Buza, N.; Ratner, E.; Silasi, D.A.; Santin, A.D.; Azodi, M.; Schwartz, P.E.; Rutherford, T.J. The significance of perineural invasion in early-stage cervical cancer. Gynecol. Oncol. 2011, 123, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.C.; Kim, H.; Cho, H.Y.; Kim, K.; No, J.H.; Kim, Y.B. Prognostic significance of perineural invasion in cervical cancer. Int. J. Gynecol. Pathol. 2013, 32, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Tanaka, Y.; Eto, K.; Ukai, N.; Sonobe, S.; Takahashi, H.; Ikegami, M.; Shimoda, M. S100-stained perineural invasion is associated with worse prognosis in stage I/II colorectal cancer: Its possible association with immunosuppression in the tumor. Pathol. Int. 2022, 72, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Conte, G.A.; Qari, O.; Fasano, G.A.; Guinto, R.K.; Palo, L.; Parker, G.S.; Rangwala, A.F.; Minassian, H.; Greenberg, P.J.; Dewan, A.A.; et al. S100 Staining Adds to the Prognostic Significance of the Combination of Perineural Invasion and Lymphovascular Invasion in Colorectal Cancer. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.R.; Wang, Y.P.; Chang, J.Y.; Yu, S.Y.; Chen, H.M.; Chiang, C.P. Perineural invasion and expression of nerve growth factor can predict the progression and prognosis of oral tongue squamous cell carcinoma. J. Oral. Pathol. Med. 2014, 43, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Lanzel, E.; Robinson, R.A.; Zimmerman, M.B.; Pourian, A.; Hellstein, J.W. The use of immunohistochemistry in detection of perineural invasion in mucoepidermoid carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 121, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Feng, F.; Liu, Z.; Liu, S.; Zheng, G.; Xiao, S.; Cai, L.; Yang, X.; Li, G.; Lian, X.; et al. Prognosis and Progression of ESCC Patients with Perineural Invasion. Sci. Rep. 2017, 7, 43828. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.H.; Xu, G.F.; Zhang, W.J.; Zhao, H.B.; Wu, Y.Y. Reevaluating significance of perineural invasion in gastric cancer based on double immunohistochemical staining. Arch. Pathol. Lab. Med. 2014, 138, 229–234. [Google Scholar] [CrossRef]
- Berlingeri-Ramos, A.C.; Detweiler, C.J.; Wagner, R.F., Jr.; Kelly, B.C. Dual S-100-AE1/3 Immunohistochemistry to Detect Perineural Invasion in Nonmelanoma Skin Cancers. J. Skin. Cancer 2015, 2015, 620235. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferrari, F.; Forte, S.; Ardighieri, L.; Bonetti, E.; Fernando, B.; Sartori, E.; Odicino, F. Multivariate analysis of prognostic factors in primary squamous cell vulvar cancer: The role of perineural invasion in recurrence and survival. Eur. J. Surg. Oncol. 2019, 45, 2115–2119. [Google Scholar] [CrossRef] [PubMed]
- Holthoff, E.R.; Jeffus, S.K.; Gehlot, A.; Stone, R.; Erickson, S.W.; Kelly, T.; Quick, C.M.; Post, S.R. Perineural Invasion Is an Independent Pathologic Indicator of Recurrence in Vulvar Squamous Cell Carcinoma. Am. J. Surg. Pathol. 2015, 39, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Skret, A.; Skret-Magierlo, J.E.; Ksiazek, M.; Gawlik, B.; Bielatowicz, J.; Barnas, E. The Diagnosis of Perineural Invasion: A Crucial Factor in Novel Algorithm of Coexistence of Conventional and Nerve-Sparing Radical Hysterectomy. Diagnostics 2021, 11, 1308. [Google Scholar] [CrossRef] [PubMed]
- Penn, R.; Abemayor, E.; Nabili, V.; Bhuta, S.; Kirsch, C. Perineural invasion detected by high-field 3.0-T magnetic resonance imaging. Am. J. Otolaryngol. 2010, 31, 482–484. [Google Scholar] [CrossRef] [PubMed]
- Capek, S.; Howe, B.M.; Amrami, K.K.; Spinner, R.J. Perineural spread of pelvic malignancies to the lumbosacral plexus and beyond: Clinical and imaging patterns. Neurosurg. Focus. 2015, 39, E14. [Google Scholar] [CrossRef] [PubMed]
- Gil, Z.; Kelly, K.J.; Brader, P.; Shah, J.P.; Fong, Y.; Wong, R.J. Utility of a herpes oncolytic virus for the detection of neural invasion by cancer. Neoplasia 2008, 10, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, J.; Wu, G.; Chen, S.; Pc, F.J.; Xie, W.; Tang, W. Development and Validation of a Nomogram for Preoperative Prediction of Perineural Invasion in Colorectal Cancer. Med. Sci. Monit. 2019, 25, 1709–1717. [Google Scholar] [CrossRef]
- Liu, S.H.; Hou, X.Y.; Zhang, X.X.; Liu, G.W.; Xin, F.J.; Wang, J.G.; Zhang, D.L.; Wang, D.S.; Lu, Y. Establishment and validation of a predictive nomogram model for advanced gastric cancer with perineural invasion. Zhonghua Wei Chang Wai Ke Za Zhi 2020, 23, 1059–1066. [Google Scholar] [CrossRef]
- Jia, H.; Li, R.; Liu, Y.; Zhan, T.; Li, Y.; Zhang, J. Preoperative Prediction of Perineural Invasion and Prognosis in Gastric Cancer Based on Machine Learning through a Radiomics-Clinicopathological Nomogram. Cancers 2024, 16, 614. [Google Scholar] [CrossRef]
- Wan, T.; Cai, G.; Gao, S.; Feng, Y.; Huang, H.; Liu, L.; Liu, J. Preoperative Evaluation of Perineural Invasion in Cervical Cancer: Development and Independent Validation of a Novel Predictive Nomogram. Front. Oncol. 2021, 11, 774459. [Google Scholar] [CrossRef] [PubMed]
- Dunn, M.; Morgan, M.B.; Beer, T.W. Perineural invasion: Identification, significance, and a standardized definition. Dermatol. Surg. 2009, 35, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Rodin, A.E.; Larson, D.L.; Roberts, D.K. Nature of the perineural space invaded by prostatic carcinoma. Cancer 1967, 20, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Kobayashi, S.; Yayama, T.; Muramatsu, J.; Kurokawa, T.; Imamura, Y.; Baba, H. Metastatic involvement of sacral nerve roots from uterine carcinoma: A case report. Spine J. 2008, 8, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Retamales-Ortega, R.; Orostica, L.; Vera, C.; Cuevas, P.; Hernandez, A.; Hurtado, I.; Vega, M.; Romero, C. Role of Nerve Growth Factor (NGF) and miRNAs in Epithelial Ovarian Cancer. Int. J. Mol. Sci. 2017, 18, 507. [Google Scholar] [CrossRef] [PubMed]
- Brushart, T.M.; Aspalter, M.; Griffin, J.W.; Redett, R.; Hameed, H.; Zhou, C.; Wright, M.; Vyas, A.; Hoke, A. Schwann cell phenotype is regulated by axon modality and central-peripheral location, and persists in vitro. Exp. Neurol. 2013, 247, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R.; Lloyd, A.C. Schwann Cells: Development and Role in Nerve Repair. Cold Spring Harb. Perspect. Biol. 2015, 7, a020487. [Google Scholar] [CrossRef] [PubMed]
- Shurin, G.V.; Kruglov, O.; Ding, F.; Lin, Y.; Hao, X.; Keskinov, A.A.; You, Z.; Lokshin, A.E.; LaFramboise, W.A.; Falo, L.D., Jr.; et al. Melanoma-Induced Reprogramming of Schwann Cell Signaling Aids Tumor Growth. Cancer Res. 2019, 79, 2736–2747. [Google Scholar] [CrossRef]
- Dvorak, H.F. Tumors: Wounds that do not heal-redux. Cancer Immunol. Res. 2015, 3, 1–11. [Google Scholar] [CrossRef]
- Long, Y.; Yao, D.S.; Wei, Y.S.; Wu, G.T. Effects of Nerve Growth Factor Expression on Perineural Invasion and Worse Prognosis in Early-Stage Cervical Cancer. Chin. Med. J. 2018, 131, 2360–2363. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Wang, R.; Chen, H.; Wang, R.; Wang, W.; Yang, X. NGF Signaling Interacts With the Hippo/YAP Pathway to Regulate Cervical Cancer Progression. Front. Oncol. 2021, 11, 688794. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Wang, Y.; Wang, Z.; Cui, Y.; Sun, X.; Wang, Y. Weighted Gene Co-Expression Network Analysis Identified Cancer Cell Proliferation as a Common Phenomenon During Perineural Invasion. OncoTargets Ther. 2019, 12, 10361–10374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, R.; Yang, W.; Zhang, Y.; Yuan, Q.; Wang, J.; Liu, Y.; Chen, S.; Zhang, S.; Zhang, W.; et al. Autophagic Schwann cells promote perineural invasion mediated by the NGF/ATG7 paracrine pathway in pancreatic cancer. J. Exp. Clin. Cancer Res. 2022, 41, 48. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Xiao, Y.; Qian, W.; Wang, X.; Gong, M.; Wang, Q.; An, R.; Han, L.; Duan, W.; Ma, Q.; et al. HGF/c-Met pathway facilitates the perineural invasion of pancreatic cancer by activating the mTOR/NGF axis. Cell Death Dis. 2022, 13, 387. [Google Scholar] [CrossRef] [PubMed]
- Lucido, C.T.; Wynja, E.; Madeo, M.; Williamson, C.S.; Schwartz, L.E.; Imblum, B.A.; Drapkin, R.; Vermeer, P.D. Innervation of cervical carcinoma is mediated by cancer-derived exosomes. Gynecol. Oncol. 2019, 154, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Wei, J.; Hou, R.; Wu, B.; Yang, Z.; Wang, L.; Lei, D.; Yang, X. Schwann cells promote EMT and the Schwann-like differentiation of salivary adenoid cystic carcinoma cells via the BDNF/TrkB axis. Oncol. Rep. 2016, 35, 427–435. [Google Scholar] [CrossRef]
- Yuan, Y.; Ye, H.Q.; Ren, Q.C. Upregulation of the BDNF/TrKB pathway promotes epithelial-mesenchymal transition, as well as the migration and invasion of cervical cancer. Int. J. Oncol. 2018, 52, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Ye, H.Q.; Ren, Q.C. Proliferative role of BDNF/TrkB signaling is associated with anoikis resistance in cervical cancer. Oncol. Rep. 2018, 40, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Li, W.; Kang, S.; Chen, L.; Hao, M.; Wang, W.; Ling, B.; Cui, Z.; Liang, C.; He, J.; et al. Expression of BDNF, TrkB, VEGF and CD105 is associated with pelvic lymph node metastasis and prognosis in IB2-stage squamous cell carcinoma. Exp. Ther. Med. 2019, 18, 4221–4230. [Google Scholar] [CrossRef]
- Sirico, A.; Simonelli, S.; Pignatiello, S.; Fulgione, C.; Sarno, L.; Chiuso, F.; Maruotti, G.M.; Sansone, M.; Guida, M.; Insabato, L. BDNF and NGF Expression in Preneoplastic Cervical Disease According to HIV Status. Int. J. Mol. Sci. 2023, 24, 729. [Google Scholar] [CrossRef]
- Chen, S.H.; Zhang, B.Y.; Zhou, B.; Zhu, C.Z.; Sun, L.Q.; Feng, Y.J. Perineural invasion of cancer: A complex crosstalk between cells and molecules in the perineural niche. Am. J. Cancer Res. 2019, 9, 1–21. [Google Scholar] [PubMed]
- He, S.; He, S.; Chen, C.H.; Deborde, S.; Bakst, R.L.; Chernichenko, N.; McNamara, W.F.; Lee, S.Y.; Barajas, F.; Yu, Z.; et al. The chemokine (CCL2-CCR2) signaling axis mediates perineural invasion. Mol. Cancer Res. 2015, 13, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Bakst, R.L.; Xiong, H.; Chen, C.H.; Deborde, S.; Lyubchik, A.; Zhou, Y.; He, S.; McNamara, W.; Lee, S.Y.; Olson, O.C.; et al. Inflammatory Monocytes Promote Perineural Invasion via CCL2-Mediated Recruitment and Cathepsin B Expression. Cancer Res. 2017, 77, 6400–6414. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Fan, Q.; Wang, Y.; Cui, Y.; Wang, Z.; Yang, L.; Sun, X.; Wang, Y. Schwann Cell-Derived CCL2 Promotes the Perineural Invasion of Cervical Cancer. Front. Oncol. 2020, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Navarro, X.; Vivo, M.; Valero-Cabre, A. Neural plasticity after peripheral nerve injury and regeneration. Prog. Neurobiol. 2007, 82, 163–201. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, C.S.; Banerjee, R.; Inglehart, R.C.; Liu, M.; Russo, N.; Hariharan, A.; van Tubergen, E.A.; Corson, S.L.; Asangani, I.A.; Mistretta, C.M.; et al. Galanin modulates the neural niche to favour perineural invasion in head and neck cancer. Nat. Commun. 2015, 6, 6885. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, G.; Ma, Q.; Li, W.; Liu, J.; Han, L.; Duan, W.; Xu, Q.; Liu, H.; Wang, Z.; et al. Neurotransmitter substance P mediates pancreatic cancer perineural invasion via NK-1R in cancer cells. Mol. Cancer Res. 2013, 11, 294–302. [Google Scholar] [CrossRef]
- Huang, C.; Li, Y.; Guo, Y.; Zhang, Z.; Lian, G.; Chen, Y.; Li, J.; Su, Y.; Li, J.; Yang, K.; et al. MMP1/PAR1/SP/NK1R paracrine loop modulates early perineural invasion of pancreatic cancer cells. Theranostics 2018, 8, 3074–3086. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Liu, Z.; Wang, X.; Ji, T. The neuropeptide calcitonin gene-related peptide links perineural invasion with lymph node metastasis in oral squamous cell carcinoma. BMC Cancer 2021, 21, 1254. [Google Scholar] [CrossRef]
- Gomez-Sanchez, J.A.; Pilch, K.S.; van der Lans, M.; Fazal, S.V.; Benito, C.; Wagstaff, L.J.; Mirsky, R.; Jessen, K.R. After Nerve Injury, Lineage Tracing Shows That Myelin and Remak Schwann Cells Elongate Extensively and Branch to Form Repair Schwann Cells, Which Shorten Radically on Remyelination. J. Neurosci. 2017, 37, 9086–9099. [Google Scholar] [CrossRef]
- Chen, G.; Zheng, Z.; Sun, H.; You, J.; Chu, J.; Gao, J.; Qiu, L.; Liu, X. Dedifferentiated Schwann cells promote perineural invasion mediated by the PACAP paracrine signalling in cervical cancer. J. Cell Mol. Med. 2023, 27, 3692–3705. [Google Scholar] [CrossRef]
- Demir, I.E.; Boldis, A.; Pfitzinger, P.L.; Teller, S.; Brunner, E.; Klose, N.; Kehl, T.; Maak, M.; Lesina, M.; Laschinger, M.; et al. Investigation of Schwann cells at neoplastic cell sites before the onset of cancer invasion. J. Natl. Cancer Inst. 2014, 106, dju184. [Google Scholar] [CrossRef] [PubMed]
- Azam, S.H.; Pecot, C.V. Cancer’s got nerve: Schwann cells drive perineural invasion. J. Clin. Invest. 2016, 126, 1242–1244. [Google Scholar] [CrossRef]
- Deborde, S.; Omelchenko, T.; Lyubchik, A.; Zhou, Y.; He, S.; McNamara, W.F.; Chernichenko, N.; Lee, S.Y.; Barajas, F.; Chen, C.H.; et al. Schwann cells induce cancer cell dispersion and invasion. J. Clin. Invest. 2016, 126, 1538–1554. [Google Scholar] [CrossRef] [PubMed]
- Huang, T. Cancer Cell-Derived NMB Initiating the Perineural Invasion of Cervical Cancer. Master’s Thesis, Shanghai Jiao Tong University, Shanghai, China, 2020. [Google Scholar] [CrossRef]
- Swanson, B.J.; McDermott, K.M.; Singh, P.K.; Eggers, J.P.; Crocker, P.R.; Hollingsworth, M.A. MUC1 is a counter-receptor for myelin-associated glycoprotein (Siglec-4a) and their interaction contributes to adhesion in pancreatic cancer perineural invasion. Cancer Res. 2007, 67, 10222–10229. [Google Scholar] [CrossRef] [PubMed]
- Roy, L.D.; Sahraei, M.; Subramani, D.B.; Besmer, D.; Nath, S.; Tinder, T.L.; Bajaj, E.; Shanmugam, K.; Lee, Y.Y.; Hwang, S.I.; et al. MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene 2011, 30, 1449–1459. [Google Scholar] [CrossRef]
- Van der Sluis, M.; De Koning, B.A.; De Bruijn, A.C.; Velcich, A.; Meijerink, J.P.; Van Goudoever, J.B.; Buller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef]
- Betge, J.; Schneider, N.I.; Harbaum, L.; Pollheimer, M.J.; Lindtner, R.A.; Kornprat, P.; Ebert, M.P.; Langner, C. MUC1, MUC2, MUC5AC, and MUC6 in colorectal cancer: Expression profiles and clinical significance. Virchows Arch. 2016, 469, 255–265. [Google Scholar] [CrossRef]
- Chiang, J.M.; Yeh, C.Y.; Changchien, C.R.; Chen, J.S.; Tang, R.; Chen, J.R. Mucinous adenocarcinoma showing different clinicopathological and molecular characteristics in relation to different colorectal cancer subgroups. Int. J. Colorectal Dis. 2010, 25, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Gundamaraju, R.; Chong, W.C. Consequence of distinctive expression of MUC2 in colorectal cancers: How much is actually bad? Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188579. [Google Scholar] [CrossRef]
- Chou, C.L.; Chen, T.J.; Tian, Y.F.; Chan, T.C.; Yeh, C.F.; Li, W.S.; Tsai, H.H.; Li, C.F.; Lai, H.Y. Upregulated MUC2 Is an Unfavorable Prognostic Indicator for Rectal Cancer Patients Undergoing Preoperative CCRT. J. Clin. Med. 2021, 10, 3030. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, M.J.; Walsh, J.C.; Parfitt, J.; Chakrabarti, S.; Correa, R.J.; MacKenzie, M.J.; Driman, D.K. CDX2 and Muc2 immunohistochemistry as prognostic markers in stage II colon cancer. Hum. Pathol. 2019, 90, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Astashchanka, A.; Shroka, T.M.; Jacobsen, B.M. Mucin 2 (MUC2) modulates the aggressiveness of breast cancer. Breast Cancer Res. Treat. 2019, 173, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Bie, L.; Sun, L.; Yue, Y. Neural activities are unfavorable for the prognosis of ovarian cancer through mRNA expression analysis. Biomark. Med. 2019, 13, 663–673. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, X.; Chen, G.; Chen, J.; Zhu, X.; Teng, Y. Transcriptome analyses reveal new insights on key determinants of perineural invasion in high-grade serous ovarian cancer. Front. Cell Dev. Biol. 2023, 11, 1109710. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Yao, D.S.; Wei, Y.S.; Wei, C.H.; Chen, X.Y. Prognostic significance of perineural invasion in vulvar squamous cell carcinoma. Cancer Manag. Res. 2019, 11, 4461–4469. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Angelico, G.; Travaglino, A.; Inzani, F.; Arciuolo, D.; Valente, M.; D’Alessandris, N.; Scaglione, G.; Piermattei, A.; Cianfrini, F.; et al. Prognostic role of perineural invasion in vulvar squamous cell carcinoma: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2022, 48, 2354–2359. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, M.P.; Sood, A.K.; Dos Reis, R.; Ramalingam, P.; Chen, C.; Frumovitz, M.; Jhingran, A.; Pitcher, B.; Ramirez, P.T.; Schmeler, K.M. Perineural invasion (PNI) in vulvar carcinoma: A review of 421 cases. Gynecol. Oncol. 2019, 152, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Pistolesi, S.; Cosio, S.; Naccarato, A.G. Is Perineural Invasion a Novel Prognostic Factor Useful to Tailor Adjuvant Treatment in Patients Treated With Primary Surgery for Cervical and Vulvar Carcinoma? Anticancer Res. 2020, 40, 3031–3037. [Google Scholar] [CrossRef]
- Ni, T.; Huang, T.; Gu, S.L.; Wang, J.; Liu, Y.; Sun, X.; Wang, Y.D. DRG Neurons Promote Perineural Invasion of Endometrial Cancer via GluR2. J. Cancer 2020, 11, 2518–2528. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Deivendran, S.; Marzook, K.H.; Radhakrishna Pillai, M. The role of inflammation in cervical cancer. Adv. Exp. Med. Biol. 2014, 816, 377–399. [Google Scholar] [CrossRef] [PubMed]
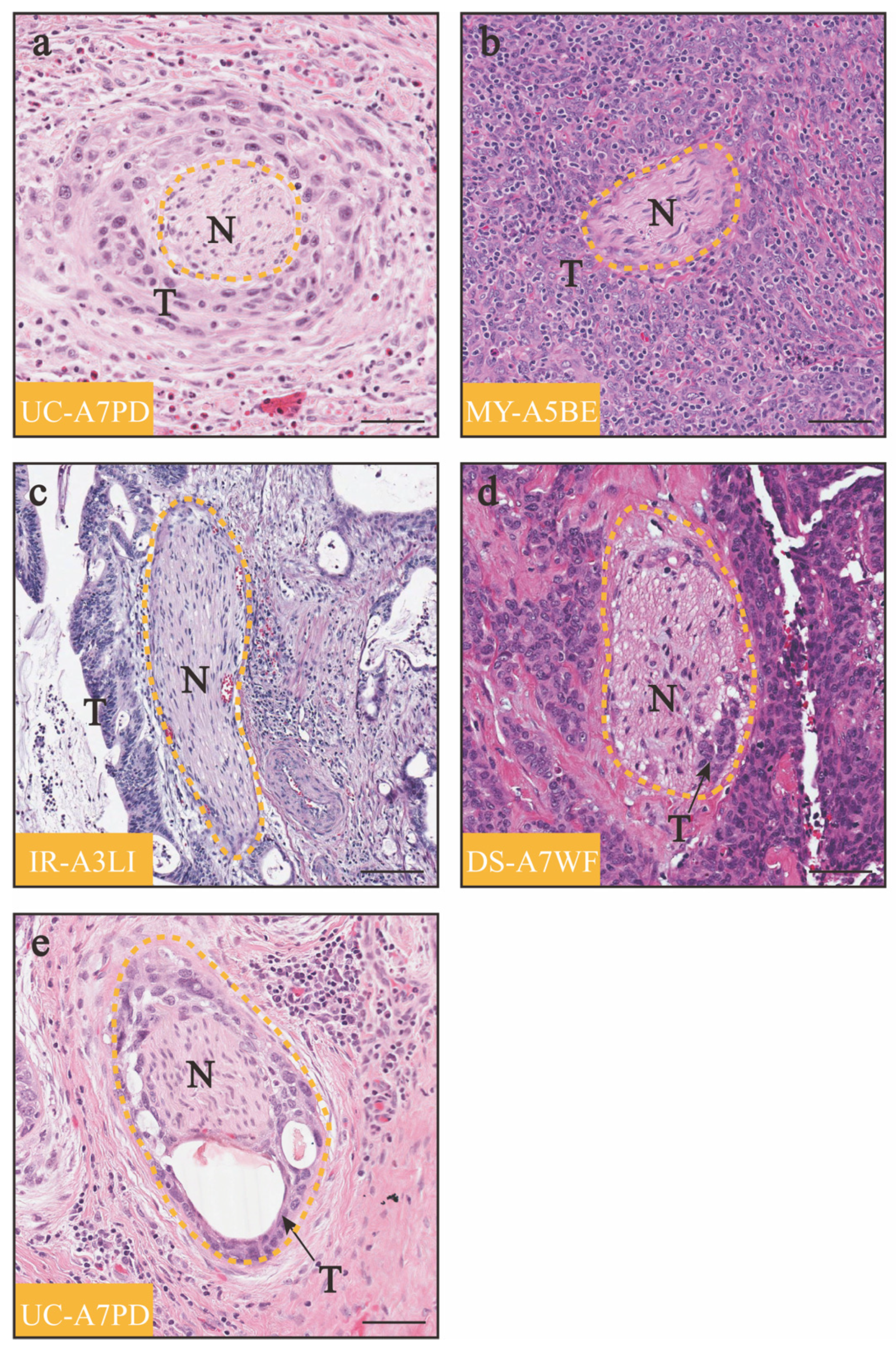
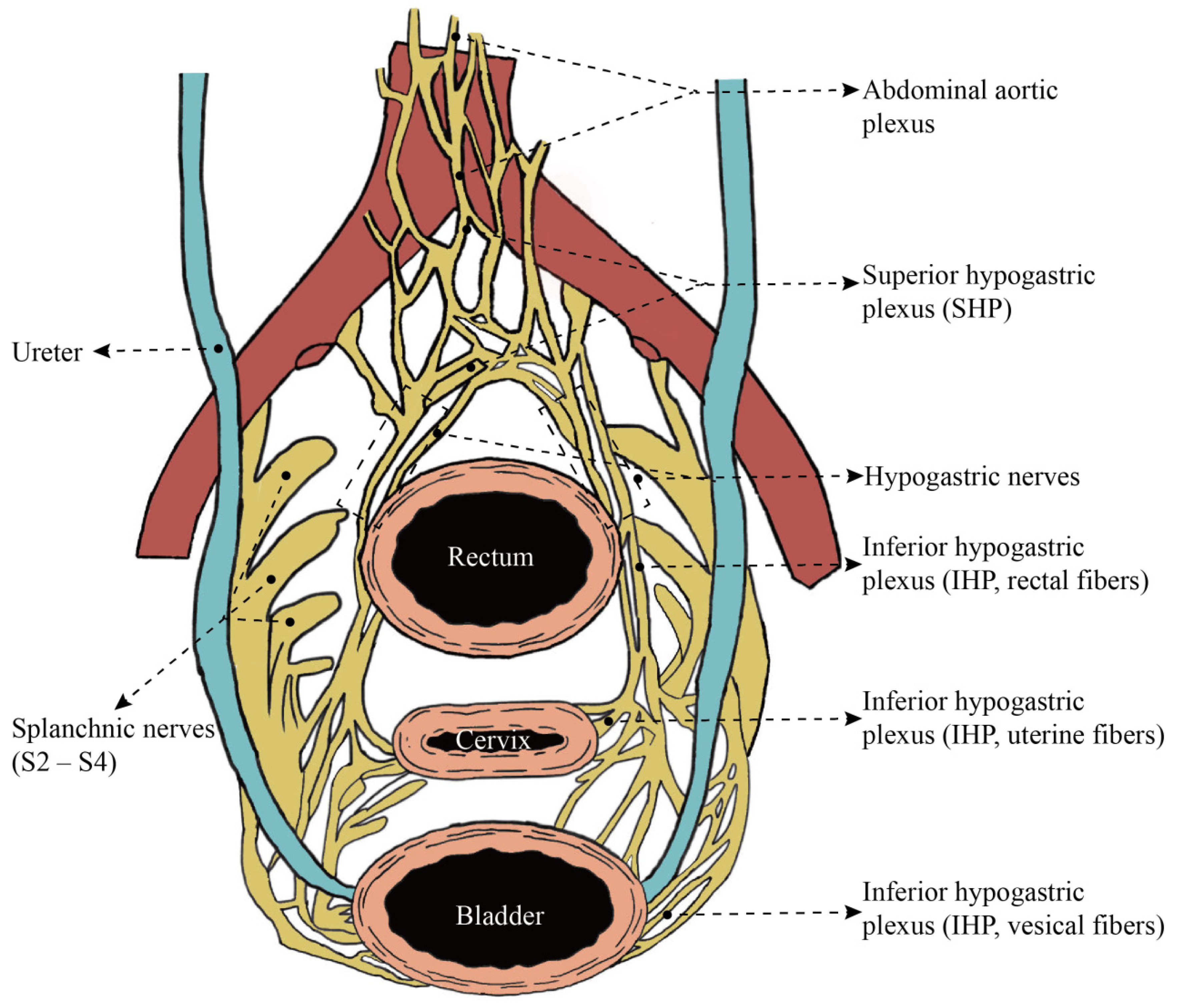
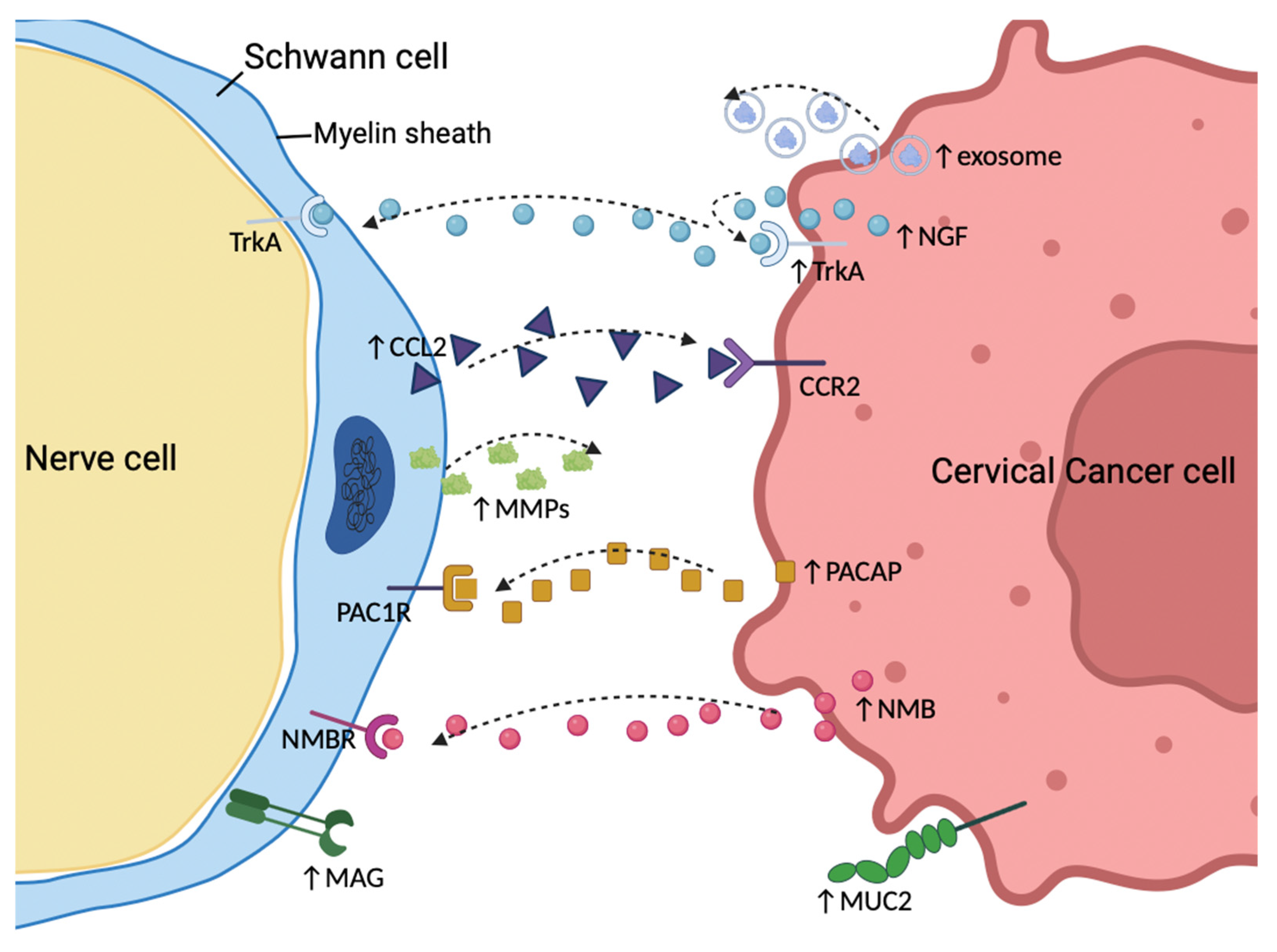
| Reference | Number and Incidence | OS | DFS or PFS | Advanced Clinical Stage | Advanced Tumor Grade | Increased Tumor Size | Deep Cervical Stromal Invasion | Parametrial Invasion | Lymphovascular Space Invasion | Positive Lymph Nodes | Positive Margins | More Adjuvant Therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Horn, L.C. 2010 [16] Meinel, A. 2011 [42] | 35.1% (68/194) | OS ↓ | NS | + | NS | / | + | / | / | / | / | / |
| Elsahwi, K.S. 2011 [43] | 12.5% (24/192) | NS | NS | + | NS | + | NS | + | + | NS | NS | + |
| Cho, H.C. 2013 [44] | 7% (13/185) | NS | NS | + | / | NS | + | + | + | + | + | + |
| Skret-Magierlo, J.E. 2014 [41] | 18% (9/50) | / | NS | + | NS | + | + | NS | NS | NS | / | / |
| Wei, Y.S. 2016 [40] | 16% (33/206) | / | / | + | + | + | + | / | + | + | / | + |
| Zhu, Y. 2018 [37] | 8.57% (18/210) | OS ↓ | DFS ↓ | / | / | + | + | + | + | + | / | + |
| Tang, M. 2019 [35] | 10.59% (43/406) | OS ↓ | DFS ↓ | NS | / | NS | + | / | + | + | + | / |
| Wan, T. 2021 [36] | 8.8% (162/1836) | OS ↓ | PFS ↓ | / | / | / | + | + | / | + | + | + |
| Wei, W.W. 2022 [38] | 12.1% (21/174) | OS ↓ | DFS ↓ | / | / | / | / | / | / | / | / | / |
| Chen, X.L. 2024 [39] | 22.6% (273/1208) | OS ↓ | DFS ↓ | NS | / | NS | + | + | + | + | NS | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Sun, H.; Chen, Y.; Wang, L.; Song, O.; Zhang, J.; Li, D.; Liu, X.; Feng, L. Perineural Invasion in Cervical Cancer: A Hidden Trail for Metastasis. Diagnostics 2024, 14, 1517. https://doi.org/10.3390/diagnostics14141517
Chen G, Sun H, Chen Y, Wang L, Song O, Zhang J, Li D, Liu X, Feng L. Perineural Invasion in Cervical Cancer: A Hidden Trail for Metastasis. Diagnostics. 2024; 14(14):1517. https://doi.org/10.3390/diagnostics14141517
Chicago/Turabian StyleChen, Guoqiang, Hao Sun, Yunxia Chen, Li Wang, Ouyi Song, Jili Zhang, Dazhi Li, Xiaojun Liu, and Lixia Feng. 2024. "Perineural Invasion in Cervical Cancer: A Hidden Trail for Metastasis" Diagnostics 14, no. 14: 1517. https://doi.org/10.3390/diagnostics14141517
APA StyleChen, G., Sun, H., Chen, Y., Wang, L., Song, O., Zhang, J., Li, D., Liu, X., & Feng, L. (2024). Perineural Invasion in Cervical Cancer: A Hidden Trail for Metastasis. Diagnostics, 14(14), 1517. https://doi.org/10.3390/diagnostics14141517






