Renal Function Preservation in Purely Off-Clamp Sutureless Robotic Partial Nephrectomy: Initial Experience and Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Enrollment
2.2. Preoperative Assessments
- –
- Clinical evaluation (age, body mass index, comorbidities using the Charlson score, diabetes mellitus, arterial hypertension, pathologies affecting the cardiovascular system, and ASA score);
- –
- Blood chemistry tests (creatinine and urea nitrogen) and estimated glomerular filtration rate (eGFR);
- –
- Total and separate renal function of both kidneys, assessed by sequential renal scintigraphy performed with radio—drug 99MTc—DTPA;
- –
- Imaging (abdominal CT) and nephrometric score grading (PADUA score).
2.3. Preoperative Preparation and Surgical Technique
2.4. Intraoperative Assessments
- –
- Total operative time (OT);
- –
- Estimated blood loss (EBL);
- –
- Intraoperative complications according to Clavien-Dindo (CD) classification.
2.5. Postoperative Assessments
2.6. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kowalewski, K.F.; Neuberger, M.; Sidoti Abate, M.A.; Kirchner, M.; Haney, C.M.; Siegel, F.; Westhoff, N.; Michel, M.S.; Honeck, P.; Nuhn, P.; et al. Randomized Controlled Feasibility Trial of Robot-Assisted Versus Conventional Open Partial Nephrectomy: The ROBOCOP II Study. Eur. Urol. Oncol. 2024, 7, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Peyronnet, B.; Seisen, T.; Oger, E.; Vaessen, C.; Grassano, Y.; Benoit, T.; Carrouget, J.; Pradère, B.; Khene, Z.; Giwerc, A.; et al. Comparison of 1800 Robotic and Open Partial Nephrectomies for Renal Tumors. Ann. Surg. Oncol. 2016, 23, 4277–4283. [Google Scholar] [CrossRef] [PubMed]
- Long, J.A.; Fiard, G.; Giai, J.; Teyssier, Y.; Fontanell, A.; Overs, C.; Poncet, D.; Descotes, J.L.; Rambeaud, J.J.; Moreau-Gaudry, A.; et al. Superselective Ischemia in Robotic Partial Nephrectomy Does Not Provide Better Long-Term Renal Function than Renal Artery Clamping in a Randomized Controlled Trial (EMERALD): Should We Take the Risk? Eur. Urol. Focus 2022, 8, 769–776. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zhang, W.; Yu, S.B.; Zhan, Y.H.; Fan, Y.F.; Zhang, X.P. The Efficacy of Modified Binding Technique for Renorrhaphy during Robotic Partial Nephrectomy: Surgical and Functional Outcomes from Single-Center Experience. Surg. Endosc. 2023, 37, 391–401. [Google Scholar] [CrossRef]
- Klatte, T.; Ficarra, V.; Gratzke, C.; Kaouk, J.; Kutikov, A.; Macchi, V.; Mottrie, A.; Porpiglia, F.; Porter, J.; Rogers, C.G.; et al. A Literature Review of Renal Surgical Anatomy and Surgical Strategies for Partial Nephrectomy. Eur. Urol. 2015, 68, 980–992. [Google Scholar] [CrossRef]
- Brassetti, A.; Misuraca, L.; Anceschi, U.; Bove, A.M.; Costantini, M.; Ferriero, M.C.; Guaglianone, S.; Mastroianni, R.; Torregiani, G.; Covotta, M.; et al. Sutureless Purely Off-Clamp Robot-Assisted Partial Nephrectomy: Avoiding Renorrhaphy Does Not Jeopardize Surgical and Functional Outcomes. Cancers 2023, 15, 698. [Google Scholar] [CrossRef]
- Anderson, B.G.; Potretzke, A.M.; Du, K.; Vetter, J.M.; Bergeron, K.; Paradis, A.G.; Figenshau, R.S. Comparing Off-Clamp and On-Clamp Robot-Assisted Partial Nephrectomy: A Prospective Randomized Trial. Urology 2019, 126, 102–109. [Google Scholar] [CrossRef]
- De Nunzio, C.; Tema, G.; Brassetti, A.; Anceschi, U.; Bove, A.M.; D’Annunzio, S.; Ferriero, M.; Mastroianni, R.; Misuraca, L.; Guaglianone, S.; et al. Purely Off-Clamp Sutureless Robotic Partial Nephrectomy for Novice Robotic Surgeons: A Multi-Institutional Propensity Score-Matched Analysis. J. Clin. Med. 2024, 13, 3553. [Google Scholar] [CrossRef]
- Greco, F.; Autorino, R.; Altieri, V.; Campbell, S.; Ficarra, V.; Gill, I.; Kutikov, A.; Mottrie, A.; Mirone, V.; van Poppel, H. Ischemia Techniques in Nephron-Sparing Surgery: A Systematic Review and Meta-Analysis of Surgical, Oncological, and Functional Outcomes. Eur. Urol. 2019, 75, 477–491. [Google Scholar] [CrossRef]
- Ferriero, M.; Brassetti, A.; Mastroianni, R.; Costantini, M.; Tuderti, G.; Anceschi, U.; Bove, A.M.; Misuraca, L.; Guaglianone, S.; Gallucci, M.; et al. Off-Clamp Robot-Assisted Partial Nephrectomy for Purely Hilar Tumors: Technique, Perioperative, Oncologic and Functional Outcomes from a Single Center Series. Eur. J. Surg. Oncol. 2022, 48, 1848–1853. [Google Scholar] [CrossRef]
- Tuderti, G.; Mastroianni, R.; Anceschi, U.; Bove, A.M.; Brassetti, A.; Ferriero, M.; Misuraca, L.; Guaglianone, S.; Costantini, M.; Torregiani, G.; et al. Assessing the Trade-off Between the Safety and Effectiveness of Off-Clamp Robotic Partial Nephrectomy for Renal Masses with a High RENAL Score: A Propensity Score–Matched Comparison of Perioperative and Functional Outcomes in a Multicenter Analysis. Eur. Urol. Focus 2023, 9, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.J.; Cai, J.; Simmons, M.N.; Gill, I.S. “Trifecta” in Partial Nephrectomy. J. Urol. 2013, 189, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Golan, S.; Patel, A.R.; Eggener, S.E.; Shalhav, A.L. The Volume of Nonneoplastic Parenchyma in a Minimally Invasive Partial Nephrectomy Specimen: Predictive Factors and Impact on Renal Function. J. Endourol. 2014, 28, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Lane, B.R.; Russo, P.; Uzzo, R.G.; Hernandez, A.V.; Boorjian, S.A.; Thompson, R.H.; Fergany, A.F.; Love, T.E.; Campbell, S.C. Comparison of Cold and Warm Ischemia during Partial Nephrectomy in 660 Solitary Kidneys Reveals Predominant Role of Nonmodifiable Factors in Determining Ultimate Renal Function. J. Urol. 2011, 185, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Simmons, M.N.; Hillyer, S.P.; Lee, B.H.; Fergany, A.F.; Kaouk, J.; Campbell, S.C. Functional Recovery after Partial Nephrectomy: Effects of Volume Loss and Ischemic Injury. J. Urol. 2012, 187, 1667–1673. [Google Scholar] [CrossRef]
- Mir, M.C.; Campbell, R.A.; Sharma, N.; Remer, E.M.; Li, J.; Demirjian, S.; Kaouk, J.; Campbell, S.C. Parenchymal Volume Preservation and Ischemia during Partial Nephrectomy: Functional and Volumetric Analysis. Urology 2013, 82, 263–269. [Google Scholar] [CrossRef]
- Choi, J.E.; You, J.H.; Kim, D.K.; Rha, K.H.; Lee, S.H. Comparison of Perioperative Outcomes between Robotic and Laparoscopic Partial Nephrectomy: A Systematic Review and Meta-Analysis. Eur. Urol. 2015, 67, 891–901. [Google Scholar] [CrossRef]
- Thompson, R.H.; Lane, B.R.; Lohse, C.M.; Leibovich, B.C.; Fergany, A.; Frank, I.; Gill, I.S.; Blute, M.L.; Campbell, S.C. Every Minute Counts When the Renal Hilum Is Clamped during Partial Nephrectomy. Eur. Urol. 2010, 58, 340–345. [Google Scholar] [CrossRef]
- Antonelli, A.; Cindolo, L.; Sandri, M.; Veccia, A.; Annino, F.; Bertagna, F.; Carini, M.; Celia, A.; D’Orta, C.; De Concilio, B.; et al. Is Off-Clamp Robot-Assisted Partial Nephrectomy Beneficial for Renal Function? Data from the CLOCK Trial. BJU Int. 2022, 129, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.G.; Gong, I.H.; Hwang, J.H.; Choi, D.K.; Lee, S.R.; Park, D.S. Prognostic Significance of Preoperative Kidney Volume for Predicting Renal Function in Renal Cell Carcinoma Patients Receiving a Radical or Partial Nephrectomy. BJU Int. 2012, 109, 1468–1473. [Google Scholar] [CrossRef]
- Song, C.; Park, S.; Jeong, I.G.; Hong, J.H.; Park, H.K.; Kim, C.S.; Ahn, H. Followup of Unilateral Renal Function after Laparoscopic Partial Nephrectomy. J. Urol. 2011, 186, 53–58. [Google Scholar] [CrossRef]
- Song, C.; Bang, J.K.; Park, H.K.; Ahn, H. Factors Influencing Renal Function Reduction after Partial Nephrectomy. J. Urol. 2009, 181, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.K.; Gill, I.S.; Patil, M.B.; Hung, A.J.; Berger, A.K.; De Castro Abreu, A.L.; Nakamoto, M.; Eisenberg, M.S.; Ukimura, O.; Thangathurai, D.; et al. Anatomic Renal Artery Branch Microdissection to Facilitate Zero-Ischemia Partial Nephrectomy. Eur. Urol. 2012, 61, 67–74. [Google Scholar] [CrossRef]
- Simone, G.; Papalia, R.; Guaglianone, S.; Carpanese, L.; Gallucci, M. Zero Ischemia Laparoscopic Partial Nephrectomy after Superselective Transarterial Tumor Embolization for Tumors with Moderate Nephrometry Score: Long-Term Results of a Single-Center Experience. J. Endourol. 2011, 25, 1443–1446. [Google Scholar] [CrossRef] [PubMed]
- Sherer, M.V.; Deka, R.; Salans, M.A.; Nelson, T.J.; Sheridan, P.; Rose, B.S. Androgen Deprivation Therapy and Acute Kidney Injury in Patients with Prostate Cancer Undergoing Definitive Radiotherapy. Prostate Cancer Prostatic Dis. 2023, 26, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Bahler, C.D.; Sundaram, C.P. Effect of Renal Reconstruction on Renal Function after Partial Nephrectomy. J. Endourol. 2016, 30. [Google Scholar] [CrossRef]
- Zabell, J.R.; Wu, J.; Suk-Ouichai, C.; Campbell, S.C. Renal Ischemia and Functional Outcomes Following Partial Nephrectomy. Urol. Clin. N. Am. 2017, 44, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Gao, S.; Zhao, Y.; Wu, B.; Chen, X. Comparison of Sutureless and Conventional Laparoscopic Partial Nephrectomy: A Propensity Score-Matching Analysis. Front. Oncol. 2021, 11, 649356. [Google Scholar] [CrossRef] [PubMed]
- Bertolo, R.; Campi, R.; Klatte, T.; Kriegmair, M.C.; Mir, M.C.; Ouzaid, I.; Salagierski, M.; Bhayani, S.; Gill, I.; Kaouk, J.; et al. Suture Techniques during Laparoscopic and Robot-Assisted Partial Nephrectomy: A Systematic Review and Quantitative Synthesis of Peri-Operative Outcomes. BJU Int. 2019, 123, 923–946. [Google Scholar] [CrossRef]
- Jin, D.; Ren, D.; Zhang, J.; Xu, G.; Ge, C.; Jiang, Q.; Wang, D.; Zhang, W.; Zhang, Y. A Propensity Score-Matched Comparison between Sutureless and Suture Techniques in Laparoscopic Nephron-Sparing Surgery: A Retrospective Non-Randomized Observational Study. J. Laparoendosc. Adv. Surg. Tech. 2020, 30, 1314–1319. [Google Scholar] [CrossRef]
- Moreno Cortés, J.C.; González García, J.; Caño Velasco, J.; Aragón Chamizo, J.; Subirá Rios, D. Reconstruction Techniques after Partial Nephrectomy: Classic vs. Sutureless Approach—A Narrative Review. Curr. Urol. Rep. 2024, 25, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Minervini, A.; Mari, A.; Bertolo, R.; Bianchi, G.; Lapini, A.; Longo, N.; Martorana, G.; Mirone, V.; Morgia, G.; et al. TriMatch Comparison of the Efficacy of FloSeal versus TachoSil versus No Hemostatic Agents for Partial Nephrectomy: Results from a Large Multicenter Dataset. Int. J. Urol. 2015, 22, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Bertolo, R.; Bove, P.; Sandri, M.; Celia, A.; Cindolo, L.; Cipriani, C.; Falsaperla, M.; Leonardo, C.; Mari, A.; Parma, P.; et al. Randomized Clinical Trial Comparing On-Clamp Versus Off-Clamp Laparoscopic Partial Nephrectomy for Small Renal Masses (CLOCK II Laparoscopic Study): A Intention-to-Treat Analysis of Perioperative Outcomes. Eur. Urol. Open Sci. 2022, 46, 75–81. [Google Scholar] [CrossRef]
- Brassetti, A.; Anceschi, U.; Bertolo, R.; Ferriero, M.; Tuderti, G.; Capitanio, U.; Larcher, A.; Garisto, J.; Antonelli, A.; Mottire, A.; et al. Surgical Quality, Cancer Control and Functional Preservation: Introducing a Novel Trifecta for Robot-Assisted Partial Nephrectomy. Minerva Urol. Nefrol. 2020, 72, 82–90. [Google Scholar] [CrossRef] [PubMed]


| Variable | Median (IQR); Mean ± SD; n (%) |
|---|---|
| Patients’ Characteristics | |
| Age (yy) | 64 (52/70) 62 ± 10 |
| BMI (Kg/m2) | 29 (27/31) 28 ± 3 |
| Charlson Comorbidity Index | 4 (4/5) |
| Arterial hypertension | 11/21 (52%) |
| Diabetes mellitus | 2/21 (10%) |
| Metabolic Syndrome | 2/21 (10%) |
| Ischemic heart disease | 0/21 (0%) |
| ASA score > 2 | 9/21(41%) |
| Tumors’ Characteristics | |
| Single | 18/21 (82%) |
| DX SN | 10/21 (48%) 11/21 (52%) |
| T1a T1b | 10/21 (48%) 11/21 (52%) |
| Dimension (mm) | 40 (29/45) 38 ± 10 |
| PADUA score | 4 (3.5/5) 4.1 ± 0.8 |
| Preoperative | Postoperative 1M | Postoperative 3M | Delta (∆) | |
|---|---|---|---|---|
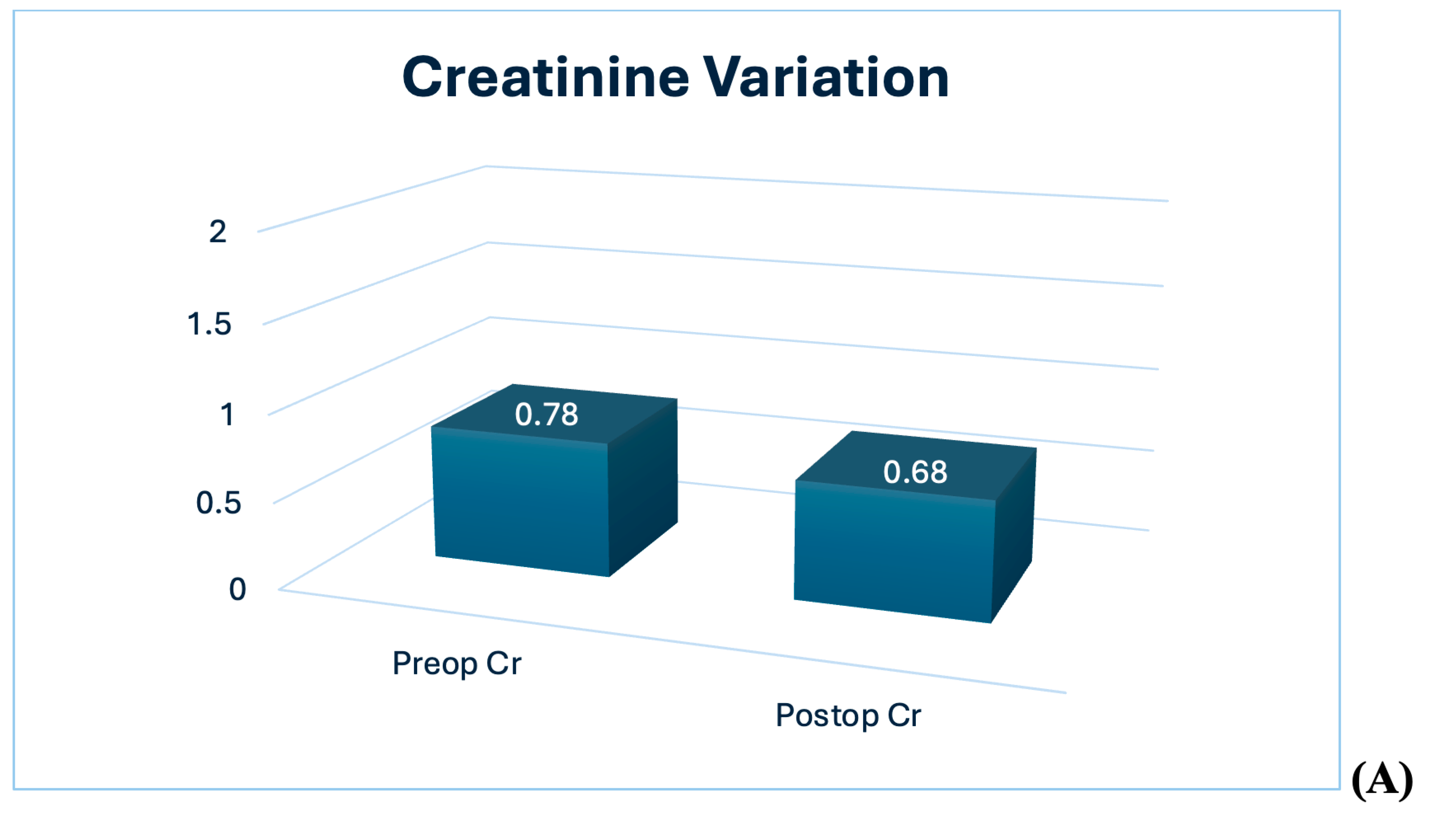
| Creatinine, mg/dL | 0.78 (0.70/0.81) | 0.66 (0.63/0.76) | 0.68 (0.66/0.74) | −0.1 |
| BUN, mg/dL | 16 (14/23) | 17 (13/28) | 16 (14/27) | 0 |
| eGFR, mL/min/1.73 m2 | 90 (60/100) | 81 (55/90) | 84 (59/90) | −10 |
| GFR scintigraphy, mL/min | 81 (60/100) | 74 (56/90) | 75 (58/90) | −9 |
| Author(s) | Type of Study | Sample Size | Ischemia Technique | Renal Function Outcomes |
|---|---|---|---|---|
| Thompson 2010 [18] | Retrospective | 362 | Warm ischemia | -AKI: 19% -New CKD IV: 17% -Longer WIT: OR 1.05 × min |
| Mir 2013 [16] | Retrospective | 35 57 | Hypothermia Limited warm | -GP/VS: 101% vs. 92% -%PVS: Effect 36.9 (29.5,43.3) |
| Greco 2019 [9] | Systematic review | 22,626 | Cold Warm Zero | Log2 GFR mean changes: −1.37 (−3.42 to 0.68) −1.00 (−2.04 to 0.03) −0.71 (−1.15 to−0.27) |
| Anderson 2019 [7] | RCT | 40 40 | Zero Warm | %GFR change: −10.7% vs. −9.4% %SRF change: −11.2% vs. −11.8% |
| Antonelli 2021 [19] | RCT | 164 160 | Zero Warm | %GFR change: −5.1% vs. −6.2% %SRF change: −2% vs. −2.5% |
| Present study | Retrospective | 21 | Zero | -%GFR change: −12% -New CDK IV: 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, A.; Riolo, S.; Tema, G.; Guidotti, A.; Brassetti, A.; Anceschi, U.; Bove, A.M.; D’Annunzio, S.; Ferriero, M.; Mastroianni, R.; et al. Renal Function Preservation in Purely Off-Clamp Sutureless Robotic Partial Nephrectomy: Initial Experience and Technique. Diagnostics 2024, 14, 1579. https://doi.org/10.3390/diagnostics14151579
Franco A, Riolo S, Tema G, Guidotti A, Brassetti A, Anceschi U, Bove AM, D’Annunzio S, Ferriero M, Mastroianni R, et al. Renal Function Preservation in Purely Off-Clamp Sutureless Robotic Partial Nephrectomy: Initial Experience and Technique. Diagnostics. 2024; 14(15):1579. https://doi.org/10.3390/diagnostics14151579
Chicago/Turabian StyleFranco, Antonio, Sara Riolo, Giorgia Tema, Alessio Guidotti, Aldo Brassetti, Umberto Anceschi, Alfredo Maria Bove, Simone D’Annunzio, Mariaconsiglia Ferriero, Riccardo Mastroianni, and et al. 2024. "Renal Function Preservation in Purely Off-Clamp Sutureless Robotic Partial Nephrectomy: Initial Experience and Technique" Diagnostics 14, no. 15: 1579. https://doi.org/10.3390/diagnostics14151579
APA StyleFranco, A., Riolo, S., Tema, G., Guidotti, A., Brassetti, A., Anceschi, U., Bove, A. M., D’Annunzio, S., Ferriero, M., Mastroianni, R., Misuraca, L., Guaglianone, S., Tuderti, G., Leonardo, C., Cicione, A., Licari, L. C., Bologna, E., Flammia, R. S., Nacchia, A., ... De Nunzio, C. (2024). Renal Function Preservation in Purely Off-Clamp Sutureless Robotic Partial Nephrectomy: Initial Experience and Technique. Diagnostics, 14(15), 1579. https://doi.org/10.3390/diagnostics14151579






