Early Presentation of Boerhaave Syndrome in the Emergency Department: A Case Report and Review of the Literature
Abstract
1. Introduction
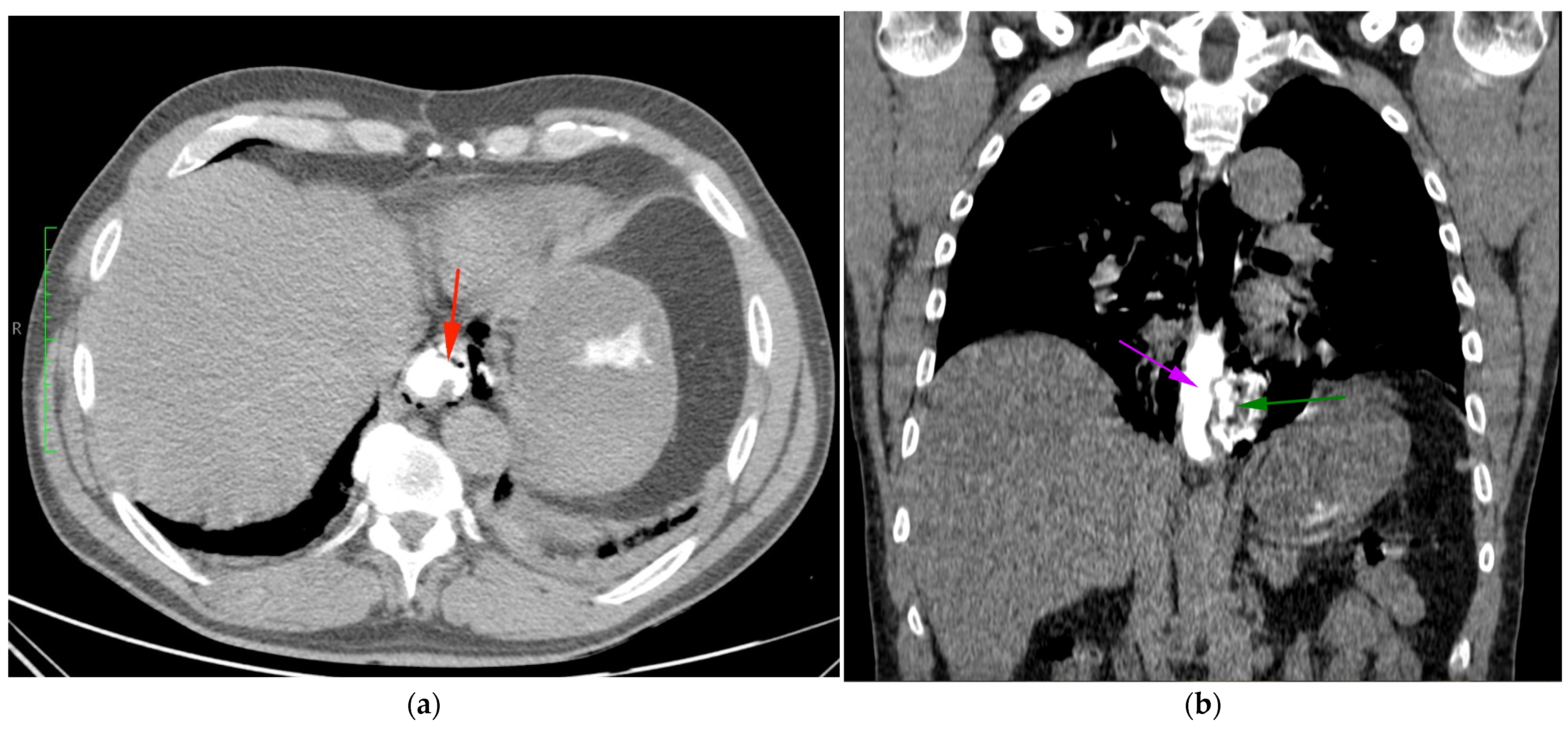
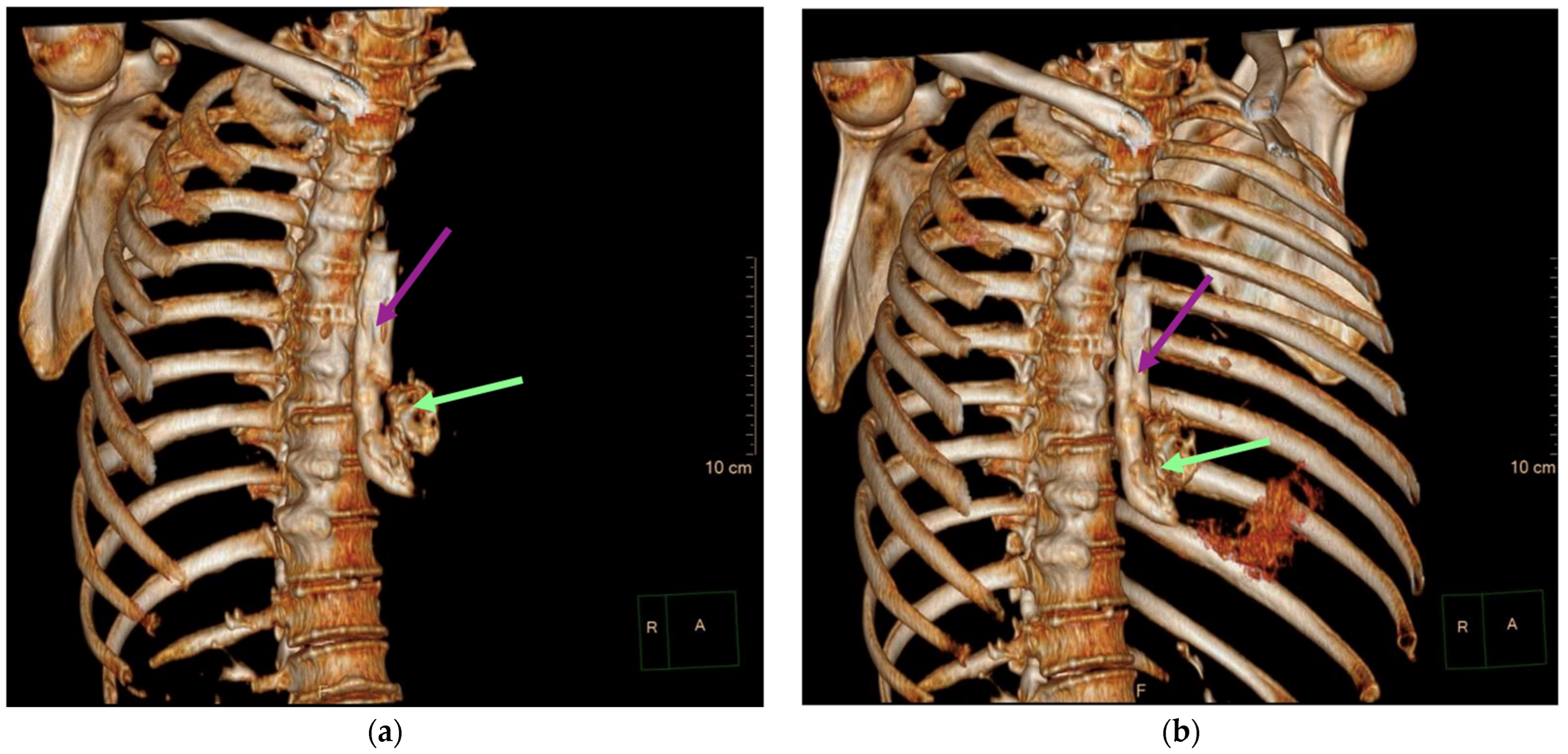
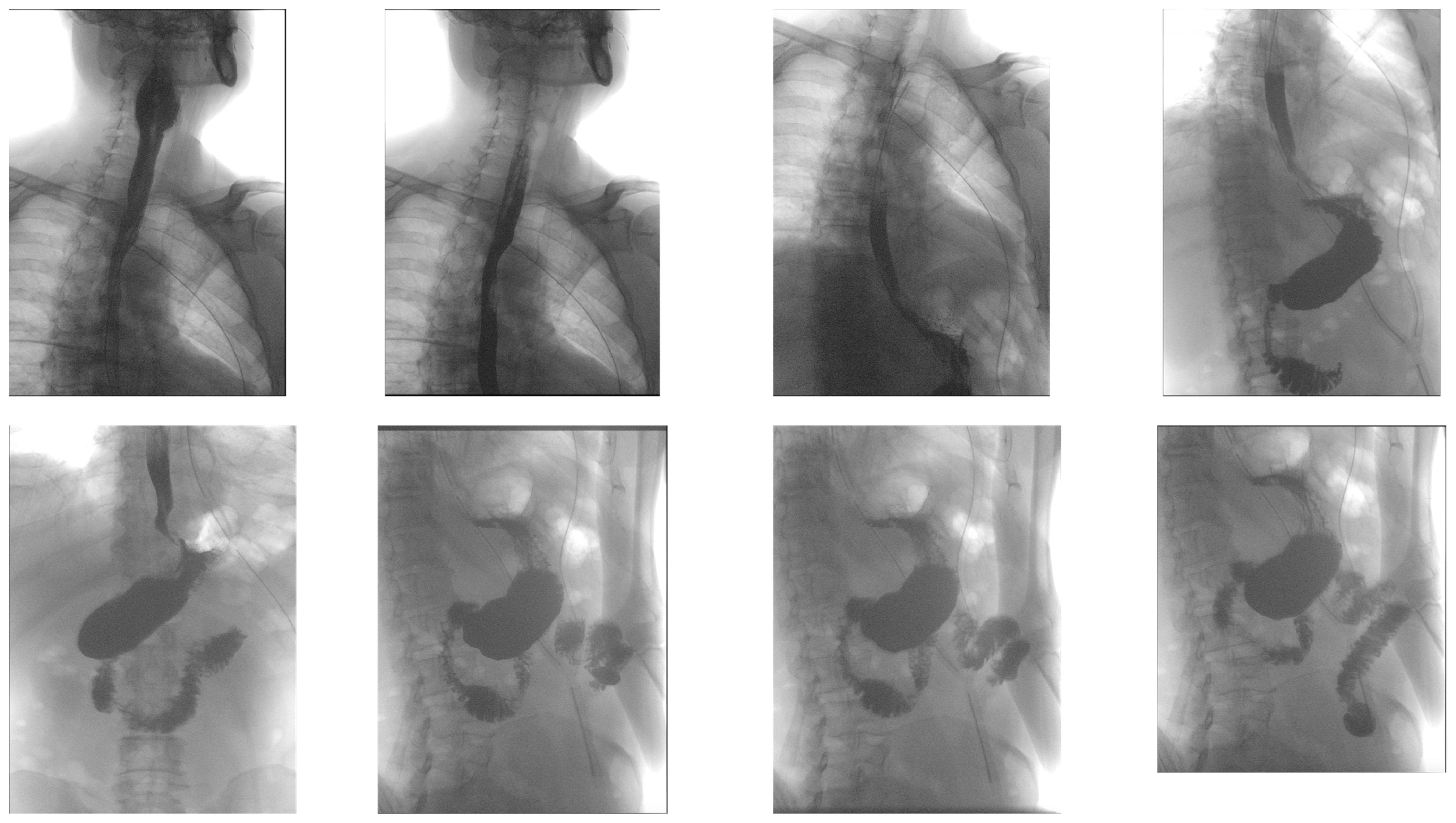
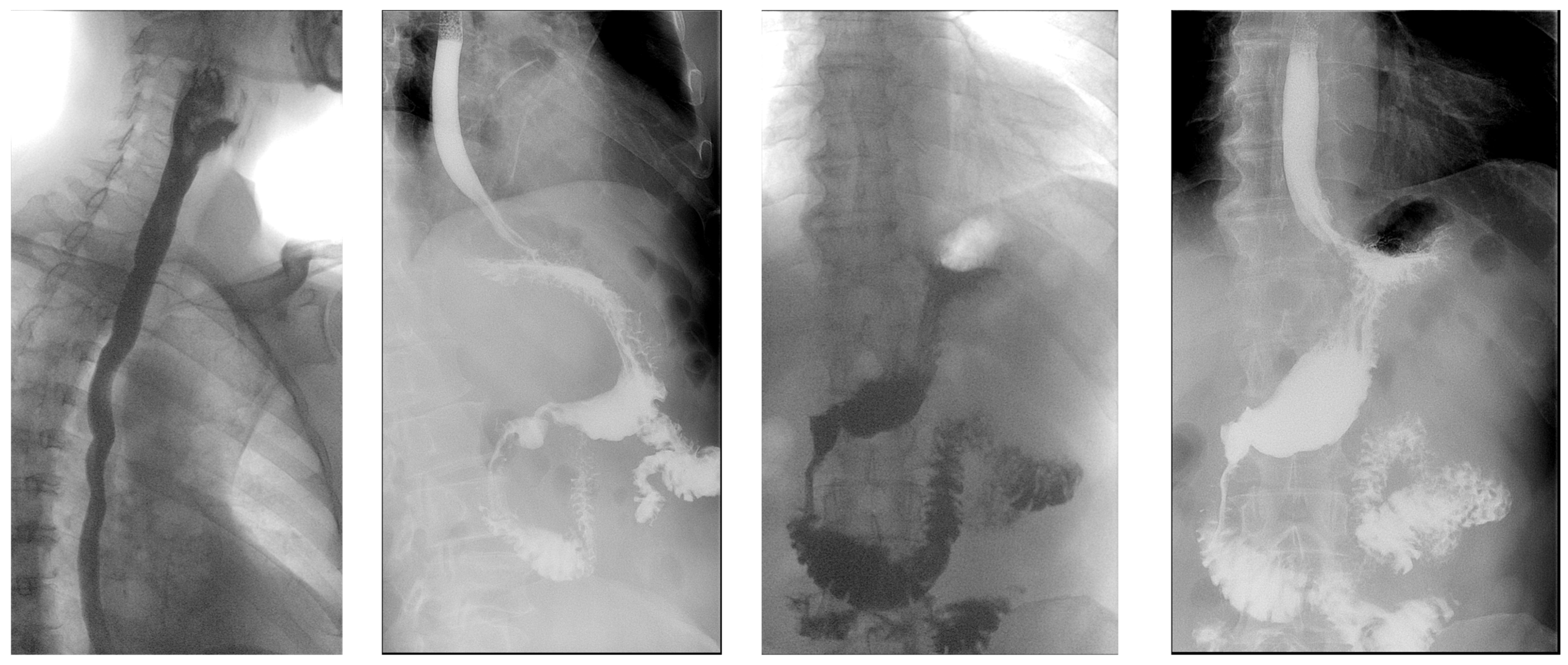
2. Case
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whyte, R.I. Boerhaave’s syndrome. N. Engl. J. Med. 2001, 344, 139. [Google Scholar] [CrossRef] [PubMed]
- Derbes, V.J.; Mitchell, R.E., Jr. Hermann Boerhaave’s Atrocis, nec descripti prius, morbi historia, the first translation of the classic case report of rupture of the esophagus, with annotations. Bull. Med. Libr. Assoc. 1955, 43, 217–240. [Google Scholar] [PubMed] [PubMed Central]
- Tamatey, M.N.; Sereboe, L.A.; Tettey, M.M.; Entsua-Mensah, K.; Gyan, B. Boerhaave’s syndrome: Diagnosis and successful primary repair one month after the oesophageal perforation. Ghana Med. J. 2013, 47, 53–55. [Google Scholar] [PubMed] [PubMed Central]
- Turner, A.R.; Collier, S.A.; Turner, S.D. Boerhaave Syndrome. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Chew, F.Y.; Yang, S.T. Boerhaave syndrome. CMAJ 2021, 193, E1499. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dinic, B.R.; Ilic, G.; Rajkovic, S.T.; Stoimenov, T.J. Boerhaave syndrome—Case report. Sao Paulo Med. J. 2017, 135, 71–75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Garas, G.; Zarogoulidis, P.; Efthymiou, A.; Athanasiou, T.; Tsakiridis, K.; Mpaka, S.; Zacharakis, E. Spontaneous esophageal rupture as the underlying cause of pneumothorax: Early recognition is crucial. J. Thorac. Dis. 2014, 6, 1655–1658. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Venø, S.; Eckardt, J. Boerhaave’s syndrome and tension pneumothorax secondary to Norovirus induced forceful emesis. J. Thorac. Dis. 2013, 5, E38–E40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsuura, N.; Saitou, K. Boerhaave’s Syndrome. Intern. Med. 2022, 61, 265–266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haba, Y.; Yano, S.; Akizuki, H.; Hashimoto, T.; Naito, T.; Hashiguchi, N. Boerhaave syndrome due to excessive alcohol consumption: Two case reports. Int. J. Emerg. Med. 2020, 13, 56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bury, J.; Fratczak, A.; Nielson, J.A. Atypical Presentation of Boerhaave Syndrome With Hypoxia and Unresponsiveness. Cureus 2022, 14, e27848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salvador-Ibarra, I.J.; Pizaña-Davila, A. Boerhaave syndrome. Case report and literature review. Cirugía Cir. 2021, 89, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Sdralis, E.I.K.; Petousis, S.; Rashid, F.; Lorenzi, B.; Charalabopoulos, A. Epidemiology, diagnosis, and management of esophageal perforations: Systematic review. Dis. Esophagus 2017, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- de Schipper, J.P.; Pull ter Gunne, A.F.; Oostvogel, H.J.; van Laarhoven, C.J. Spontaneous rupture of the oesophagus: Boerhaave’s syndrome in 2008. Literature review and treatment algorithm. Dig. Surg. 2009, 26, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sancheti, M.S.; Fernandez, F.G. Surgical Management of Esophageal Perforation. Oper. Tech. Thorac. Cardiovasc. Surg. 2015, 20, 234–250. [Google Scholar] [CrossRef]
- Brinster, C.J.; Singhal, S.; Lee, L.; Marshall, M.B.; Kaiser, L.R.; Kucharczuk, J.C. Evolving options in the management of esophageal perforation. Ann. Thorac. Surg. 2004, 77, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Vidarsdottir, H.; Blondal, S.; Alfredsson, H.; Geirsson, A.; Gudbjartsson, T. Oesophageal perforations in Iceland: A whole population study on incidence, aetiology and surgical outcome. Thorac. Cardiovasc. Surg. 2010, 58, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, B.D.; van der Leeden, B.; Ali, J.T.; Gudbjartsson, T.; Hermansson, M.; Low, D.E.; Adler, D.G.; Botha, A.J.; D’Journo, X.B.; Eroglu, A.; et al. Early diagnosis is associated with improved clinical outcomes in benign esophageal perforation: An individual patient data meta-analysis. Surg. Endosc. 2021, 35, 3492–3505. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khadka, B.; Khanal, K.; Dahal, P.; Adhikari, H. A rare case of Boerhaave syndrome with cervico-thoracic esophageal junction rupture causing bilateral empyema; case report from Nepal. Int. J. Surg. Case Rep. 2023, 105, 108018. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rokicki, M.; Rokicki, W.; Rydel, M. Boerhaave’s Syndrome—Over 290 Yrs of Surgical Experiences. Epidemiology, Pathophysiology, Diagnosis. Pol. J. Surg. 2016, 88, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Bladergroen, M.R.; Lowe, J.E.; Postlethwait, R.W. Diagnosis and recommended management of esophageal perforation and rupture. Ann. Thorac. Surg. 1986, 42, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Korn, O.; Oñate, J.C.; López, R. Anatomy of the Boerhaave syndrome. Surgery 2007, 141, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Yap, D.; Ng, M.; Mbakada, N. A rare complication of ileostomy obstruction: Boerhaave syndrome. Ann. R. Coll. Surg. Engl. 2018, 100, e1–e4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lieu, M.T.; Layoun, M.E.; Dai, D.; Soo Hoo, G.W.; Betancourt, J. Tension hydropneumothorax as the initial presentation of Boerhaave syndrome. Respir. Med. Case Rep. 2018, 25, 100–103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kopelman, Y.; Troiza, A.; Hebron, D. Boerhaave syndrome in an elderly man successfully treated with 3-month indwelling esophageal stent. Radiol. Case Rep. 2018, 13, 1084–1086. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dodds, W.J.; Stewart, E.T.; Vlymen, W.J. Appropriate contrast media for evaluation of esophageal disruption. Radiology 1982, 144, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Tonolini, M.; Bianco, R. Spontaneous esophageal perforation (Boerhaave syndrome): Diagnosis with CT-esophagography. J. Emergencies Trauma Shock. 2013, 6, 58–60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Lutio di Castelguidone, E.; Merola, S.; Pinto, A.; Raissaki, M.; Gagliardi, N.; Romano, L. Esophageal injuries: Spectrum of multidetector row CT findings. Eur. J. Radiol. 2006, 59, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Kim, R. Boerhaave syndrome treated with endoscopic suturing. VideoGIE 2019, 4, 118–119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Curci, J.J.; Horman, M.J. Boerhaave’s syndrome: The importance of early diagnosis and treatment. Ann. Surg. 1976, 183, 401–408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Türüt, H.; Gulhan, E.; Adams, P.Y.; Cetin, G. Successful conservative management of Boerhaave’s syndrome with late presentation. J. Natl. Med. Assoc. 2006, 98, 1857–1859. [Google Scholar] [PubMed] [PubMed Central]
- Cross, M.R.; Greenwald, M.F.; Dahhan, A. Esophageal Perforation and Acute Bacterial Mediastinitis: Other Causes of Chest Pain That Can Be Easily Missed. Medicine 2015, 94, e1232. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ionescu Miron, A.I.; Anghel, A.V.; Antone-Iordache, I.L.; Atasiei, D.I.; Anghel, C.A.; Barnonschi, A.A.; Bobolocu, A.M.; Verga, C.; Șandru, F.; Lișcu, H.D. Assessing the Impact of Organ Failure and Metastases on Quality of Life in Breast Cancer Patients: A Prospective Study Based on Utilizing EORTC QLQ-C30 and EORTC QLQ-BR45 Questionnaires in Romania. J. Pers. Med. 2024, 14, 214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miron, A.I.; Anghel, A.V.; Barnonschi, A.A.; Mitre, R.; Liscu, H.D.; Găinariu, E.; Pătru, R.; Coniac, S. Real-World Outcomes of CDK4/6 Inhibitors Treatment in Metastatic Breast Cancer in Romania. Diagnostics 2023, 13, 1938. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ionescu Miron, A.I.; Atasiei, D.I.; Ionescu, R.T.; Ultimescu, F.; Barnonschi, A.A.; Anghel, A.V.; Anghel, C.A.; Antone-Iordache, I.L.; Mitre, R.; Bobolocu, A.M.; et al. Prediction of Subclinical and Clinical Multiple Organ Failure Dysfunction in Breast Cancer Patients-A Review Using AI Tools. Cancers 2024, 16, 381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dagash, H.I.; Baillie, C.; Lawson, R.A.; Will, A.M. Boerhaave syndrome following chemotherapy in a child with acute lymphoblastic leukemia. Pediatr. Blood Cancer 2004, 43, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Uemura, N.; Nishikawa, D.; Oguri, K.; Abe, T.; Higaki, E.; Hosoi, T.; An, B.; Hasegawa, Y.; Shimizu, Y. Boerhaave syndrome due to hypopharyngeal stenosis associated with chemoradiotherapy for hypopharyngeal cancer: A case report. Surg. Case Rep. 2018, 4, 54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hauge, T.; Kleven, O.C.; Johnson, E.; Hofstad, B.; Johannessen, H.O. Outcome after stenting and débridement for spontaneous esophageal rupture. Scand. J. Gastroenterol. 2018, 53, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.T.; Rice, R.D.; David, E.A.; Spicer, J.D.; Dubose, J.J.; Bonavina, L.; Siboni, S.; O’Callaghan, T.A.; Luo-Owen, X.; Harrison, S.; et al. Perforated esophageal intervention focus (PERF) study: A multi-center examination of contemporary treatment. Dis. Esophagus 2017, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schweigert, M.; Beattie, R.; Solymosi, N.; Booth, K.; Dubecz, A.; Muir, A.; Moskorz, K.; Stadlhuber, R.J.; Ofner, D.; McGuigan, J.; et al. Endoscopic stent insertion versus primary operative management for spontaneous rupture of the esophagus (Boerhaave syndrome): An international study comparing the outcome. Am. Surg. 2013, 79, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Dasari, B.V.; Neely, D.; Kennedy, A.; Spence, G.; Rice, P.; Mackle, E.; Epanomeritakis, E. The role of esophageal stents in the management of esophageal anastomotic leaks and benign esophageal perforations. Ann. Surg. 2014, 259, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Hanajima, T.; Kataoka, Y.; Masuda, T.; Asari, Y. Usefulness of lavage and drainage using video-assisted thoracoscopic surgery for Boerhaave’s syndrome: A retrospective analysis. J. Thorac. Dis. 2021, 13, 3420–3425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| Blood Test Category | Blood Test | Result | Reference Interval |
|---|---|---|---|
| Blood Count | White blood cell | 15.09 | 4–10 × m/mm3 |
| Lymphocytes % | 12.2 | 20–40% | |
| Monocytes % | 9.5 | 3–10% | |
| Neutrophiles % | 77.9 | 30–70% | |
| Eosinophiles % | 0.0 | 0–7% | |
| Basophils % | 0.4% | <1% | |
| Red blood cell % | 5.56 | 3.8–6 M/mm3 | |
| Mean corpuscular volume (MCV) | 97.5 | 80–100 fL | |
| Mean corpuscular hemoglobin (MCH) | 27.8 | 25–32 pg | |
| Mean corpuscular hemoglobin concentration (MCHC) | 28.5 | 28–36 g/dL | |
| Hematocrit | 54.2 | 33–54% | |
| Hemoglobin | 15.5 | 10–16.5 g/dL | |
| Platelet count | 408 | 100–450 m/mm3 | |
| Mean platelet volume (MPV) | 7.9 | 6–13 fL | |
| Biochemistry Tests | Glucose | 173 | 74–106 mg/dL |
| Urea | 40.9 | 17–43 mg/dL | |
| Creatinine | 1.39 | 0.67–1.17 mg/dL | |
| Alanine aminotransferase (ALT) | 72 | 0–50 U/L | |
| Aspartate aminotransferase (AST) | 97 | 0–50 U/L | |
| Bilirubin total | 0.52 | 0.3–1.2 mg/dL | |
| Bilirubin direct | 0.09 | 0–0.2 mg/dL | |
| Amylase | 155 | 28–100 U/L | |
| Lipase | 87 | 8–78 U/L | |
| Creatine kinase (CK) | 6480 | 0–171 U/L | |
| Creatine kinase MB (CK-MB) | 96 | 0–25 U/L | |
| Sodium | 142 | 136–146 mmol/L | |
| Potassium | 3.58 | 3.5–5.1 mmol/L | |
| hs-cTnI | 18 | <28.9 ng/L | |
| Coagulogram | Prothrombin time (PT) | 12.9 | 9.4–12.5 s |
| INR | 1.17 | 0.800–1.140 | |
| Activated partial thromboplastin time (aPTT) | 22.7 | 22.0–36.0 s | |
| Fibrinogen | 351 | 238–498 mg/dL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eremia, I.-A.; Anghel, C.-A.; Cofaru, F.-A.; Nica, S. Early Presentation of Boerhaave Syndrome in the Emergency Department: A Case Report and Review of the Literature. Diagnostics 2024, 14, 1592. https://doi.org/10.3390/diagnostics14151592
Eremia I-A, Anghel C-A, Cofaru F-A, Nica S. Early Presentation of Boerhaave Syndrome in the Emergency Department: A Case Report and Review of the Literature. Diagnostics. 2024; 14(15):1592. https://doi.org/10.3390/diagnostics14151592
Chicago/Turabian StyleEremia, Irina-Anca, Cătălin-Alexandru Anghel, Florina-Alexandra Cofaru, and Silvia Nica. 2024. "Early Presentation of Boerhaave Syndrome in the Emergency Department: A Case Report and Review of the Literature" Diagnostics 14, no. 15: 1592. https://doi.org/10.3390/diagnostics14151592
APA StyleEremia, I.-A., Anghel, C.-A., Cofaru, F.-A., & Nica, S. (2024). Early Presentation of Boerhaave Syndrome in the Emergency Department: A Case Report and Review of the Literature. Diagnostics, 14(15), 1592. https://doi.org/10.3390/diagnostics14151592








