Advancing Pathogen Identification: The Role of Digital PCR in Enhancing Diagnostic Power in Different Settings
Abstract
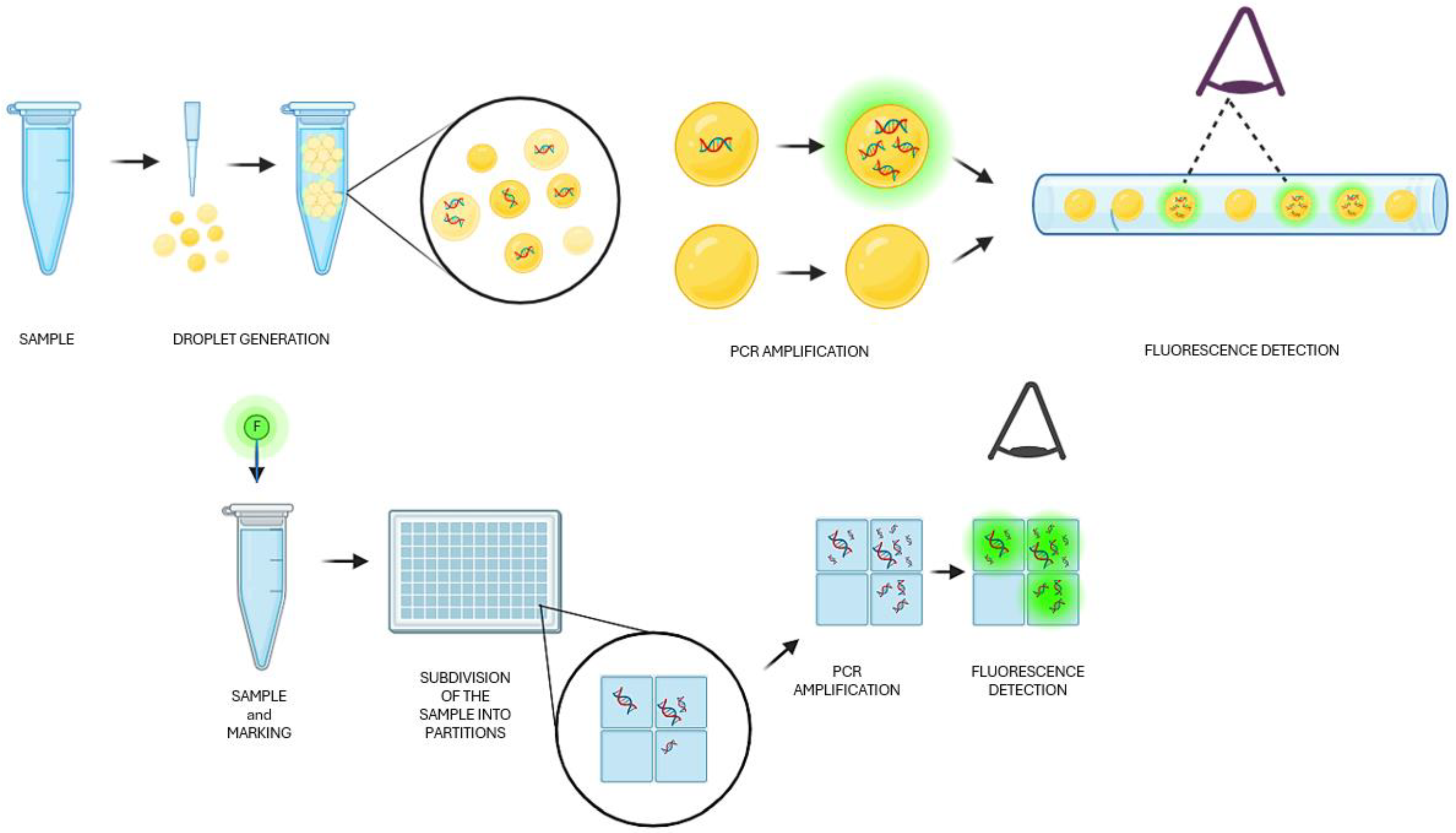
:1. Introduction
2. Digital PCR: Brief History and Concept
3. Commercially Available Digital Platform
3.1. Droplet Digital PCR (ddPCR)
3.1.1. Bio-Rad QX200™ Droplet Digital PCR System (Bio-Rad Laboratories S.r.l, Milan, Italy)
3.1.2. Bio-Rad QX ONE™ Droplet Digital PCR System (Bio-Rad Laboratories S.r.l, Milan, Italy)
3.1.3. Bio-Rad ThunderBolts™ Digital PCR System (Bio-Rad Laboratories S.r.l, Milan, Italy)
3.2. Digital PCR (dPCR)
3.2.1. Thermo Fisher Scientific’s QuantStudio 3D Digital PCR System (Thermo Fisher Scientific Inc., Waltham, MA, USA)
3.2.2. Stilla Technologies’ Naica™ System (Stilla Technologies, Villejuif, France)
3.2.3. QIAcuity Digital PCR System by QIAGEN (QUIAGEN, Hilden, Germany)
3.2.4. Digital LightCycler® System Developed by Roche Diagnostics (Hoffmann-La Roche, Basilea, Switzerland)
3.2.5. Lab on an Array (LOAA) Digital Real-Time PCR Analyzer System by OPTOLANE (OPTOLANE Technologies Inc., Giheung-gu, Republic of Korea)
3.3. Technical Features
3.3.1. Bio-Rad QX200™ Droplet Digital PCR (ddPCR) System
3.3.2. QIAcuity Digital PCR System by QIAGEN
3.3.3. Digital LightCycler® System Developed by Roche Diagnostics
4. Digital PCR in Water Microbiology
4.1. dPCR in SARS-CoV-2 Wastewater-Based Epidemiology
4.2. dPCR in Wastewater Monitoring
4.3. dPCR in Surface Water Monitoring
4.4. dPCR in Drinking Water Monitoring
5. Clinical Diagnostic Application
6. Future Prospects of dPCR
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuypers, J.; Jerome, K.R. Applications of Digital PCR for Clinical Microbiology. J. Clin. Microbiol. 2017, 55, 1621–1628. [Google Scholar] [CrossRef]
- Quan, P.-L.; Sauzade, M.; Brouzes, E. dPCR: A Technology Review. Sensors 2018, 18, 1271. [Google Scholar] [CrossRef]
- Arya, M.; Shergill, I.S.; Williamson, M.; Gommersall, L.; Arya, N.; Patel, H.R. Basic principles of real-time quantitative PCR. Expert Rev. Mol. Diagn. 2005, 5, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Liu, C.; Tong, H.; Chen, Y.; Liu, K. Principles of digital PCR and its applications in current obstetrical and gynecological diseases. Am. J. Transl. Res. 2019, 11, 7209–7222. [Google Scholar] [PubMed]
- Svec, D.; Tichopad, A.; Novosadova, V.; Pfaffl, M.W.; Kubista, M. How good is a PCR efficiency estimate: Recommendations for precise and robust qPCR efficiency assessments. Biomol. Detect. Quantif. 2015, 3, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Day, E.; Dear, P.H.; McCaughan, F. Digital PCR strategies in the development and analysis of molecular biomarkers for personalized medicine. Methods 2013, 59, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Kinzler, K.W. Digital PCR. Proc. Natl. Acad. Sci. USA 1999, 96, 9236–9241. [Google Scholar] [CrossRef] [PubMed]
- Sykes, P.J.; Neoh, S.H.; Brisco, M.J.; Hughes, E.; Condon, J.; Morley, A.A. Quantitation of targets for PCR by use of limiting dilution. BioTechniques 1992, 13, 444–449. [Google Scholar] [PubMed]
- Baker, M. Digital PCR hits its stride. Nat. Methods 2012, 9, 541–544. [Google Scholar] [CrossRef]
- Feng, Z.; Shu, Y. An Overview of Digital PCR. Bing Xue Bao Chin. J. Virol. 2017, 33, 103–107. [Google Scholar]
- Whale, A.S.; Cowen, S.; Foy, C.A.; Huggett, J.F. Methods for applying accurate digital PCR analysis on low copy DNA samples. PLoS ONE 2013, 8, e58177. [Google Scholar] [CrossRef] [PubMed]
- Dube, S.; Qin, J.; Ramakrishnan, R. Mathematical analysis of copy number variation in a DNA sample using digital PCR on a nanofluidic device. PLoS ONE 2008, 3, e2876. [Google Scholar] [CrossRef] [PubMed]
- Wainman, L.M.; Sathyanarayana, S.H.; Lefferts, J.A. Applications of Digital Polymerase Chain Reaction (dPCR) in Molecular and Clinical Testing. J. Appl. Lab. Med. 2024, 9, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Ahmed, W.; Oikarinen, S.; Sherchan, S.P.; Heikinheimo, A.; Jiang, G.; Simpson, S.L.; Greaves, J.; Bivins, A. Application of digital PCR for public health-related water quality monitoring. Sci. Total Environ. 2022, 837, 155663. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Shen, S.; Jiang, H.; Chen, Z. Application of Digital PCR in Detecting Human Diseases Associated Gene Mutation. Cell. Physiol. Biochem. 2017, 43, 1718–1730. [Google Scholar] [CrossRef] [PubMed]
- Kokkoris, V.; Vukicevich, E.; Richards, A.; Thomsen, C.; Hart, M.M. Challenges Using Droplet Digital PCR for Environmental Samples. Appl. Microbiol. 2021, 1, 74–88. [Google Scholar] [CrossRef]
- Tamura, T.; Imaizumi, T.; Shimojima Yamamoto, K.; Yamamoto, T. Genomic Copy Number Analysis Using Droplet Digital PCR: A Simple Method with EvaGreen Single-Color Fluorescent Design. Methods Mol. Biol. 2024, 2794, 293–304. [Google Scholar] [PubMed]
- Scaini, M.C.; Catoni, C.; Poggiana, C.; Pigozzo, J.; Piccin, L.; Leone, K.; Scarabello, I.; Facchinetti, A.; Menin, C.; Elefanti, L.; et al. A multiparameter liquid biopsy approach allows to track melanoma dynamics and identify early treatment resistance. NPJ Precis. Oncol. 2024, 8, 78. [Google Scholar] [CrossRef]
- Tumpach, C.; Rhodes, A.; Kim, Y.; Ong, J.; Liu, H.; Chibo, D.; Druce, J.; Williamson, D.; Hoh, R.; Deeks, S.G.; et al. Adaptation of Droplet Digital PCR-Based HIV Transcription Profiling to Digital PCR and Association of HIV Transcription and Total or Intact HIV DNA. Viruses 2023, 15, 1606. [Google Scholar] [CrossRef]
- Liu, D.; Yin, H.; Wang, Y.; Cao, Y.; Yin, J.; Zhang, J.; Yin, H.; Zhao, X. Development of a highly sensitive digital PCR assay to quantify long non-coding RNA MYU in urine samples which exhibited great potential as an alternative diagnostic biomarker for prostate cancer. Transl. Androl. Urol. 2021, 10, 3815–3825. [Google Scholar] [CrossRef]
- Maggi, R.G.; Richardson, T.; Breitschwerdt, E.B.; Miller, J.C. Development and validation of a droplet digital PCR assay for the detection and quantification of Bartonella species within human clinical samples. J. Microbiol. Methods 2020, 176, 106022. [Google Scholar] [CrossRef] [PubMed]
- Specchiarello, E.; Carletti, F.; Matusali, G.; Abbate, I.; Rozera, G.; Minosse, C.; Petrivelli, E.; Ferraioli, V.; Sciamanna, R.; Maggi, F. Development and validation of a nanoplate-based digital PCR assay for absolute MPXV quantification. J. Virol. Methods 2023, 321, 114802. [Google Scholar] [CrossRef] [PubMed]
- Hyung, J.-H.; Moon, S.J.; Kim, E.J.; Kim, D.W.; Park, J. Quantification of Alexandrium catenella (Group I) using sxtA4-based digital PCR for screening of paralytic shellfish toxins in Jinhae-Masan Bay, Korea. Mar. Pollut. Bull. 2024, 200, 116048. [Google Scholar] [CrossRef] [PubMed]
- Kanagal-Shamanna, R. Emulsion PCR: Techniques and Applications. Methods Mol. Biol. 2016, 1392, 33–42. [Google Scholar] [PubMed]
- Guo, Q.; Wang, L.; Liang, X.; Zhao, M.; Huang, X.; Xu, W.; Lou, J.; Qiao, L. Comparative analysis of QS3D versus droplet digital PCR for quantitative measures of EGFR T790M mutation from identical plasma. Heliyon 2022, 8, e11339. [Google Scholar] [CrossRef] [PubMed]
- Dioni, L.; Orlandi, A.; Uceda Renteria, S.; Favero, C.; Solazzo, G.; Oggioni, M.; Bollati, V. Digital RT-PCR Chip method for detection of SARS-CoV-2 virus. J. Immunol. Methods 2022, 509, 113339. [Google Scholar] [CrossRef] [PubMed]
- Bogožalec Košir, A.; Muller, S.; Žel, J.; Milavec, M.; Mallory, A.C.; Dobnik, D. Fast and Accurate Multiplex Identification and Quantification of Seven Genetically Modified Soybean Lines Using Six-Color Digital PCR. Foods 2023, 12, 4156. [Google Scholar] [CrossRef] [PubMed]
- Corné, J.; Quillien, V.; Godey, F.; Cherel, M.; Cochet, A.; Le Du, F.; Robert, L.; Bourien, H.; Brunot, A.; Crouzet, L.; et al. Plasma-based analysis of ERBB2 mutational status by multiplex digital PCR in a large series of patients with metastatic breast cancer. Mol. Oncol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, K.; Wells, J.; Patel, A.; Mendonca Torres, M.; Costello, J.; Jensen, K.; Vasko, V. Clinical and molecular characterization of thyroid cancer when seen as a second malignant neoplasm. Ther. Adv. Endocrinol. Metab. 2021, 12, 20420188211058327. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-J.; Shin, W.; Song, M.; Shin, S.-S.; Park, Y.; Srikulnath, K.; Kim, D.H.; Han, K. Comparison of digital PCR platforms using the molecular marker. Genom. Inform. 2023, 21, e24. [Google Scholar] [CrossRef]
- Boxman, I.L.A.; Molin, R.; Persson, S.; Juréus, A.; Jansen, C.C.C.; Sosef, N.P.; Le Guyader, S.F.; Ollivier, J.; Summa, M.; Hautaniemi, M.; et al. An international inter-laboratory study to compare digital PCR with ISO standardized qPCR assays for the detection of norovirus GI and GII in oyster tissue. Food Microbiol. 2024, 120, 104478. [Google Scholar] [CrossRef]
- Dhar, B.C.; Delgado Santander, R.; Aćimović, S.G. Improved Canker Processing and Viability Droplet Digital PCR Allow Detection of Erwinia amylovora Viable Nonculturable Cells in Apple Bark. Microorganisms 2024, 12, 376. [Google Scholar] [CrossRef] [PubMed]
- Kløve-Mogensen, K.; Terp, S.K.; Steffensen, R. Comparison of real-time quantitative PCR and two digital PCR platforms to detect copy number variation in FCGR3B. J. Immunol. Methods 2024, 526, 113628. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martín, V.; López-López, E.; Reguero-Paredes, D.; Godoy-Ortiz, A.; Domínguez-Recio, M.E.; Jiménez-Rodríguez, B.; Alba-Bernal, A.; Elena Quirós-Ortega, M.; Roldán-Díaz, M.D.; Velasco-Suelto, J.; et al. Comparative study of droplet-digital PCR and absolute Q digital PCR for ctDNA detection in early-stage breast cancer patients. Clin. Chim. Acta Int. J. Clin. Chem. 2024, 552, 117673. [Google Scholar] [CrossRef] [PubMed]
- Musso, N.; Bivona, D.; Bonomo, C.; Bonacci, P.; D’Ippolito, M.E.; Boccagni, C.; Rubino, F.; De Tanti, A.; Lucca, L.F.; Pingue, V.; et al. Investigating microRNAs as biomarkers in disorders of consciousness: A longitudinal multicenter study. Sci. Rep. 2023, 13, 18415. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.A.; Nazakat, R.; Muhamad Robat, R.; Ismail, R.; Suppiah, J.; Rajendran, K.; Raj Louis Masalamany, A.S.S.; Muhamad Hendri, N.A.; Mohamad, N.; Khairul Hasni, N.A.; et al. Droplet digital PCR application for the detection of SARS-CoV-2 in air sample. Front. Public Health 2023, 11, 1208348. [Google Scholar] [CrossRef] [PubMed]
- Zaytseva, M.; Usman, N.; Salnikova, E.; Sanakoeva, A.; Valiakhmetova, A.; Chervova, A.; Papusha, L.; Novichkova, G.; Druy, A. Methodological Challenges of Digital PCR Detection of the Histone H3 K27M Somatic Variant in Cerebrospinal Fluid. Pathol. Oncol. Res. POR 2022, 28, 1610024. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.A.; Marians, R.C.; Miller, K.; Brenton, M.D.; Mallo, R.L.V.; Kohler, M.E.; Fry, T.J.; Winters, A.C. Digital polymerase chain reaction strategies for accurate and precise detection of vector copy number in CAR T cell products. Cytotherapy 2023, 25, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Crucitta, S.; Ruglioni, M.; Novi, C.; Manganiello, M.; Arici, R.; Petrini, I.; Pardini, E.; Cucchiara, F.; Marmorino, F.; Cremolini, C.; et al. Comparison of digital PCR systems for the analysis of liquid biopsy samples of patients affected by lung and colorectal cancer. Clin. Chim. Acta 2023, 541, 117239. [Google Scholar] [CrossRef]
- United Nations (UN). UN-Water, 2021: Summary Progress Update 2021–SDG 6–Water and Sanitation for All. 2021. Available online: https://www.unwater.org/publications/summary-progress-update-2021-sdg-6-water-and-sanitation-all (accessed on 26 March 2024).
- Cabral, J.P.S. Water Microbiology. Bacterial Pathogens and Water. Int. J. Environ. Res. Public. Health 2010, 7, 3657–3703. [Google Scholar] [CrossRef]
- Shayo, G.M.; Elimbinzi, E.; Shao, G.N.; Fabian, C. Severity of waterborne diseases in developing countries and the effectiveness of ceramic filters for improving water quality. Bull. Natl. Res. Cent. 2023, 47, 113. [Google Scholar] [CrossRef]
- Hamza, I.A.; Bibby, K. Critical issues in application of molecular methods to environmental virology. J. Virol. Methods 2019, 266, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, A.P.; Kumar, S.; Giri, B.S.; Kim, K.-H. Antibiotic resistance in major rivers in the world: A systematic review on occurrence, emergence, and management strategies. J. Clean. Prod. 2019, 234, 1484–1505. [Google Scholar] [CrossRef]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Raith, M.R.; Griffith, J.F. Droplet digital PCR for simultaneous quantification of general and human-associated fecal indicators for water quality assessment. Water Res. 2015, 70, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T.; Inagaki, F. Molecular quantification of environmental DNA using microfluidics and digital PCR. Syst. Appl. Microbiol. 2012, 35, 390–395. [Google Scholar] [CrossRef]
- Botes, M.; De Kwaadsteniet, M.; Cloete, T.E. Application of quantitative PCR for the detection of microorganisms in water. Anal. Bioanal. Chem. 2013, 405, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Oliver, D.M.; Hanley, N.D.; Van Niekerk, M.; Kay, D.; Heathwaite, A.L.; Rabinovici, S.J.M.; Kinzelman, J.L.; Fleming, L.E.; Porter, J.; Shaikh, S.; et al. Molecular tools for bathing water assessment in Europe: Balancing social science research with a rapidly developing environmental science evidence-base. Ambio 2016, 45, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Stokdyk, J.P.; Firnstahl, A.D.; Walsh, J.F.; Spencer, S.K.; De Lambert, J.R.; Anderson, A.C.; Rezania, L.-I.W.; Kieke, B.A.; Borchardt, M.A. Viral, bacterial, and protozoan pathogens and fecal markers in wells supplying groundwater to public water systems in Minnesota, USA. Water Res. 2020, 178, 115814. [Google Scholar] [CrossRef]
- Brooks, Y.M.; Spirito, C.M.; Bae, J.S.; Hong, A.; Mosier, E.M.; Sausele, D.J.; Fernandez-Baca, C.P.; Epstein, J.L.; Shapley, D.J.; Goodman, L.B.; et al. Fecal indicator bacteria, fecal source tracking markers, and pathogens detected in two Hudson River tributaries. Water Res. 2020, 171, 115342. [Google Scholar] [CrossRef]
- An, X.-L.; Wang, J.-Y.; Pu, Q.; Li, H.; Pan, T.; Li, H.-Q.; Pan, F.-X.; Su, J.-Q. High-throughput diagnosis of human pathogens and fecal contamination in marine recreational water. Environ. Res. 2020, 190, 109982. [Google Scholar] [CrossRef]
- Fujioka, R.; Solo-Gabriele, H.; Byappanahalli, M.; Kirs, M.U.S. Recreational Water Quality Criteria: A Vision for the Future. Int. J. Environ. Res. Public. Health 2015, 12, 7752–7776. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yamahara, K.M.; Cao, Y.; Boehm, A.B. Absolute Quantification of Enterococcal 23S rRNA Gene Using Digital PCR. Environ. Sci. Technol. 2016, 50, 3399–3408. [Google Scholar] [CrossRef]
- Crain, C.; Kezer, K.; Steele, S.; Owiti, J.; Rao, S.; Victorio, M.; Austin, B.; Volner, A.; Draper, W.; Griffith, J.; et al. Application of ddPCR for detection of Enterococcus spp. in coastal water quality monitoring. J. Microbiol. Methods 2021, 184, 106206. [Google Scholar] [CrossRef]
- Jahne, M.A.; Brinkman, N.E.; Keely, S.P.; Zimmerman, B.D.; Wheaton, E.A.; Garland, J.L. Droplet digital PCR quantification of norovirus and adenovirus in decentralized wastewater and graywater collections: Implications for onsite reuse. Water Res. 2020, 169, 115213. [Google Scholar] [CrossRef]
- Varela, M.F.; Monteiro, S.; Rivadulla, E.; Santos, R.; Romalde, J.L. Development of a novel digital RT-PCR method for detection of human sapovirus in different matrices. J. Virol. Methods 2018, 254, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Pillay, L.; Amoah, I.D.; Deepnarain, N.; Pillay, K.; Awolusi, O.O.; Kumari, S.; Bux, F. Monitoring changes in COVID-19 infection using wastewater-based epidemiology: A South African perspective. Sci. Total Environ. 2021, 786, 147273. [Google Scholar] [CrossRef]
- Reynolds, L.J.; Gonzalez, G.; Sala-Comorera, L.; Martin, N.A.; Byrne, A.; Fennema, S.; Holohan, N.; Kuntamukkula, S.R.; Sarwar, N.; Nolan, T.M.; et al. SARS-CoV-2 variant trends in Ireland: Wastewater-based epidemiology and clinical surveillance. Sci. Total Environ. 2022, 838, 155828. [Google Scholar] [CrossRef] [PubMed]
- Al-Duroobi, H.; Moghadam, S.V.; Phan, D.C.; Jafarzadeh, A.; Matta, A.; Kapoor, V. Wastewater surveillance of SARS-CoV-2 corroborates heightened community infection during the initial peak of COVID-19 in Bexar County, Texas. FEMS Microbes 2021, 2, xtab015. [Google Scholar] [CrossRef]
- Róka, E.; Déri, D.; Khayer, B.; Kis, Z.; Schuler, E.; Magyar, N.; Pályi, B.; Pándics, T.; Vargha, M. SARS-CoV-2 variant detection from wastewater: Rapid spread of B.1.1.7 lineage in Hungary. J. Water Health 2022, 20, 277–286. [Google Scholar] [CrossRef]
- Van Poelvoorde, L.A.E.; Picalausa, C.; Gobbo, A.; Verhaegen, B.; Lesenfants, M.; Herman, P.; Van Hoorde, K.; Roosens, N.H.C. Development of a Droplet Digital PCR to Monitor SARS-CoV-2 Omicron Variant BA.2 in Wastewater Samples. Microorganisms 2023, 11, 729. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Stange, C.; Suhrborg, R.; Wurzbacher, C.; Drewes, J.E.; Tiehm, A. SARS-CoV-2 wastewater surveillance in Germany: Long-term RT-digital droplet PCR monitoring, suitability of primer/probe combinations and biomarker stability. Water Res. 2022, 210, 117977. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Liu, S.; Liu, C.; Bai, J.; Meng, J.; Tian, H.; Han, X.; Han, G.; Xu, X.; Li, Q. Surveillance of SARS-CoV-2 in wastewater by quantitative PCR and digital PCR: A case study in Shijiazhuang city, Hebei province, China. Emerg. Microbes Infect. 2024, 13, 2324502. [Google Scholar] [CrossRef] [PubMed]
- Toledo, D.M.; Robbins, A.A.; Gallagher, T.L.; Hershberger, K.C.; Barney, R.E.; Salmela, S.M.; Pilcher, D.; Cervinski, M.A.; Nerenz, R.D.; Szczepiorkowski, Z.M.; et al. Wastewater-Based SARS-CoV-2 Surveillance in Northern New England. Microbiol. Spectr. 2022, 10, e02207-21. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Straathof, J.; Liu, Y.; Hull, N.M. Monitoring SARS-CoV-2 RNA in Wastewater with RT-qPCR and Chip-Based RT-dPCR: Sewershed-Level Trends and Relationships to COVID-19. ACS EST Water 2022, 2, 2084–2093. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Lara, R.W.; Heijnen, L.; Oude Munnink, B.B.; Schapendonk, C.M.E.; Elsinga, G.; Langeveld, J.; Post, J.; Prasad, D.K.; Carrizosa, C.; Been, F.; et al. Rise and fall of SARS-CoV-2 variants in Rotterdam: Comparison of wastewater and clinical surveillance. Sci. Total Environ. 2023, 873, 162209. [Google Scholar] [CrossRef] [PubMed]
- Viveros, M.L.; Azimi, S.; Pichon, E.; Roose-Amsaleg, C.; Bize, A.; Durandet, F.; Rocher, V. Wild type and variants of SARS-CoV-2 in Parisian sewage: Presence in raw water and through processes in wastewater treatment plants. Environ. Sci. Pollut. Res. 2022, 29, 67442–67449. [Google Scholar] [CrossRef] [PubMed]
- Mtetwa, H.N.; Amoah, I.D.; Kumari, S.; Bux, F.; Reddy, P. Wastewater-Based Surveillance of Antibiotic Resistance Genes Associated with Tuberculosis Treatment Regimen in KwaZulu Natal, South Africa. Antibiotics 2021, 10, 1362. [Google Scholar] [CrossRef] [PubMed]
- Mtetwa, H.N.; Amoah, I.D.; Kumari, S.; Bux, F.; Reddy, P. Molecular surveillance of tuberculosis-causing mycobacteria in wastewater. Heliyon 2022, 8, e08910. [Google Scholar] [CrossRef]
- Maestre-Carballa, L.; Navarro-López, V.; Martinez-Garcia, M. City-scale monitoring of antibiotic resistance genes by digital PCR and metagenomics. Environ. Microbiome 2024, 19, 16. [Google Scholar] [CrossRef]
- Bonanno Ferraro, G.; Bonomo, C.; Brandtner, D.; Mancini, P.; Veneri, C.; Briancesco, R.; Coccia, A.M.; Lucentini, L.; Suffredini, E.; Bongiorno, D.; et al. Characterisation of microbial communities and quantification of antibiotic resistance genes in Italian wastewater treatment plants using 16S rRNA sequencing and digital PCR. Sci. Total Environ. 2024, 933, 173217. [Google Scholar] [CrossRef]
- Staley, Z.R.; Boyd, R.J.; Shum, P.; Edge, T.A. Microbial Source Tracking Using Quantitative and Digital PCR To Identify Sources of Fecal Contamination in Stormwater, River Water, and Beach Water in a Great Lakes Area of Concern. Appl. Environ. Microbiol. 2018, 84, e01634-18. [Google Scholar] [CrossRef]
- Harringer, M.; Alfreider, A. Primer evaluation and development of a droplet digital PCR protocol targeting amoA genes for the quantification of Comammox in lakes. Sci. Rep. 2021, 11, 2982. [Google Scholar] [CrossRef]
- Pendergraph, D.P.; Ranieri, J.; Ermatinger, L.; Baumann, A.; Metcalf, A.L.; DeLuca, T.H.; Church, M.J. Differentiating Sources of Fecal Contamination to Wilderness Waters Using Droplet Digital PCR and Fecal Indicator Bacteria Methods. Wilderness Environ. Med. 2021, 32, 332–339. [Google Scholar] [CrossRef]
- Vezzulli, L.; Oliveri, C.; Borello, A.; Gregory, L.; Kimirei, I.; Brunetta, M.; Stern, R.; Coco, S.; Longo, L.; Taviani, E.; et al. Aquatic reservoir of Vibrio cholerae in an African Great Lake assessed by large scale plankton sampling and ultrasensitive molecular methods. ISME Commun. 2021, 1, 20. [Google Scholar] [CrossRef]
- Coertze, R.D.; Bezuidenhout, C.C. The prevalence and diversity of AmpC β-lactamase genes in plasmids from aquatic systems. Water Sci. Technol. 2018, 2017, 603–611. [Google Scholar] [CrossRef]
- Di Cesare, A.; Petrin, S.; Fontaneto, D.; Losasso, C.; Eckert, E.M.; Tassistro, G.; Borello, A.; Ricci, A.; Wilson, W.H.; Pruzzo, C.; et al. ddPCR applied on archived Continuous Plankton Recorder samples reveals long-term occurrence of class 1 integrons and a sulphonamide resistance gene in marine plankton communities. Environ. Microbiol. Rep. 2018, 10, 458–464. [Google Scholar] [CrossRef]
- Luvhimbi, N.; Tshitangano, T.G.; Mabunda, J.T.; Olaniyi, F.C.; Edokpayi, J.N. Water quality assessment and evaluation of human health risk of drinking water from source to point of use at Thulamela municipality, Limpopo Province. Sci. Rep. 2022, 12, 6059. [Google Scholar] [CrossRef]
- Spencer-Williams, I.; Meyer, M.; DePas, W.; Elliott, E.; Haig, S.-J. Assessing the Impacts of Lead Corrosion Control on the Microbial Ecology and Abundance of Drinking-Water-Associated Pathogens in a Full-Scale Drinking Water Distribution System. Environ. Sci. Technol. 2023, 57, 20360–20369. [Google Scholar] [CrossRef]
- Logan-Jackson, A.; Rose, J.B. Cooccurrence of Five Pathogenic Legionella spp. and Two Free-Living Amoebae Species in a Complete Drinking Water System and Cooling Towers. Pathogens 2021, 10, 1407. [Google Scholar] [CrossRef]
- Kitajima, M.; Cruz, M.C.; Williams, R.B.H.; Wuertz, S.; Whittle, A.J. Microbial abundance and community composition in biofilms on in-pipe sensors in a drinking water distribution system. Sci. Total Environ. 2021, 766, 142314. [Google Scholar] [CrossRef]
- Bivins, A.; Lowry, S.; Wankhede, S.; Hajare, R.; Murphy, H.M.; Borchardt, M.; Labhasetwar, P.; Brown, J. Microbial water quality improvement associated with transitioning from intermittent to continuous water supply in Nagpur, India. Water Res. 2021, 201, 117301. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M. The third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Miglietta, L.; Moniri, A.; Pennisi, I.; Malpartida-Cardenas, K.; Abbas, H.; Hill-Cawthorne, K.; Bolt, F.; Jauneikaite, E.; Davies, F.; Holmes, A.; et al. Coupling Machine Learning and High Throughput Multiplex Digital PCR Enables Accurate Detection of Carbapenem-Resistant Genes in Clinical Isolates. Front. Mol. Biosci. 2021, 8, 775299. [Google Scholar] [CrossRef]
- Hennebique, A.; Bidart, M.; Jarraud, S.; Beraud, L.; Schwebel, C.; Maurin, M.; Boisset, S. Digital PCR for Detection and Quantification of Fluoroquinolone Resistance in Legionella pneumophila. Antimicrob. Agents Chemother. 2017, 61, e00628-17. [Google Scholar] [CrossRef]
- Yoshida, R.; Sasaki, T.; Umekage, Y.; Tanno, S.; Ono, Y.; Ogata, M.; Chiba, S.; Mizukami, Y.; Ohsaki, Y. Highly sensitive detection of ALK resistance mutations in plasma using droplet digital PCR. BMC Cancer 2018, 18, 1136. [Google Scholar] [CrossRef]
- Luo, J.; Li, J.; Yang, H.; Yu, J.; Wei, H. Accurate Detection of Methicillin-Resistant Staphylococcus aureus in Mixtures by Use of Single-Bacterium Duplex Droplet Digital PCR. J. Clin. Microbiol. 2017, 55, 2946–2955. [Google Scholar] [CrossRef]
- Pholwat, S.; Stroup, S.; Foongladda, S.; Houpt, E. Digital PCR to detect and quantify heteroresistance in drug resistant Mycobacterium tuberculosis. PLoS ONE 2013, 8, e57238. [Google Scholar] [CrossRef]
- Zheng, Y.; Jin, J.; Shao, Z.; Liu, J.; Zhang, R.; Sun, R.; Hu, B. Development and clinical validation of a droplet digital PCR assay for detecting Acinetobacter baumannii and Klebsiella pneumoniae in patients with suspected bloodstream infections. MicrobiologyOpen 2021, 10, e1247. [Google Scholar] [CrossRef]
- Chen, B.; Xie, Y.; Zhang, N.; Li, W.; Liu, C.; Li, D.; Bian, S.; Jiang, Y.; Yang, Z.; Li, R.; et al. Evaluation of Droplet Digital PCR Assay for the Diagnosis of Candidemia in Blood Samples. Front. Microbiol. 2021, 12, 700008. [Google Scholar] [CrossRef]
- Ferrer, J.; Clari, M.Á.; Giménez, E.; Carbonell, N.; Torres, I.; Blasco, M.L.; Albert, E.; Navarro, D. The Biofire® Filmarray® Pneumonia Plus panel for management of lower respiratory tract infection in mechanically-ventilated patients in the COVID-19 era: A diagnostic and cost-benefit evaluation. Diagn. Microbiol. Infect. Dis. 2023, 105, 115847. [Google Scholar] [CrossRef]
- Bian, W.; Xie, Y.; Shang, Y.; Zhao, L.; Yang, Z.; Ma, X.; He, Y.; Yu, W.; Xi, W.; Yang, D.; et al. Relationship between clinical features and droplet digital PCR copy number in non-HIV patients with pneumocystis pneumonia. BMC Infect. Dis. 2023, 23, 833. [Google Scholar] [CrossRef]
- Falzone, L.; Gattuso, G.; Lombardo, C.; Lupo, G.; Grillo, C.M.; Spandidos, D.A.; Libra, M.; Salmeri, M. Droplet digital PCR for the detection and monitoring of Legionella pneumophila. Int. J. Mol. Med. 2020, 46, 1777–1782. [Google Scholar] [CrossRef]
- Wu, J.; Tang, B.; Qiu, Y.; Tan, R.; Liu, J.; Xia, J.; Zhang, J.; Huang, J.; Qu, J.; Sun, J.; et al. Clinical validation of a multiplex droplet digital PCR for diagnosing suspected bloodstream infections in ICU practice: A promising diagnostic tool. Crit. Care 2022, 26, 243. [Google Scholar] [CrossRef]
- Bălan, A.-M.; Bodolea, C.; Trancă, S.D.; Hagău, N. Trends in Molecular Diagnosis of Nosocomial Pneumonia Classic PCR vs. Point-of-Care PCR: A Narrative Review. Healthcare 2023, 11, 1345. [Google Scholar] [CrossRef]
- Whale, A.S.; Bushell, C.A.; Grant, P.R.; Cowen, S.; Gutierrez-Aguirre, I.; O’Sullivan, D.M.; Žel, J.; Milavec, M.; Foy, C.A.; Nastouli, E.; et al. Detection of Rare Drug Resistance Mutations by Digital PCR in a Human Influenza A Virus Model System and Clinical Samples. J. Clin. Microbiol. 2016, 54, 392–400. [Google Scholar] [CrossRef]
- Lin, K.; Zhao, Y.; Xu, B.; Yu, S.; Fu, Z.; Zhang, Y.; Wang, H.; Song, J.; Fan, M.; Zhou, Y.; et al. Clinical Diagnostic Performance of Droplet Digital PCR for Suspected Bloodstream Infections. Microbiol. Spectr. 2023, 11, e0137822. [Google Scholar] [CrossRef]
- Merino, I.; de la Fuente, A.; Domínguez-Gil, M.; Eiros, J.M.; Tedim, A.P.; Bermejo-Martín, J.F. Digital PCR applications for the diagnosis and management of infection in critical care medicine. Crit. Care 2022, 26, 63. [Google Scholar] [CrossRef]
- Kalil, A.C.; Johnson, D.W.; Lisco, S.J.; Sun, J. Early Goal-Directed Therapy for Sepsis: A Novel Solution for Discordant Survival Outcomes in Clinical Trials. Crit. Care Med. 2017, 45, 607–614. [Google Scholar] [CrossRef]
- Seymour, C.W.; Gesten, F.; Prescott, H.C.; Friedrich, M.E.; Iwashyna, T.J.; Phillips, G.S.; Lemeshow, S.; Osborn, T.; Terry, K.M.; Levy, M.M. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N. Engl. J. Med. 2017, 376, 2235–2244. [Google Scholar] [CrossRef]
- Gross, P.A. Hypotension and mortality in septic shock: The “golden hour”. Crit. Care Med. 2006, 34, 1819–1820. [Google Scholar] [CrossRef]
- Liang, Q.; Chiu, J.; Chen, Y.; Huang, Y.; Higashimori, A.; Fang, J.; Brim, H.; Ashktorab, H.; Ng, S.C.; Ng, S.S.M.; et al. Fecal Bacteria Act as Novel Biomarkers for Noninvasive Diagnosis of Colorectal Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 2061–2070. [Google Scholar] [CrossRef]
- Darbà, J.; Marsà, A. Epidemiology, management and costs of sepsis in Spain (2008–2017): A retrospective multicentre study. Curr. Med. Res. Opin. 2020, 36, 1089–1095. [Google Scholar] [CrossRef]
- Arefian, H.; Hagel, S.; Heublein, S.; Rissner, F.; Scherag, A.; Brunkhorst, F.M.; Baldessarini, R.J.; Hartmann, M. Extra length of stay and costs because of health care-associated infections at a German university hospital. Am. J. Infect. Control 2016, 44, 160–166. [Google Scholar] [CrossRef]
- Brun-Buisson, C.; Roudot-Thoraval, F.; Girou, E.; Grenier-Sennelier, C.; Durand-Zaleski, I. The costs of septic syndromes in the intensive care unit and influence of hospital-acquired sepsis. Intensive Care Med. 2003, 29, 1464–1471. [Google Scholar] [CrossRef]
- Paoli, C.J.; Reynolds, M.A.; Coles, C.; Gitlin, M.; Crouser, E. Predicted Economic Benefits of a Novel Biomarker for Earlier Sepsis Identification and Treatment: A Counterfactual Analysis. Crit. Care Explor. 2019, 1, e0029. [Google Scholar] [CrossRef]
- Shen, F.; Sun, B.; Kreutz, J.E.; Davydova, E.K.; Du, W.; Reddy, P.L.; Joseph, L.J.; Ismagilov, R.F. Multiplexed quantification of nucleic acids with large dynamic range using multivolume digital RT-PCR on a rotational SlipChip tested with HIV and hepatitis C viral load. J. Am. Chem. Soc. 2011, 133, 17705–17712. [Google Scholar] [CrossRef]
- Lehmann, L.E.; Herpichboehm, B.; Kost, G.J.; Kollef, M.H.; Stüber, F. Cost and mortality prediction using polymerase chain reaction pathogen detection in sepsis: Evidence from three observational trials. Crit. Care 2010, 14, R186. [Google Scholar] [CrossRef]
- Whale, A.S.; Devonshire, A.S.; Karlin-Neumann, G.; Regan, J.; Javier, L.; Cowen, S.; Fernandez-Gonzalez, A.; Jones, G.M.; Redshaw, N.; Beck, J.; et al. International Interlaboratory Digital PCR Study Demonstrating High Reproducibility for the Measurement of a Rare Sequence Variant. Anal. Chem. 2017, 89, 1724–1733. [Google Scholar] [CrossRef]
- Schlenker, F.; Kipf, E.; Borst, N.; Hutzenlaub, T.; Zengerle, R.; von Stetten, F.; Juelg, P. Virtual Fluorescence Color Channels by Selective Photobleaching in Digital PCR Applied to the Quantification of KRAS Point Mutations. Anal. Chem. 2021, 93, 10538–10545. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhang, H.; Wang, X.; Gaňová, M.; Lednický, T.; Zhu, H.; Liu, X.; Korabečná, M.; Chang, H.; Neužil, P. An image-to-answer algorithm for fully automated digital PCR image processing. Lab Chip 2022, 22, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Choi, J.W.; Kang, T.; Chung, B.G. Deep Learning-Assisted Droplet Digital PCR for Quantitative Detection of Human Coronavirus. Biochip J. 2023, 17, 112–119. [Google Scholar] [CrossRef]
- Oon, Y.-L.; Oon, Y.-S.; Ayaz, M.; Deng, M.; Li, L.; Song, K. Waterborne pathogens detection technologies: Advances, challenges, and future perspectives. Front. Microbiol. 2023, 14, 1286923. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.A.; Joshi, K.; Bhand, S.; Roy, U. Recent developments in detection and enumeration of waterborne bacteria: A retrospective minireview. MicrobiologyOpen 2016, 5, 901–922. [Google Scholar] [CrossRef]
| Digital PCR (dPCR) | Digital Droplet PCR (ddPCR) | ||
|---|---|---|---|
| PROS | CONS | PROS | CONS |
| dPCR encompasses various partitioning methods, including microfluidic-based, chip-based, and emulsion-based techniques, providing flexibility in experimental design. | Unlike ddPCR, dPCR techniques may have less precise control over droplet size and uniformity, potentially affecting assay performance. | ddPCR offers exceptional precision and accuracy in quantifying nucleic acids, particularly for low-abundance targets, due to the ability to analyze individual droplets. | ddPCR may have higher consumable costs compared to some dPCR platforms, especially for emulsion-based systems, which require specialized reagents. |
| Some dPCR platforms may have lower consumable costs compared to ddPCR, especially for microfluidic-based systems. | Certain dPCR platforms may have higher upfront costs compared to ddPCR systems, making them less accessible for some laboratories. | ddPCR can detect and quantify rare target molecules with high sensitivity, making it ideal for applications requiring the detection of low-level genetic mutations or rare transcripts. | Some ddPCR platforms have lower throughput compared to certain dPCR systems, potentially limiting scalability for high-throughput applications. |
| Certain dPCR platforms offer higher throughput options, enabling the analysis of a larger number of samples simultaneously. | While there are multiple dPCR technologies, the availability of commercial platforms may be more limited compared to ddPCR systems, leading to fewer options for researchers. | ddPCR typically requires smaller sample volumes and reagent amounts compared to traditional PCR or dPCR, potentially reducing experimental costs. | Manual handling of samples and droplet generation can introduce the risk of cross-contamination between samples, requiring careful experimental design and handling procedures. |
| In some dPCR systems, such as microfluidic-based platforms, sample handling is automated, minimizing the risk of contamination between samples. | Depending on the platform, dPCR may have lower sensitivity compared to ddPCR, particularly in applications requiring the detection of rare target molecules. | The compartmentalization of reactions in individual droplets can help mitigate the effects of PCR inhibitors present in complex samples, enhancing assay robustness. | ddPCR platforms may have limited options for integration with other analytical techniques, potentially restricting workflow customization for specific experimental needs. |
| dPCR platforms may be more amenable to integration with other analytical techniques, such as microfluidic-based sample preparation or downstream analysis. | |||
| Technology | Bio-Rad QX200™ | QIAcuity | LightCycler® |
|---|---|---|---|
| Partitions | 20,000 | 8500–26,000 | 20,000–28,000–100,000 |
| Samples | Up to 96 for runtime | Up to 96 for runtime | Up to 96 for runtime |
| Concentration on MMx | 2×, 4× | 4× | 5× |
| Channels | 4, 6 | 2, 5 | 6 |
| Working volume | 20 µL | 12 µL, 40 µL | 15 µL, 30 µL, 45 µL |
| Medical devices | Yes | No | Yes |
| Price range | €€ | € | €€€ |
| Feature | QIAcuity One | QIAcuity Four | QIAcuity Eight |
|---|---|---|---|
| Plates processed | 1 | 4 | 8 |
| Detection channels (multiplexing) | 2 or 5 | 5 | 5 |
| Thermocyclers | 1 | 1 | 2 |
| Run time | 2 h | First plate approximately 2 h, every 80 min a following plate | First plate approximately 2 h, every 40 min a following plate |
| Throughput | Up to 384 (96-well) Up to 96 (24-well) | Up to 672 (96-well) Up to 168 (24-well) | Up to 1248 (96-well) Up to 312 (24-well) |
| Feature | High-Resolution Plate | Universal Plate | High-Sensitivity Plate |
|---|---|---|---|
| Working volume | 15 µL | 30 µL | 45 µL |
| Partitions | 100,000 | 28,000 | 20,000 |
| Sensitivity grade | Copy number variation | Gene expression | Cell-free DNA |
| Some applications | NIPT, human genetic disease | Transplant rejection | Oncology, rare mutation detection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirabile, A.; Sangiorgio, G.; Bonacci, P.G.; Bivona, D.; Nicitra, E.; Bonomo, C.; Bongiorno, D.; Stefani, S.; Musso, N. Advancing Pathogen Identification: The Role of Digital PCR in Enhancing Diagnostic Power in Different Settings. Diagnostics 2024, 14, 1598. https://doi.org/10.3390/diagnostics14151598
Mirabile A, Sangiorgio G, Bonacci PG, Bivona D, Nicitra E, Bonomo C, Bongiorno D, Stefani S, Musso N. Advancing Pathogen Identification: The Role of Digital PCR in Enhancing Diagnostic Power in Different Settings. Diagnostics. 2024; 14(15):1598. https://doi.org/10.3390/diagnostics14151598
Chicago/Turabian StyleMirabile, Alessia, Giuseppe Sangiorgio, Paolo Giuseppe Bonacci, Dalida Bivona, Emanuele Nicitra, Carmelo Bonomo, Dafne Bongiorno, Stefania Stefani, and Nicolò Musso. 2024. "Advancing Pathogen Identification: The Role of Digital PCR in Enhancing Diagnostic Power in Different Settings" Diagnostics 14, no. 15: 1598. https://doi.org/10.3390/diagnostics14151598






