Systematic Review on the Use of 3D-Printed Models for Planning, Training and Simulation in Vascular Surgery
Abstract
:1. Introduction
2. Materials and Methods
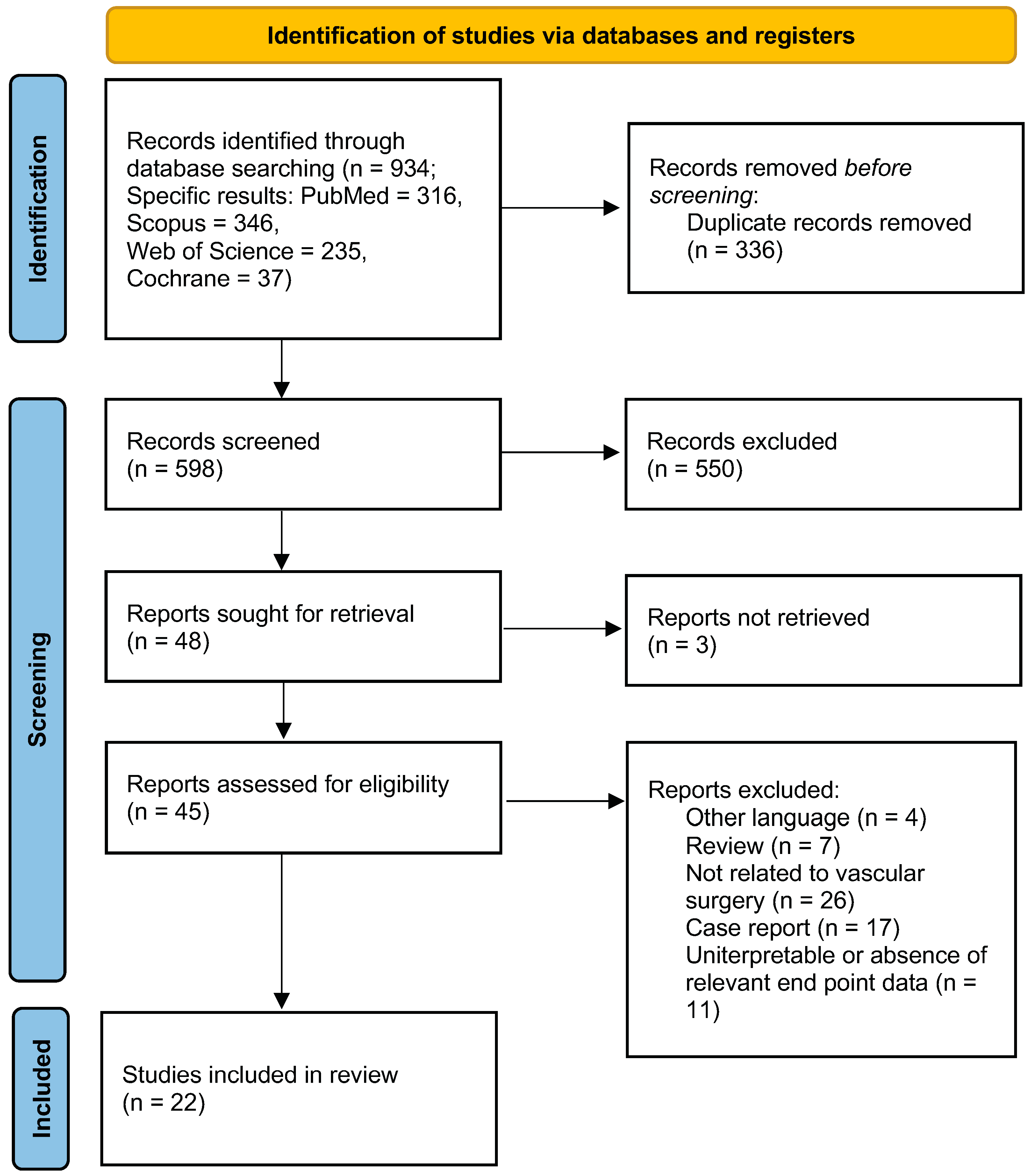
2.1. Data Sources, Search Strategy and Selection Criteria
2.2. Data Extraction, Outcome Measures, and Evaluation of Study Quality
- Diagnostic imaging technique;
- Image processing and post-processing software;
- 3D-printing technologies and materials;
- Feasibility of 3D-printing technology application in vascular surgery;
- 3D-printed models in vascular training;
- 3D-printed models in vascular planning.
2.3. Definitions
3. Results
3.1. Review Design and Baseline Characteristics
3.2. Diagnostic Imaging
3.3. Software for 3D Model Generation
3.4. 3D-Printing Technology in Vascular Surgery
3.5. Feasibility of 3D-Printing Technology for Vascular Models
3.6. 3D-Printed Models in Vascular Surgery Training
3.7. 3D-Printed Models in Vascular Surgery Planning
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wanhainen, A.; Van Herzeele, I.; Bastos Goncalves, F.; Bellmunt Montoya, S.; Berard, X.; Boyle, J.R.; D’Oria, M.; Prendes, C.F.; Karkos, C.D.; Kazimierczak, A.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 192–331. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, C.; Willaert, W.; Kawa, E.; Centola, M.; Steger, B.; Dirnhofer, R.; Mangin, P.; Grabherr, S. Postmortem Circulation: A New Model for Testing Endovascular Devices and Training Clinicians in Their Use. Clin. Anat. 2014, 27, 556–562. [Google Scholar] [CrossRef]
- Nayahangan, L.J.; Konge, L.; Schroeder, T.V.; Paltved, C.; Lindorff-Larsen, K.G.; Nielsen, B.U.; Eiberg, J.P. A National Needs Assessment to Identify Technical Procedures in Vascular Surgery for Simulation Based Training. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Goudie, C.; Kinnin, J.; Bartellas, M.; Gullipalli, R.; Dubrowski, A. The Use of 3D Printed Vasculature for Simulation-Based Medical Education within Interventional Radiology. Cureus 2019, 11, e4381. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. bmj 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q.N.; Fàbregues, S.; Bartlett, G.; Boardman, F.; Cargo, M.; Dagenais, P.; Gagnon, M.-P.; Griffiths, F.; Nicolau, B.; O’Cathain, A.; et al. The Mixed Methods Appraisal Tool (MMAT) Version 2018 for Information Professionals and Researchers. Educ. Inf. 2018, 34, 285–291. [Google Scholar] [CrossRef]
- Kaschwich, M.; Horn, M.; Matthiensen, S.; Stahlberg, E.; Behrendt, C.-A.; Matysiak, F.; Bouchagiar, J.; Dell, A.; Ellebrecht, D.; Bayer, A.; et al. Accuracy Evaluation of Patient-Specific 3D-Printed Aortic Anatomy. Ann. Anat. Anat. Anz. Off. Organ. Anat. Ges. 2021, 234, 151629. [Google Scholar] [CrossRef]
- O’Reilly, M.K.; Reese, S.; Herlihy, T.; Geoghegan, T.; Cantwell, C.P.; Feeney, R.N.M.; Jones, J.F.X. Fabrication and Assessment of 3D Printed Anatomical Models of the Lower Limb for Anatomical Teaching and Femoral Vessel Access Training in Medicine. Anat. Sci. Educ. 2016, 9, 71–79. [Google Scholar] [CrossRef]
- Shibata, E.; Takao, H.; Amemiya, S.; Ohtomo, K. 3D-Printed Visceral Aneurysm Models Based on Ct Data for Simulations of Endovascular Embolization: Evaluation of Size and Shape Accuracy. Am. J. Roentgenol. 2017, 209, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.; Stanislaus, I.; McGahon, C.; Pattabathula, K.; Bryant, S.; Pinto, N.; Jenkins, J.; Meinert, C. Quality Assurance in 3D-Printing: A Dimensional Accuracy Study of Patient-Specific 3D-Printed Vascular Anatomical Models. Front. Med. Technol. 2023, 5, 1097850. [Google Scholar] [CrossRef]
- Kärkkäinen, J.M.; Sandri, G.; Tenorio, E.R.; Alexander, A.; Bjellum, K.; Matsumoto, J.; Morris, J.; Mendes, B.C.; DeMartino, R.R.; Oderich, G.S. Simulation of Endovascular Aortic Repair Using 3D Printed Abdominal Aortic Aneurysm Model and Fluid Pump. Cardiovasc. Interv. Radiol. 2019, 42, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Matyjas, M.; Sauerbrey, M.; Wyschkon, S.; de Bucourt, M.; Scheel, M. Three-Dimensional Simulator: Training for Beginners in Endovascular Embolization with Liquid Agents. CVIR Endovasc. 2021, 4, 78. [Google Scholar] [CrossRef] [PubMed]
- Marconi, S.; Negrello, E.; Mauri, V.; Pugliese, L.; Peri, A.; Argenti, F.; Auricchio, F.; Pietrabissa, A. Toward the Improvement of 3D-Printed Vessels’ Anatomical Models for Robotic Surgery Training. Int. J. Artif. Organs 2019, 42, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Bortman, J.; Mahmood, F.; Schermerhorn, M.; Lo, R.; Swerdlow, N.; Mahmood, F.; Matyal, R. Use of 3-Dimensional Printing to Create Patient-Specific Abdominal Aortic Aneurysm Models for Preoperative Planning. J. Cardiothorac. Vasc. Anesth. 2019, 33, 1442–1446. [Google Scholar] [CrossRef] [PubMed]
- Tam, M.D.; Latham, T.R.; Lewis, M.; Khanna, K.; Zaman, A.; Parker, M.; Grunwald, I.Q. A Pilot Study Assessing the Impact of 3-D Printed Models of Aortic Aneurysms on Management Decisions in EVAR Planning. Vasc. Endovasc. Surg. 2016, 50, 4–9. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, R.P.; Chand, A.; Vidiyala, S.; Arechavala, S.M.; Mitsouras, D.; Rudin, S.; Ionita, C.N. Advanced 3D Mesh Manipulation in Stereolithographic Files and Post-Print Processing for the Manufacturing of Patient-Specific Vascular Flow Phantoms. In Proceedings of the Progress in Biomedical Optics and Imaging Proceedings of SPIE; Cook, T.S., Zhang, J., Eds.; SPIE: Bellingham, WA, USA, 2016; Volume 9789. [Google Scholar]
- Magagna, P.; Xodo, A.; Menegolo, M.; Campana, C.; Ghiotto, L.; Salvador, L.; Grego, F. Applications of Three-Dimensional Printing in the Management of Complex Aortic Diseases. AORTA Stamford Conn. 2022, 10, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Little, C.D.; Mackle, E.C.; Maneas, E.; Chong, D.; Nikitichev, D.; Constantinou, J.; Tsui, J.; Hamilton, G.; Rakhit, R.D.; Mastracci, T.M.; et al. A Patient-Specific Multi-Modality Abdominal Aortic Aneurysm Imaging Phantom. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Kaschwich, M.; Dell, A.; Matysiak, F.; Bouchagiar, J.; Bayer, A.; Scharfschwerdt, M.; Ernst, F.; Kleemann, M.; Horn, M. Development of an Ultrasound-Capable Phantom with Patient-Specific 3D-Printed Vascular Anatomy to Simulate Peripheral Endovascular Interventions. Ann. Anat. Anat. Anz. Off. Organ. Anat. Ges. 2020, 232, 151563. [Google Scholar] [CrossRef]
- Coles-Black, J.; Bolton, D.; Chuen, J. Accessing 3D Printed Vascular Phantoms for Procedural Simulation. Front. Surg. 2020, 7, 626212. [Google Scholar] [CrossRef]
- Foresti, R.; Fornasari, A.; Bianchini Massoni, C.; Mersanne, A.; Martini, C.; Cabrini, E.; Freyrie, A.; Perini, P. Surgical Medical Education via 3D Bioprinting: Modular System for Endovascular Training. Bioengineering 2024, 11, 197. [Google Scholar] [CrossRef]
- Marone, E.M.; Auricchio, F.; Marconi, S.; Conti, M.; Rinaldi, L.F.; Pietrabissa, A.; Argenteri, A. Effectiveness of 3D Printed Models in the Treatment of Complex Aortic Diseases. J. Cardiovasc. Surg. 2018, 59, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Taher, F.; Falkensammer, J.; McCarte, J.; Strassegger, J.; Uhlmann, M.; Schuch, P.; Assadian, A. The Influence of Prototype Testing in Three-Dimensional Aortic Models on Fenestrated Endograft Design. J. Vasc. Surg. 2017, 65, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Borracci, R.A.; Ferreira, L.M.; Alvarez Gallesio, J.M.; Tenorio Núñez, O.M.; David, M.; Eyheremendy, E.P. Three-Dimensional Virtual and Printed Models for Planning Adult Cardiovascular Surgery. Acta Cardiol. 2021, 76, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Torres, I.; De Luccia, N. A Simulator for Training in Endovascular Aneurysm Repair: The Use of Three Dimensional Printers. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, M.E.; Bordet, M.; Chavent, B.; Reijnen, M.M.P.J.; Frisch, N.; Midy, D.; Feugier, P.; Millon, A.; Lardenoije, J.-W.; Assadian, A.; et al. Assessment of Fenestrated Anaconda Stent Graft Design by Numerical Simulation: Results of a European Prospective Multicenter Study. J. Vasc. Surg. 2022, 75, 99–108.e2. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, R.; Zech, C.J.; Deutschmann, M.; Scharinger, B.; Hecht, S.; Hergan, K.; Rezar, R.; Hitzl, W.; Meissnitzer, M. Endovascular Embolization Techniques in Acute Thoracic and Abdominal Bleedings Can Be Technically Reproduced and Trained in a Standardized Simulation Setting Using SLA 3D Printing: A 1-Year Single-Center Study. Insights Imaging 2022, 13, 72. [Google Scholar] [CrossRef]
- Göçer, H.; Durukan, A.B.; Tunç, O.; Naser, E.; Gürbüz, H.A.; Ertuğrul, E. Evaluation of 3D Printing in Planning, Practicing, and Training for Endovascular Lower Extremity Arterial Interventions. Turk. Gogus Kalp Damar Cerrahisi Derg. 2021, 29, 20–26. [Google Scholar] [CrossRef]
- Kaschwich, M.; Sieren, M.; Matysiak, F.; Bouchagiar, J.; Dell, A.; Bayer, A.; Ernst, F.; Ellebrecht, D.; Kleemann, M.; Horn, M. Feasibility of an Endovascular Training and Research Environment with Exchangeable Patient Specific 3D Printed Vascular Anatomy: Simulator with Exchangeable Patient-Specific 3D-Printed Vascular Anatomy for Endovascular Training and Research. Ann. Anat. Anat. Anz. Off. Organ. Anat. Ges. 2020, 231, 151519. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.M.; Sonka, M.; et al. 3D Slicer as an Image Computing; Platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- ITK-SNAP Home. Available online: http://www.itksnap.org/pmwiki/pmwiki.php (accessed on 1 March 2024).
- Torres, I.; De Luccia, N. Artificial Vascular Models for Endovascular Training (3D Printing). Innov. Surg. Sci. 2018, 3, 225–234. [Google Scholar] [CrossRef]
- Marti, P.; Lampus, F.; Benevento, D.; Setacci, C. Trends in Use of 3D Printing in Vascular Surgery: A Survey. Int. Angiol. J. Int. Union Angiol. 2019, 38, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Stana, J.; Grab, M.; Kargl, R.; Tsilimparis, N. 3D Printing in the Planning and Teaching of Endovascular Procedures. Radiol. Heidelb. Ger. 2022, 62, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Lawaetz, J.; Skovbo Kristensen, J.S.; Nayahangan, L.J.; Van Herzeele, I.; Konge, L.; Eiberg, J.P. Simulation Based Training and Assessment in Open Vascular Surgery: A Systematic Review. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Maguire, S.C.; Traynor, O.; Strawbridge, J.; O’Callaghan, A.; Kavanagh, D.O. A Systematic Review of Simulation in Open Abdominal Aortic Aneurysm Repair. J. Vasc. Surg. 2020, 71, 1802–1808.e1. [Google Scholar] [CrossRef]
- Foresti, R.; Rossi, S.; Pinelli, S.; Alinovi, R.; Sciancalepore, C.; Delmonte, N.; Selleri, S.; Caffarra, C.; Raposio, E.; Macaluso, G.; et al. In-Vivo Vascular Application via Ultra-Fast Bioprinting for Future 5D Personalised Nanomedicine. Sci. Rep. 2020, 10, 3205. [Google Scholar] [CrossRef]


| Author | Year | Carotid Arteries | Thoraco-Abdominal | Infrainguinal Arteries | Visceral Arteries | Aneurysm Disease | Steno-Occlusive Disease |
|---|---|---|---|---|---|---|---|
| Foresti [21] | 2024 | X | X | ||||
| Nguyen [10] | 2023 | X | X | ||||
| Kaufmann [27] | 2022 | X | X | ||||
| Magagna [17] | 2022 | X | X | X | |||
| Little [18] | 2022 | X | X | ||||
| Göçer [28] | 2021 | X | X | ||||
| Matyjas [12] | 2021 | X | |||||
| Kliewer [26] | 2021 | X | X | X | |||
| Kaschwich [7] | 2021 | X | X | ||||
| Coles-Black [20] | 2021 | X | X | ||||
| Kaschwich [19] | 2020 | X | X | ||||
| Borracci [24] | 2020 | X | X | X | X | ||
| Kärkkäinen [11] | 2019 | X | X | ||||
| Marconi S. [13] | 2019 | X | X | ||||
| Bortman [14] | 2019 | X | X | ||||
| Marone [22] | 2018 | X | X | X | |||
| Shibata [9] | 2017 | X | X | ||||
| Taher [23] | 2017 | X | X | X | |||
| Torres [25] | 2017 | X | X | ||||
| Tam [15] | 2016 | X | X | ||||
| O’Hara [16] | 2016 | X | X | ||||
| O’Reilly [8] | 2015 | X | X | X | X |
| Software | Supplier | Category | Model Design | Image Processing/3D Reconstruction | .STL File Generation | .STL File Post-Processing | Author |
|---|---|---|---|---|---|---|---|
| Autodesk fusion 360 | Autodesk, Inc. (San Francisco, CA, USA) | CAD/CAM | x | x | x | Matyjas [12] | |
| SolidWorks® v. 2015 | Solidsolution (Vélizy-Villacoublay, France) | CAD | x | x | Foresti [21] | ||
| Mimics | Materialise NV (Leuven, Belgium) | MI | x | x | Nguyen [10]; Kärkkäinen [11]; Bortman [14]; Taher [23] | ||
| OsiriX | Pixmeo (Geneva, Switzerland) | MI | x | x | Shibata [9]; Tam [15] | ||
| 3D Slicer | Open-source (www.slicer.org) | MI | x | x | Little [18]; Coles-Black [20] | ||
| ITK-Snap | Open-source (http://www.itksnap.org/) | MI | x | Marconi [13]; Marone [22] | |||
| ImageJ | Open-source (https://imagej.nih.gov/ij/index.html accessed on 1 March 204) | MI | x | x | Kaufmann [27] | ||
| InVesalius | Open-source (https://www.cti.gov.br/invesalius/ accessed on 1 March 2024) | MI | x | x | Magagna [17] | ||
| Mimics Innovation Suite | Materialise NV (Leuven, Belgium) | MI | x | x | Göçer [28] | ||
| Vascular Modelling Toolkit | Open-source (http://www.vmtk.org/) | MI | x | x | x | Marconi [13] | |
| TeraRecon iNtuition Unlimited | TeraRecon (Durham, NC, USA) | MI | x | x | Torres [32] | ||
| Vitrea 3D Station | Vital Images, Inc. (Minnetonka, MN, USA) | MI | x | x | O’Hara [16] | ||
| Syngo.via * | Siemens Healthineers (Herlangen, Germany) | MI | x | x | Kaschwich [19,29] | ||
| Blender | Open-source (www.blender.org) | ME | x | x | x | Kaufmann [27] | |
| Meshmixer | Open-source (San Francisco, CA, USA) | ME | x | x | Little [18]; Magagna [17]; Matyas [12]; Coles-Black [20]; Kaschwich [19,29]; Borracci [24]; Torres [25]; O’Hara [16] | ||
| 3-matic | Materialise NV (Leuven, Belgium) | ME | x | Nguyen [10]; Kärkkäinen [11]; Bortman [14] | |||
| Meshlab | Open-source (www.meshlab.net) | ME | x | Marconi [13] | |||
| Magics | Materialise NV (Leuven, Belgium) | AM | x | Torres [25] | |||
| Netfabb | Autodesk, Inc. (San Francisco, CA, USA) | AM | x | Marone [22] | |||
| Slic3r | Open-source (https://slic3r.org/) | AM | x | x | Foresti [21] | ||
| MATLAB * | MathWorks, Inc. (Natick, MA, USA) | MC | Marconi [13], Shibata [9] |
| Printer | Suppliers |
|---|---|
| CubePro | 3D Systems Corporation (Rock Hill, SC, USA) |
| ProJet 3500 | 3D Systems Corporation (Rock Hill, SC, USA) |
| Projet460 Plus | 3D Systems Corporation (Rock Hill, SC, USA) |
| ZPrinterVR 250 | 3D Systems Corporation (Rock Hill, SC, USA) |
| sPro 60 | 3D Systems Corporation (Rock Hill, SC, USA) |
| Felix 3 | FELIXprinters (Ijsselstein, The Netherlands) |
| Form 1+ | Formlabs (Somerville, MA, USA) |
| Form 2 | Formlabs (Somerville, MA, USA) |
| Form 3 | Formlabs (Somerville, MA, USA) |
| Ultimaker S3 | Ultimaker B.V. (Utrecht, The Netherlands) |
| Ultimaker S5 | Ultimaker B.V. (Utrecht, The Netherlands) |
| MakerBot Replicator 2X | Stratasys (Eden Prairie, MN, USA)/MakerBot (New York City, NY, USA) |
| Objet260 Connex3 | Stratasys (Eden Prairie, MN, USA) |
| Objet30 Prime | Stratasys (Eden Prairie, MN, USA) |
| Stratasys J750 | Stratasys (Eden Prairie, MN, USA) |
| J750 Digital Anatomy | Stratasys (Eden Prairie, MN, USA) |
| Objet350 Connex | Stratasys (Eden Prairie, MN, USA) |
| Objet500 Connex3 | Stratasys (Eden Prairie, MN, USA) |
| Objet Eden 260V | Stratasys (Eden Prairie, MN, USA) |
| FlashForge Creator Pro | Flashforge (Zhejiang, China) |
| Prusa i3 MK3S+ | Prusa Research (Prague, Czech Republic) |
| ZPrinter 450 | Z Corporation (3D Systems Corporation, Rock Hill, SC, USA) |
| Orcabot 3D printer | Mendel-Parts (Prodim International, Helmond, The Netherlands) |
| Author | Year | Printer | 3D-Printing Technology | Model Material | Model Hardness | Model Appearance | Printing Time (h) | Cost (EUR) | Accuracy * |
|---|---|---|---|---|---|---|---|---|---|
| Foresti [21] | 2024 | Form 2 | SLA | M-based resin | rigid | transparent | 21 | 200 | high |
| Nguyen [10] | 2023 | Ultimaker S5 | FDM | PLA | rigid | opaque | n.a. | n.a. | high |
| sPro 60 | SLS | nylon | rigid | opaque | n.a. | n.a. | high | ||
| J750 Digital Anatomy | PolyJet | PUR-based resin | (1) rigid; (2) flexible | opaque | n.a. | n.a. | high | ||
| Form 3 | SLA | M-based resin | rigid | opaque | n.a. | n.a. | high | ||
| Kaufmann [27] | 2022 | Form 3 | SLA | M-based resin | flexible | transparent | n.a. | low | high |
| Magagna [17] | 2022 | n.a. | n.a. | silicone | rigid | opaque | 24–72 | 1000–1500 | high |
| Little [18] | 2022 | Ultimaker S3 | FDM | PVA | rigid | opaque | n.a. | 100 | high |
| Göçer [28] | 2021 | Form 2 | SLA | M-based resin | rigid | transparent | 6 | 400 | high |
| Matyjas [12] | 2021 | Form 2 | SLA | M-based resin | rigid | transparent | 8 | low | high |
| Kliewer [26] | 2021 | External provider ** | n.a. | n.a. | rigid | transparent | n.a. | n.a. | high |
| Kaschwich [7] | 2021 | Objet500 Connex3 | PolyJet | PUR-based resin | flexible + rigid | opaque | n.a. | n.a. | high |
| Coles-Black [20] | 2021 | Objet500 Connex3Stratasys J750ProJet 3500 | PolyJet | PUR-based resin | flexible | transparent | n.a. | 650–930 | high |
| Form 2 | SLA | M-based resin | rigid or flexible | opaque or transparent | n.a. | 50–100 | high | ||
| FlashForge Creator Pro Prusa i3 MK3S + Ultimaker S5MakerBot Replicator 2X | FDM | ABS | rigid | opaque | 24–48 | 10–20 | high | ||
| Kaschwich [29] | 2020 | Felix3 | FDM | silicone | rigid | opaque | n.a. | low | n.a. |
| Borracci [24] | 2020 | External provider § | FDM | n.a. | rigid or flexible | opaque or transparent | n.a. | 90–460 | high |
| Kärkkäinen [11] | 2019 | Objet500 Connex3 | PolyJet | PUR-based resin | flexible + rigid | opaque | 24–36 | 280–370 | high |
| Marconi [13] | 2019 | Objet260 Connex3 | PolyJet | PUR-based resin | flexible + rigid | opaque | 10 | n.a. | high |
| Bortman [14] | 2019 | Objet30 Prime | PolyJet | PUR-based resin | rigid | opaque | 3 | 30 | high |
| Marone [22] | 2018 | Projet460 Plus | ColorJet | silicone | rigid | opaque | 8 | 100–150 | high |
| Shibata [9] | 2017 | CubePro | FDM | nylon | rigid | n.a. | n.a. | low | high |
| Taher [23] | 2017 | External provider * | SLA | M-based resin | rigid | transparent | n.a. | n.a. | high |
| Torres [25] | 2017 | Form 1+ | SLA | M-based resin | flexible | transparent | n.a. | 150 | high |
| MakerBot Replicator 2X | FDM | silicone | rigid | opaque | n.a. | 120 | high | ||
| Objet350 Connex | PolyJet | PUR-based resin | (1) flexible; (2) rigid; (3) flexible + rigid | (1) opaque; (2) transparent; (3) opaque | n.a. | 475 | high | ||
| Tam [15] | 2016 | ZPrinter 450 | ColorJet | plaster | rigid | opaque | 24 | 185 | good |
| Orcabot 3D printer | FDM | PLA | rigid | opaque | 24 | 185 | high | ||
| O’Hara [16] | 2016 | Objet Eden 260V | PolyJet | PUR-based resin | flexible | opaque | 24 | n.a. | high |
| O’Reilly [8] | 2015 | ZPrinterVR 250 | ColorJet | silicone | rigid | opaque | n.a. | low | high |
| Author | Year | Patient-Specific 3D Model | In-House Designed Set-Up | 3D-Printed Model Only | Simulated Technique |
|---|---|---|---|---|---|
| Foresti [21] | 2023 | no | yes | no | PTA |
| Nguyen [10] | 2023 | yes | yes | no | EVAR |
| Kaufmann [27] | 2022 | yes | yes | no | Endovascular embolization |
| Magagna [17] | 2022 | yes | yes | no | EVAR |
| Little [18] | 2022 | yes | no | yes | EVAR |
| Göçer [28] | 2021 | yes | no | yes | PTA |
| Matyjas [12] | 2021 | no | yes | no | Endovascular embolization |
| Kaschwich [19] | 2020 | yes | yes | no | DUS guided peripheral endovascular intervention |
| Kärkkäinen [11] | 2019 | yes | yes | no | EVAR |
| Torres [25] | 2017 | yes | no | yes | EVAR |
| O’Reilly [8] | 2015 | yes | yes | no | Femoral artery access with DUS imaging |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catasta, A.; Martini, C.; Mersanne, A.; Foresti, R.; Bianchini Massoni, C.; Freyrie, A.; Perini, P. Systematic Review on the Use of 3D-Printed Models for Planning, Training and Simulation in Vascular Surgery. Diagnostics 2024, 14, 1658. https://doi.org/10.3390/diagnostics14151658
Catasta A, Martini C, Mersanne A, Foresti R, Bianchini Massoni C, Freyrie A, Perini P. Systematic Review on the Use of 3D-Printed Models for Planning, Training and Simulation in Vascular Surgery. Diagnostics. 2024; 14(15):1658. https://doi.org/10.3390/diagnostics14151658
Chicago/Turabian StyleCatasta, Alexandra, Chiara Martini, Arianna Mersanne, Ruben Foresti, Claudio Bianchini Massoni, Antonio Freyrie, and Paolo Perini. 2024. "Systematic Review on the Use of 3D-Printed Models for Planning, Training and Simulation in Vascular Surgery" Diagnostics 14, no. 15: 1658. https://doi.org/10.3390/diagnostics14151658







