Utilizing Next-Generation Sequencing: Advancements in the Diagnosis of Fungal Infections
Abstract
:1. Introduction
2. Next-Generation Sequencing over Conventional Testing
3. Application of NGS in Diagnoses of Fungal Infection
4. Metagenomics and Metabarcoding
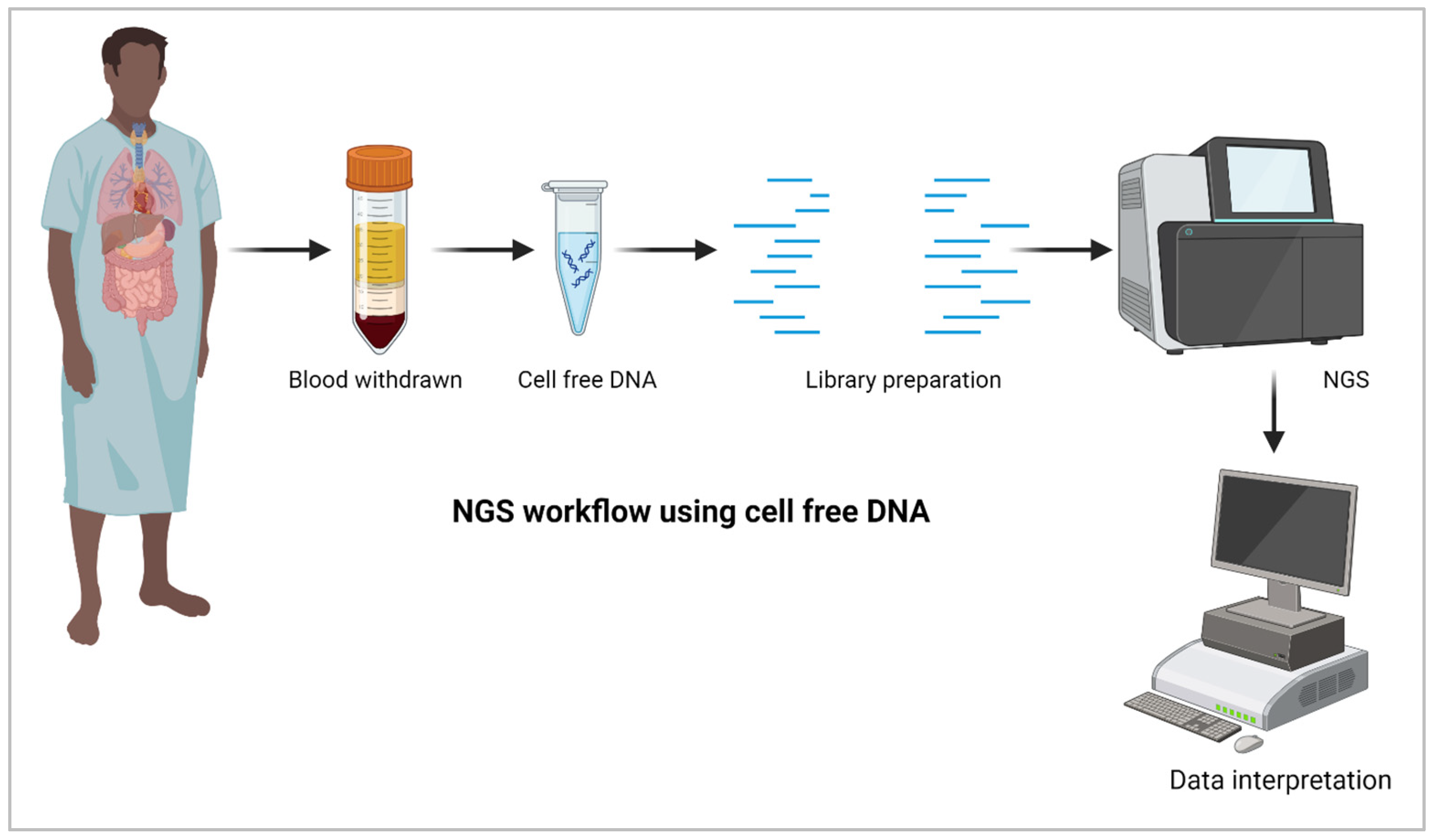
5. NGS for Liquid Biopsy
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fisher, M.C.; Denning, D.W. The WHO fungal priority pathogens list as a game-changer. Nat. Rev. Microbiol. 2023, 21, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Leck, A.K.; Gichangi, M.; Burton, M.J.; Denning, D.W. The global incidence and diagnosis of fungal keratitis. Lancet Infect. Dis. 2021, 21, e49–e57. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.L.; Nosanchuk, J.D. Fungal diseases as neglected pathogens: A wake-up call to public health officials. PLoS Negl. Trop. Dis. 2020, 14, e0007964. [Google Scholar] [CrossRef] [PubMed]
- Hoang, M.T.V.; Irinyi, L.; Hu, Y.; Schwessinger, B.; Meyer, W. Long-Reads-Based Metagenomics in Clinical Diagnosis With a Special Focus on Fungal Infections. Front. Microbiol. 2021, 12, 708550. [Google Scholar] [CrossRef] [PubMed]
- Angeletti, S. Matrix assisted laser desorption time of flight mass spectrometry (MALDI-TOF MS) in clinical microbiology. J. Microbiol. Methods 2017, 138, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Irinyi, L.; Lackner, M.; de Hoog, G.S.; Meyer, W. DNA barcoding of fungi causing infections in humans and animals. Fungal Biol. 2016, 120, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Burillo, A.; Bouza, E. Use of rapid diagnostic techniques in ICU patients with infections. BMC Infect. Dis. 2014, 14, 593. [Google Scholar] [CrossRef]
- Hilt, E.E.; Ferrieri, P. Next Generation and Other Sequencing Technologies in Diagnostic Microbiology and Infectious Diseases. Genes 2022, 13, 1566. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Chen, Y.; Han, S.; Lv, L.; Li, L. Next-Generation Sequencing Applications for the Study of Fungal Pathogens. Microorganisms 2022, 10, 1882. [Google Scholar] [CrossRef] [PubMed]
- Gouba, N.; Drancourt, M. Digestive tract mycobiota: A source of infection. Med. Mal. Infect. 2015, 45, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Consortium, O.; Gabaldon, T. Recent trends in molecular diagnostics of yeast infections: From PCR to NGS. FEMS Microbiol. Rev. 2019, 43, 517–547. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lu, A.D.; Zhang, L.P.; Zuo, Y.X.; Jia, Y.P. Study of clinical outcome and prognosis in pediatric core binding factor-acute myeloid leukemia. Zhonghua Xue Ye Xue Za Zhi 2019, 40, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.C.; Teng, J.L.L.; Lau, S.K.P.; Woo, P.C.Y. Rapid Genomic Diagnosis of Fungal Infections in the Age of Next-Generation Sequencing. J. Fungi 2021, 7, 636. [Google Scholar] [CrossRef] [PubMed]
- Gaston, D.C.; Miller, H.B.; Fissel, J.A.; Jacobs, E.; Gough, E.; Wu, J.; Klein, E.Y.; Carroll, K.C.; Simner, P.J. Evaluation of Metagenomic and Targeted Next-Generation Sequencing Workflows for Detection of Respiratory Pathogens from Bronchoalveolar Lavage Fluid Specimens. J. Clin. Microbiol. 2022, 60, e0052622. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.S.; Henson, A.; Gerrish, A.; Jones, L.; Williams, J.; Kidd, E.J. Decreasing the expression of PICALM reduces endocytosis and the activity of beta-secretase: Implications for Alzheimer’s disease. BMC Neurosci. 2016, 17, 50. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Sun, W.; He, Y. Comparison of the next-generation sequencing (NGS) technology with culture methods in the diagnosis of bacterial and fungal infections. J. Thorac. Dis. 2020, 12, 4924–4929. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Y.; Zhang, M.; Li, M.; Zhang, R.; Lu, X.; Gao, W.; Li, Q.; Xia, Y.; Pan, P.; et al. Etiology of Severe Community-Acquired Pneumonia in Adults Based on Metagenomic Next-Generation Sequencing: A Prospective Multicenter Study. Infect. Dis. Ther. 2020, 9, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Larkin, P.M.K.; Lawson, K.L.; Contreras, D.A.; Le, C.Q.; Trejo, M.; Realegeno, S.; Hilt, E.E.; Chandrasekaran, S.; Garner, O.B.; Fishbein, G.A.; et al. Amplicon-Based Next-Generation Sequencing for Detection of Fungi in Formalin-Fixed, Paraffin-Embedded Tissues: Correlation with Histopathology and Clinical Applications. J. Mol. Diagn. 2020, 22, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.; Acharya, N.R.; Pinsky, B.A.; Sahoo, M.K.; Chow, E.D.; Banaei, N.; Budvytiene, I.; Cevallos, V.; Zhong, L.; Zhou, Z.; et al. Metagenomic DNA Sequencing for the Diagnosis of Intraocular Infections. Ophthalmology 2017, 124, 1247–1248. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wang, Z.; Zhu, C.; Yang, Q.; Lu, Y.; Yu, X.; Hong, B.; Wang, X.; Zhang, Y. Case Report: Proven Diagnosis of Culture-Negative Chronic Disseminated Candidiasis in a Patient Suffering From Hematological Malignancy: Combined Application of mNGS and CFW Staining. Front. Med. 2021, 8, 627166. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.R.; O’Donovan, B.D.; Gelfand, J.M.; Sample, H.A.; Chow, F.C.; Betjemann, J.P.; Shah, M.P.; Richie, M.B.; Gorman, M.P.; Hajj-Ali, R.A.; et al. Chronic Meningitis Investigated via Metagenomic Next-Generation Sequencing. JAMA Neurol. 2018, 75, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.W.; Zhang, J.T.; Ma, Y.B.; Zheng, N.; Yang, F.; Yu, S.Y. Apparent performance of metagenomic next-generation sequencing in the diagnosis of cryptococcal meningitis: A descriptive study. J. Med. Microbiol. 2019, 68, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, C.; Chen, F.; Huang, Z.; Fang, X.; Li, W.; Yang, B.; Zhang, W. Metagenomic Next-Generation Sequencing Technique Helps Identify Cryptococcal Infection in the Rib: A Report of 2 Cases and Review of the Literature. JBJS Case Connect. 2019, 9, e0367. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Li, Z.; Chen, G.; Liu, X.; Ding, L. Metagenomic next-generation sequencing identified Histoplasma capsulatum in the lung and epiglottis of a Chinese patient: A case report. Int. J. Infect. Dis. 2020, 101, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Hu, Q.; Xie, Z.; Li, C. Case Report: Infective Endocarditis Caused by Aspergillus flavus in a Hemodialysis Patient. Front. Med. 2021, 8, 655640. [Google Scholar] [CrossRef]
- He, B.C.; Liu, L.L.; Chen, B.L.; Zhang, F.; Su, X. The application of next-generation sequencing in diagnosing invasive pulmonary aspergillosis: Three case reports. Am. J. Transl. Res. 2019, 11, 2532–2539. [Google Scholar] [PubMed]
- Alonso, R.; Pisa, D.; Aguado, B.; Carrasco, L. Identification of Fungal Species in Brain Tissue from Alzheimer’s Disease by Next-Generation Sequencing. J. Alzheimers Dis. 2017, 58, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Pisa, D.; Carrasco, L. Brain Microbiota in Huntington’s Disease Patients. Front. Microbiol. 2019, 10, 2622. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Pisa, D.; Fernandez-Fernandez, A.M.; Carrasco, L. Infection of Fungi and Bacteria in Brain Tissue from Elderly Persons and Patients with Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Shivaji, S.; Jayasudha, R.; Sai Prashanthi, G.; Kalyana Chakravarthy, S.; Sharma, S. The Human Ocular Surface Fungal Microbiome. Investig. Ophthalmol. Vis. Sci. 2019, 60, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, D.; Joseph, J.; Chakrabarti, M.; Sharma, S.; Jayasudha, R.; Sama, K.C.; Sontam, B.; Tyagi, M.; Narayanan, R.; Shivaji, S. New insights into culture negative endophthalmitis by unbiased next generation sequencing. Sci. Rep. 2019, 9, 844. [Google Scholar] [CrossRef] [PubMed]
- Imabayashi, Y.; Moriyama, M.; Takeshita, T.; Ieda, S.; Hayashida, J.N.; Tanaka, A.; Maehara, T.; Furukawa, S.; Ohta, M.; Kubota, K.; et al. Molecular analysis of fungal populations in patients with oral candidiasis using next-generation sequencing. Sci. Rep. 2016, 6, 28110. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.; Litlekalsoy, J.; Ahmed, I.A.; Martinsen, E.M.H.; Furriol, J.; Javier-Lopez, R.; Elsheikh, M.; Gaafar, N.M.; Morgado, L.; Mundra, S.; et al. Analysis of Salivary Mycobiome in a Cohort of Oral Squamous Cell Carcinoma Patients from Sudan Identifies Higher Salivary Carriage of Malassezia as an Independent and Favorable Predictor of Overall Survival. Front. Cell Infect. Microbiol. 2021, 11, 673465. [Google Scholar] [CrossRef] [PubMed]
- Francoise, A.; Hery-Arnaud, G. The Microbiome in Cystic Fibrosis Pulmonary Disease. Genes 2020, 11, 536. [Google Scholar] [CrossRef] [PubMed]
- Brunkhorst, F.M.; Oppert, M.; Marx, G.; Bloos, F.; Ludewig, K.; Putensen, C.; Nierhaus, A.; Jaschinski, U.; Meier-Hellmann, A.; Weyland, A.; et al. Effect of empirical treatment with moxifloxacin and meropenem vs meropenem on sepsis-related organ dysfunction in patients with severe sepsis: A randomized trial. JAMA 2012, 307, 2390–2399. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.E.; Rossoff, J.; Hollemon, D.; Hong, D.K.; Muller, W.J.; Chaudhury, S. Cell-free DNA next-generation sequencing successfully detects infectious pathogens in pediatric oncology and hematopoietic stem cell transplant patients at risk for invasive fungal disease. Pediatr. Blood Cancer 2019, 66, e27734. [Google Scholar] [CrossRef] [PubMed]
- Decker, S.O.; Kruger, A.; Wilk, H.; Grumaz, S.; Vainshtein, Y.; Schmitt, F.C.F.; Uhle, F.; Bruckner, T.; Zimmermann, S.; Mehrabi, A.; et al. New approaches for the detection of invasive fungal diseases in patients following liver transplantation-results of an observational clinical pilot study. Langenbecks Arch. Surg. 2019, 404, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.E.; Heuser, R.; Missan, D.S.; Martinez, D.; Heningburg, A.; Shabilla, M.; Schwartz, R.; Fry, S. Evidence for polymicrobial communities in explanted vascular filters and atheroma debris. Mol. Cell Probes 2017, 33, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Mwaigwisya, S.; Assiri, R.A.; O’Grady, J. Emerging commercial molecular tests for the diagnosis of bloodstream infection. Expert. Rev. Mol. Diagn. 2015, 15, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Durand, C.; Maubon, D.; Cornet, M.; Wang, Y.; Aldebert, D.; Garnaud, C. Can We Improve Antifungal Susceptibility Testing? Front. Cell Infect. Microbiol. 2021, 11, 720609. [Google Scholar] [CrossRef] [PubMed]
- Abril, M.K.; Barnett, A.S.; Wegermann, K.; Fountain, E.; Strand, A.; Heyman, B.M.; Blough, B.A.; Swaminathan, A.C.; Sharma-Kuinkel, B.; Ruffin, F.; et al. Diagnosis of Capnocytophaga canimorsus Sepsis by Whole-Genome Next-Generation Sequencing. Open Forum Infect. Dis. 2016, 3, ofw144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Vlaminck, I.; Martin, L.; Kertesz, M.; Patel, K.; Kowarsky, M.; Strehl, C.; Cohen, G.; Luikart, H.; Neff, N.F.; Okamoto, J.; et al. Noninvasive monitoring of infection and rejection after lung transplantation. Proc. Natl. Acad. Sci. USA 2015, 112, 13336–13341. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aubert, O.; Ursule-Dufait, C.; Brousse, R.; Gueguen, J.; Racapé, M.; Raynaud, M.; Van Loon, E.; Pagliazzi, A.; Huang, E.; Jordan, S.C.; et al. Cell-free DNA for the detection of kidney allograft rejection. Nat. Med. 2024. [Google Scholar] [CrossRef]
- Mitchell, S.L.; Simner, P.J. Next-Generation Sequencing in Clinical Microbiology: Are We There Yet? Clin. Lab. Med. 2019, 39, 405–418. [Google Scholar] [CrossRef] [PubMed]

| Decade | Technique | Description | Applications |
|---|---|---|---|
| 1950s | Electrophoresis | Separation of molecules based on charge and size | Analysis of protein and nucleic acid molecules |
| 1960s | Gel electrophoresis | Separation of DNA fragments by size | Analysis of genetic variation |
| 1970s | DNA sequencing | Determination of nucleotide sequence of DNA | Analysis of genetic variation |
| 1975 | Southern blotting | Transfer of DNA fragments from a gel to a membrane for detection | Identification of specific DNA sequences |
| 1980s | PCR | Amplification of specific DNA sequences | Analysis of specific regions of the genome |
| 1983 | DNA fingerprinting | Analysis of genetic variation using variable number tandem repeats (VNTRs) | Identification of individuals and populations |
| 1985 | DNA hybridization | Detection of complementary DNA sequences using labeled probes | Identification of specific DNA sequences |
| 1986 | DNA cloning | Insertion of a DNA fragment into a vector for replication and expression | Production of recombinant proteins and study of gene function |
| 1990s | Fluorescent in situ hybridization (FISH) | Detection of specific DNA sequences in cells using fluorescently labeled probes | Visualization of microbial communities and identification of individual microbial species |
| 1990s | 18S rRNA/ITS sequencing (DNA barcoding) | Amplification and analysis of specific regions of the fungal genome | Identification of individual fungal species |
| 1990s | Random amplified polymorphic DNA (RAPD) | Amplification of random regions of the genome for analysis of genetic variation | Identification of genetic markers and analysis of genetic diversity |
| 1990s | Restriction fragment length polymorphism (RFLP) | Analysis of genetic variation based on restriction enzyme cleavage patterns | Identification of genetic markers and analysis of genetic diversity |
| 1996 | Differential display PCR | Analysis of differences in gene expression between cell populations | Identification of differentially expressed genes |
| 2000s | Microarrays | Analysis of gene expression, SNP genotyping, and comparative genomic hybridization | Study of gene expression, detection of genetic variation, and identification of chromosomal abnormalities |
| 2000s | Metagenomics | Comprehensive analysis of entire fungal communities | Identification of rare and uncultivable fungi |
| 2000s | Metabarcoding | Comprehensive analysis of fungal communities based on barcode regions | Identification of rare and uncultivable fungi |
| Late 2000s | Next-generation sequencing (NGS) | Sequencing of entire genomes, transcriptomes, and epigenomes | Identification of genetic variation within and between fungal populations |
| Late 2000s | RNA sequencing | Analysis of gene expression and identification of novel transcripts | Study of gene regulation and identification of new genes |
| Recent years | Single-cell sequencing | Sequencing of individual microbial cells | Analysis of genomic variation at the single-cell level |
| Recent years | Metatranscriptomics | Analysis of gene expression in fungal communities | Study of fungal function and activity in different environments |
| Recent years | CRISPR (clustered regularly interspaced palindromic repeats)-Cas9 | Targeted genome editing using RNA-guided endonucleases | Study of gene function and development of gene therapy treatments |
| Criteria | Conventional Testing | Next-Generation Sequencing |
|---|---|---|
| Method | Culture-based methods, microscopy, and biochemical tests | High-throughput DNA sequencing |
| Sample types | Swabs, tissue samples, body fluids, food, environmental samples | DNA or RNA extracted from any sample |
| Detection limit | 102–103 CFU/mL | 1–10 CFU/mL |
| Speed of analysis | Hours to two weeks | Six h to 7 days |
| Level of detail | Limited identification to species level | Exact identification, including strain level |
| Sample size | Small to medium | Small to large |
| Cost | Low to moderate | High |
| Data output | Qualitative | Qualitative and quantitative |
| Data analysis | Manual interpretation required | Automated, computer-based analysis |
| Applications |
|
|
| Sensitivity and specificity | Moderate to high, depending on the method and organism | Very high |
| Advantages |
|
|
| Disadvantages |
|
|
| Parameter | Metabarcoding | Metagenomics |
|---|---|---|
| Target | Specific regions of the genome (18S rRNA or ITS regions for fungi) | The entire genetic content of the sample |
| PCR amplification | Yes | No |
| Sequence depth | Millions of reads per sample | Billions of reads per sample |
| Bias and errors | It may introduce biases and errors due to PCR amplification | Fewer biases and errors |
| Data Analysis | Clustering sequences into operational taxonomic units (OTUs) and assigning taxonomy based on a reference database | Assembling reads into contiguous sequences and assigning taxonomy based on sequence homology to known genomes. |
| Applications | Targeted analysis, studying specific taxonomic groups | Comprehensive analysis, identifying rare or low-abundance microorganisms, outbreak tracing, virulence detection, resistance detection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naik, S.; Kashyap, D.; Deep, J.; Darwish, S.; Cross, J.; Mansoor, E.; Garg, V.K.; Honnavar, P. Utilizing Next-Generation Sequencing: Advancements in the Diagnosis of Fungal Infections. Diagnostics 2024, 14, 1664. https://doi.org/10.3390/diagnostics14151664
Naik S, Kashyap D, Deep J, Darwish S, Cross J, Mansoor E, Garg VK, Honnavar P. Utilizing Next-Generation Sequencing: Advancements in the Diagnosis of Fungal Infections. Diagnostics. 2024; 14(15):1664. https://doi.org/10.3390/diagnostics14151664
Chicago/Turabian StyleNaik, Sheetal, Dharambir Kashyap, Jashan Deep, Saif Darwish, Joseph Cross, Edmond Mansoor, Vivek Kumar Garg, and Prasanna Honnavar. 2024. "Utilizing Next-Generation Sequencing: Advancements in the Diagnosis of Fungal Infections" Diagnostics 14, no. 15: 1664. https://doi.org/10.3390/diagnostics14151664







