Computed Tomography Angiography Identified High-Risk Coronary Plaques: From Diagnosis to Prognosis and Future Management
Abstract
:1. Introduction
2. Vulnerable Plaque Pathophysiology
2.1. Atherosclerosis Progression
2.2. Plaque Vulnerability
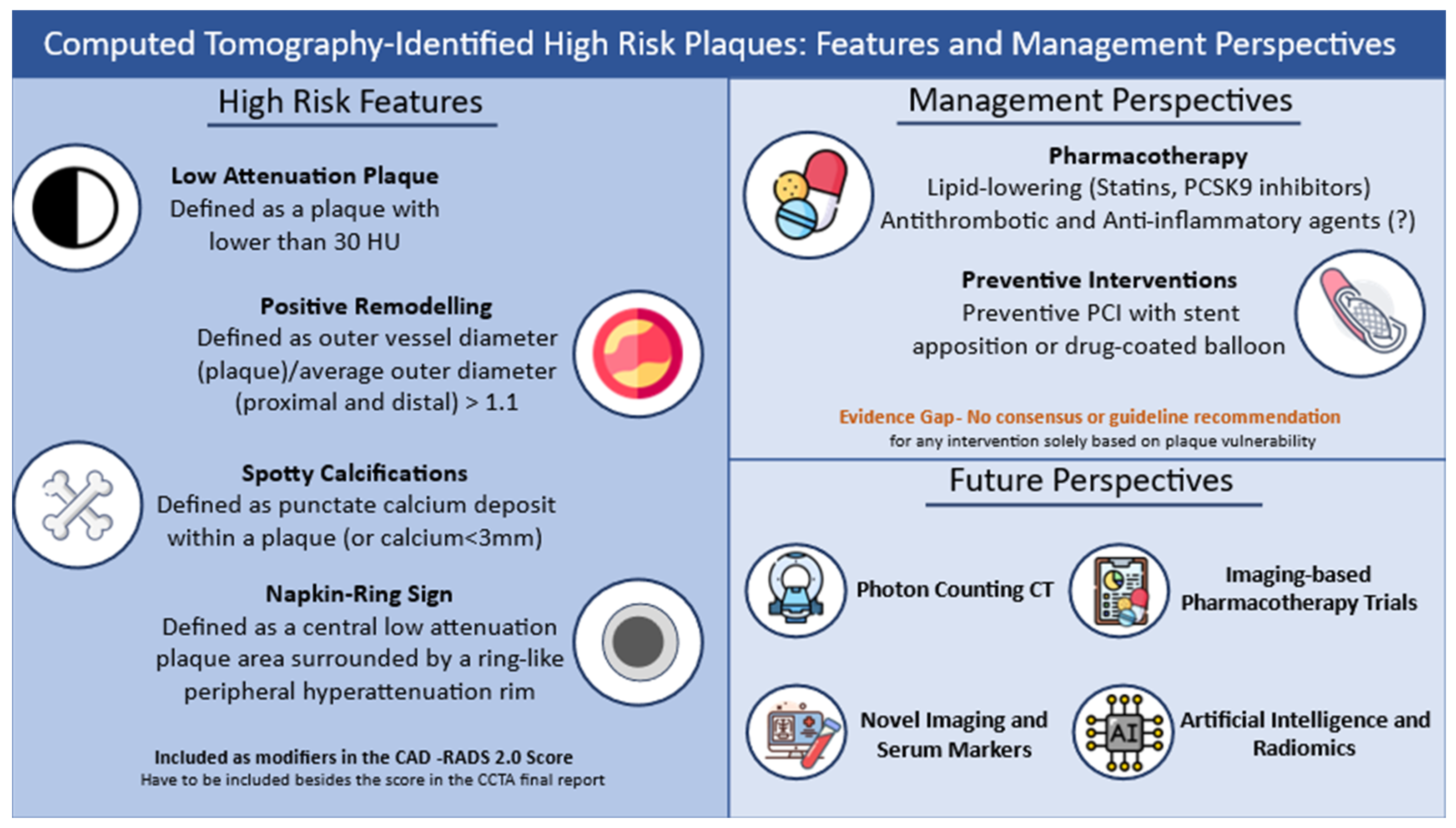
3. CCTA Characteristics to Identify Vulnerable Plaques
4. Vulnerable Plaques Prognosis
| Study | Year | Study Type | Setting | Number of Participants | Plaque Characteristic Assessed | Follow Up | ACS or MACE Prediction Rates | Other Outcomes |
|---|---|---|---|---|---|---|---|---|
| Motoyama et al. [69] | 2009 | Observational | Suspected CAD | 892 patients | LAP, PR | 27 ± 10 months | ACS rate was predicted by: Both features: 22.6% (10) One feature: 3.7% (1) None feature: 0.5% (4) | HRP independent predictor of ACS (HR: 22.8, 95% CI: 6.9–75.2) |
| Motoyama et al. [70] | 2015 | Observational | Suspected CAD | 3158 patients | LAP, PR | 3.9 ± 2.4 years | ACS rate: 16.3% (48) versus 1.4% (40) | Plaque progression independently predicted ACS, regardless of HRP(+) (27%) or HRP(-) (10%) compared to HRP(-)/PP(-) (0.3%) |
| Yamamoto et al. [71] | 2013 | Observational | Suspected CAD | 453 patients | LAP, PR | 3.3 ± 1.2 years | Adjusted HR for mortality, non-fatal MI and unstable angina: 11.2 (95% CI: 3.71–36.7; p < 0.0001) | - |
| Nakanishi et al. [72] | 2012 | Observational | Suspected CAD and hypertension | 77 patients | LAP, PR | 39 ± 10 months | The number of LAP (p < 0.001) was an independent predictor of ACS in multivariate analysis | Total LAP: 1.6 0.6 (ACS) vs. 0.05 0.23 (non-ACS) (p < 0.001) Total PR: 1.0 1.0 (ACS) vs. 0.1 0.3 (non-ACS) (p < 0.001) |
| Otsuka et al. [73] | 2013 | Observational | Suspected CAD | 895 patients | LAP, PR, NRS | 2.3 ± 0.8 years | 2.6% developed ACS PR (p < 0.001), LAP (p = 0.007), and NRS (p < 0.0001) were independent predictors of future ACS | Events developed in 41% of NRS plaques |
| Ferencik et al. [75] | 2018 | RCT, secondary analysis | Stable, symptomatic CAD | 4415 patients | LAP, PR, NRS | 25 months | MACE rate: 3% HRP was associated with a higher MACE rate (6.4% vs. 2.4%; HR: 2.73; 95% CI: 1.89–3.93) | HRP increased MACE risk among patients with nonobstructive coronary artery disease compared to non-HRP (HR: 4.31 vs. 2.64; 95% CI: 2.25–8.26 vs. 1.49–4.69) HRP was a stronger predictor of MACE in women and younger patients |
| Taron et al. [76] | 2021 | Observational | Stable CAD | 2890 patients | LAP, PR, NRS | 26 months | Presence of two or more HRP features predicted MACE (HR: 2.40; p = 0.008) | Patients with ASCVD ≥ 7.5%, any HRP, and mild/moderate stenosis had significantly higher events than those not meeting these criteria (3.0% vs. 6.2%; p = 0.007) |
| Chang et al. [77] | 2019 | Observational | ACS and stable CAD | 468 patients | PR, LAP, SC | 3.4 ± 2.1 years | Future ACS events were predicted by: HRP presence: HR: 1.59; 95% CI: 1.22 to 2.08, LAP presence: HR: 1.38; 95% CI: 1.05–1.81 SC presence: HR: 1.54; 95% CI: 1.17–2.04 | HRP was observed in 31.01% of future culprit lesion (LAP: 24.03%, PR: 76.74%, and SC: 17.83%) |
| Williams et al. [78] | 2019 | RCT, post-hoc analysis | Stable CAD | 1769 patients | PR, LAP, SC, NRS | 4.7 years (IQR 4.0–5.7) | MACE events: HRP 4.1% vs. non-HRP 1.4%; p < 0.001 In HRP, obstructive CAD: HRP 4.9% vs. non-HRP 2.4%; p = 0.036 | Patients with HRP and obstructive disease had the highest event rate (HR: 11.50; 95% CI: 3.39–39.04; p < 0.001) |
| Feuchtner et al. [80] | 2017 | Observational | Suspected CAD | 1469 patients | PR, LAP, SC, NRS | 7.8 years | MACE group showed: Decreased LAP (35.2 HU ± 32 vs. 108.8 HU ± 53; p < 0.001) Increased NRS presence (63.4% vs. 28%; p < 0.001) LAP < 30, <60, and <90 HU presence (46.3–78% vs. 2.4–7%; p < 0.001). | After adjustment, LAP < 60 and NRS were independent predictors of MACE |
| Senoner et al. [81] | 2020 | Observational | Suspected CAD | 1430 patients | PR, LAP, SC, NRS | 10.55 ± 1.98 years | MACE, but not mortality, were predicted by: LAP < 60 HU (HR: 4.00, 95% CI: 1.52–10.52, p = 0.005) NRS (HR: 4.11, 95% CI: 1.77–9.52, p = 0.001) Non-significance of SC and PR | HRP features, when added to CAD-RADS and calcium score for MACE prediction, perform superior to solely calcium score (c = 0.816 vs. 0.716, p < 0.001) |
| Yang et al. [84] | 2022 | Observational | Stable CAD | 335 patients | LAP, PR | 2.9 years | MACE were predicted by HRP (HR: 2.70; 95% CI: 1.10–6.50; p = 0.02) | HRP predicted MACE irrespective of flow or pressure gradient parameters |
5. High-Risk Features Quantification: The Use of AI, ML and Radiomics
5.1. AI and ML
5.2. Radiomics
| Study | Year | Study Type | Type of Modality Used | Comparator Group | Number of Participants | Plaque Characteristic Assessed | Outcomes |
|---|---|---|---|---|---|---|---|
| Artificial Intelligence and Machine Learning | |||||||
| Masuda et al. [89] | 2019 | Observational | ML, CCTA | Median cut-off CT number | 78 | Fatty–Fibrofatty composition | ML-yielded results showed a significantly higher AUC compared to the conventional measurement (ML-AUC: 0.92; 95% CI: 0.86–0.92 vs. Conventional-AUC: 0.83; 95% CI: 0.75–0.92; p = 0.001). |
| Choi et al. [90] | 2021 | Observational | AI, CCTA | CCTA expert readers | 232 | CAD-RADS HRP Features | HRP features were recoginzed in 21.1% by AI vs. 13.4% by expert readers (82% agreement) |
| Lin et al. [91] | 2022 | Observational | ML, CCTA | CCTA expert readers | 1196 | Good agreement regarding plaque volume [ICC 0.964] and good correlation with IVUS-derived characteristics. ML-derived plaque volume > 238.5 mm2 associated with MI (HR: 5.36; 95% CI: 1.70–16.86) Significant less time for plaque analysis by ML (mean 5.56 s vs. 25.66 min). | |
| Koo et al. [92] | 2024 | Observational | AI, CCTA | Reference CAD-RADS model | 351 | CAD-RADS and AI-derived feauters (relation with ACS prediction model) | Best predictive HRP features (included in the AI model): plaque burden, total plaque volume, low-attenuation plaque volume Validation: Higher prediction of future ACS-related plaques than conventional reference methods (AI-AUC: 0.84 VS Reference-AUC: 0.78; p < 0.001) |
| Tzimas et al. [93] | 2024 | Observational | AI, CCTA | N/A | 11,808 | Plaque volume (AI-identified normographic values) | Significantly higher plaque volume in males compared to feales (360 mm3 vs. 108 mm3; p < 0.0001). Total plaque volume increased with age in both genders. |
| Radiomics | |||||||
| Kolossvary et al. [95] | 2017 | Observational | Radiomics, CCTA | Conventional NRS sings | 60 | NRS | No conventional sign significantly differentiated NRS from non-NRS 20.6% of radiomic features were signifcicantly different in NRS vs. non-NRS, with 50% of these having an AUC > 0.80 |
| Lin et al. [96] | 2022 | Observational | Radiomics, CCTA | Conventional qualitative HRP measurement | 60 | Conventional HRP characteristics | Radiomic features identify HRP lesions with higher efficacy compared to conventional qualitative techniques (AUC 0.86 vs. 0.76; p = 0.004). |
| Kolossvary et al. [97] | 2019 | Observational (ex vivo) | Radiomics, CCTA | Histology assessment | 7 | Fatty and fibrous tissue composition; HRP characteristics | The radiomics model outperformed visual assessment (AUC = 0.73 vs. 0.65; p = 0.04) and histogram-based areas of low attenuation (AUC = 0.55 vs. 0.68; p = 0.01) in the assesment of advanced atherosclerosis |
| Chen et al. [98] | 2023 | Observational | Radiomics, CCTA | Conventional CCTA morpological assesment | 214 | Rapid plaque progression | Radiomics significantly outperformed morphological assesment |
| Chen et al. [99] | 2023 | Observational | Radiomics, CCTA | IVUS | 255 | HRP features and relation to prognosis | The radiomics model had moderate–good AUCs from training through calidation, internal and external sets (0.81, 0.75, 0.80 and 0.77). Radiomic signature equal or greater to 1.07 was significantly related to MACE at 3 years (HR: 2.01) |
6. Management of Vulnerable Plaques: Potential Indications
6.1. The Effect of Statin Treatment
6.2. The Use of PCSK9 Inhibitors
6.3. Preventive Interventional Plaque Modification
7. Future Directions
8. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2021 Causes of Death Collaborators. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2100–2132. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.; Gilbert, G.; Almansoor, Z.; Agrawal, S.; Junejo, S.; Raja, Y. Is Ct Coronary Angiography (ctca) a New Gold Standard for Diagnosis of Coronary Artery Disease?—Comparison of Ctca and Invasive Coronary Angiography (ica). J. Cardiovasc. Comput. Tomogr. 2023, 17, S11. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients with Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2023, 148, e9–e119. [Google Scholar] [CrossRef] [PubMed]
- Liga, R.; Vontobel, J.; Rovai, D.; Marinelli, M.; Caselli, C.; Pietila, M.; Teresinska, A.; Aguadé-Bruix, S.; Pizzi, M.N.; Todiere, G.; et al. Multicentre multi-device hybrid imaging study of coronary artery disease: Results from the EValuation of INtegrated Cardiac Imaging for the Detection and Characterization of Ischaemic Heart Disease (EVINCI) hybrid imaging population. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 951–960. [Google Scholar] [CrossRef] [PubMed]
- SCOT-HEART Investigators; Newby, D.E.; Adamson, P.D.; Berry, C.; Boon, N.A.; Dweck, M.R.; Flather, M.; Forbes, J.; Hunter, A.; Lewis, S.; et al. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N. Engl. J. Med. 2018, 379, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Douglas, P.S.; Hoffmann, U.; Patel, M.R.; Mark, D.B.; Al-Khalidi, H.R.; Cavanaugh, B.; Cole, J.; Dolor, R.J.; Fordyce, C.B.; Huang, M.; et al. Outcomes of Anatomical versus Functional Testing for Coronary Artery Disease. N. Engl. J. Med. 2015, 372, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Shao, C.; Wang, J.; Tian, J.; da Tang, Y. Coronary Artery Disease: From Mechanism to Clinical Practice. Adv. Exp. Med. Biol. 2020, 1177, 1–36. [Google Scholar] [CrossRef]
- Hermida, N.; Balligand, J.L. Low-Density Lipoprotein-Cholesterol-Induced Endothelial Dysfunction and Oxidative Stress: The Role of Statins. Antioxid. Redox Signal 2014, 20, 1216–1237. [Google Scholar] [CrossRef] [PubMed]
- Pyrpyris, N.; Dimitriadis, K.; Beneki, E.; Iliakis, P.; Soulaidopoulos, S.; Tsioufis, P.; Adamopoulou, E.; Kasiakogias, A.; Sakalidis, A.; Koutsopoulos, G.; et al. LOX-1 Receptor: A Diagnostic Tool and Therapeutic Target in Atherogenesis. Curr. Probl. Cardiol. 2024, 49, 102117. [Google Scholar] [CrossRef] [PubMed]
- Teh, Y.C.; Ding, J.L.; Ng, L.G.; Chong, S.Z. Capturing the Fantastic Voyage of Monocytes Through Time and Space. Front. Immunol. 2019, 10, 834. [Google Scholar] [CrossRef]
- Lara-Guzmán, O.J.; Gil-Izquierdo, Á.; Medina, S.; Osorio, E.; Álvarez-Quintero, R.; Zuluaga, N.; Oger, C.; Galano, J.M.; Durand, T.; Muñoz-Durango, K. Oxidized LDL triggers changes in oxidative stress and inflammatory biomarkers in human macrophages. Redox Biol. 2018, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Chappell, J.; Harman, J.L.; Narasimhan, V.M.; Yu, H.; Foote, K.; Simons, B.D.; Bennett, M.R.; Jørgensen, H.F. Extensive Proliferation of a Subset of Differentiated, yet Plastic, Medial Vascular Smooth Muscle Cells Contributes to Neointimal Formation in Mouse Injury and Atherosclerosis Models. Circ. Res. 2016, 119, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Herranz, L.; Albarrán-Juárez, J.; Bentzon, J.F. Mechanisms of fibrous cap formation in atherosclerosis. Front. Cardiovasc. Med. 2023, 10, 1254114. [Google Scholar] [CrossRef]
- Nakagawa, K.; Tanaka, M.; Hahm, T.H.; Nguyen, H.N.; Matsui, T.; Chen, Y.X.; Nakashima, Y. Accumulation of Plasma-Derived Lipids in the Lipid Core and Necrotic Core of Human Atheroma: Imaging Mass Spectrometry and Histopathological Analyses. Arterioscler. Thromb. Vasc. Biol. 2021, 41, e498–e511. [Google Scholar] [CrossRef]
- Gonzalez, L.; Trigatti, B.L. Macrophage Apoptosis and Necrotic Core Development in Atherosclerosis: A Rapidly Advancing Field with Clinical Relevance to Imaging and Therapy. Can. J. Cardiol. 2017, 33, 303–312. [Google Scholar] [CrossRef]
- Jaminon, A.; Reesink, K.; Kroon, A.; Schurgers, L. The Role of Vascular Smooth Muscle Cells in Arterial Remodeling: Focus on Calcification-Related Processes. Int. J. Mol. Sci. 2019, 20, 5694. [Google Scholar] [CrossRef]
- Abbasian, N. Vascular Calcification Mechanisms: Updates and Renewed Insight into Signaling Pathways Involved in High Phosphate-Mediated Vascular Smooth Muscle Cell Calcification. Biomedicines 2021, 9, 804. [Google Scholar] [CrossRef] [PubMed]
- Neels, J.G.; Leftheriotis, G.; Chinetti, G. Atherosclerosis Calcification: Focus on Lipoproteins. Metabolites 2023, 13, 457. [Google Scholar] [CrossRef]
- Shi, X.; Gao, J.; Lv, Q.; Cai, H.; Wang, F.; Ye, R.; Liu, X. Calcification in Atherosclerotic Plaque Vulnerability: Friend or Foe? Front. Physiol. 2020, 11, 56. [Google Scholar] [CrossRef]
- Muller, J.E.; Tofler, G.H.; Stone, P.H. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation 1989, 79, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Schaar, J.A.; Muller, J.E.; Falk, E.; Virmani, R.; Fuster, V.; Serruys, P.W.; Colombo, A.; Stefanadis, C.; Casscells, S.W.; Moreno, P.R. Terminology for high-risk and vulnerable coronary artery plaques. Eur. Heart J. 2004, 25, 1077–1082. [Google Scholar] [CrossRef]
- Davies, M.J. Anatomic features in victims of sudden coronary death. Coronary artery pathology. Circulation 1992, 85 (Suppl. S1), I19–I24. [Google Scholar]
- Burke, A.P.; Farb, A.; Malcom, G.T.; Liang, Y.H.; Smialek, J.; Virmani, R. Coronary Risk Factors and Plaque Morphology in Men with Coronary Disease Who Died Suddenly. N. Engl. J. Med. 1997, 336, 1276–1282. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from Sudden Coronary Death. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef]
- Varnava, A.M.; Mills, P.G.; Davies, M.J. Relationship Between Coronary Artery Remodeling and Plaque Vulnerability. Circulation 2002, 105, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Tang, Z.; Yan, B.; Yin, H.; Tai, S.; Peng, J.; Cui, Y.; Gui, Y.; Belke, D.; Zhou, S.; et al. PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9) Triggers Vascular Smooth Muscle Cell Senescence and Apoptosis: Implication of Its Direct Role in Degenerative Vascular Disease. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 67–86. [Google Scholar] [CrossRef]
- Libby, P.; Pasterkamp, G.; Crea, F.; Jang, I.K. Reassessing the Mechanisms of Acute Coronary Syndromes. Circ. Res. 2019, 124, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Ekstrand, J.; Razuvaev, A.; Folkersen, L.; Roy, J.; Hedin, U. Tissue factor pathway inhibitor-2 is induced by fluid shear stress in vascular smooth muscle cells and affects cell proliferation and survival. J. Vasc. Surg. 2010, 52, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Samady, H.; Eshtehardi, P.; McDaniel, M.C.; Suo, J.; Dhawan, S.S.; Maynard, C.; Timmins, L.H.; Quyyumi, A.A.; Giddens, D.P. Coronary Artery Wall Shear Stress Is Associated with Progression and Transformation of Atherosclerotic Plaque and Arterial Remodeling in Patients with Coronary Artery Disease. Circulation 2011, 124, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Candreva, A.; Buongiorno, A.L.; Matter, M.A.; Rizzini, M.L.; Giacobbe, F.; Ravetti, E.; Giannino, G.; Carmagnola, L.; Gilhofer, T.; Gallo, D.; et al. Impact of endothelial shear stress on coronary atherosclerotic plaque progression and composition: A meta-analysis and systematic review. Int. J. Cardiol. 2024, 407, 132061. [Google Scholar] [CrossRef]
- Bajraktari, A.; Bytyçi, I.; Henein, M.Y. High Coronary Wall Shear Stress Worsens Plaque Vulnerability: A Systematic Review and Meta-Analysis. Angiology 2021, 72, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Agasthi, P.; Kanmanthareddy, A.; Khalil, C.; Egbuche, O.; Yarlagadda, V.; Sachdeva, R.; Arsanjani, R. Comparison of Computed Tomography derived Fractional Flow Reserve to invasive Fractional Flow Reserve in Diagnosis of Functional Coronary Stenosis: A Meta-Analysis. Sci. Rep. 2018, 8, 11535. [Google Scholar] [CrossRef] [PubMed]
- Douglas, P.S.; De Bruyne, B.; Pontone, G.; Patel, M.R.; Norgaard, B.L.; Byrne, R.A.; Curzen, N.; Purcell, I.; Gutberlet, M.; Rioufol, G.; et al. 1-Year Outcomes of FFRCT-Guided Care in Patients with Suspected Coronary Disease. J. Am. Coll. Cardiol. 2016, 68, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.T.; Ferencik, M.; Roberts, R.S.; Lee, K.L.; Ivanov, A.; Adami, E.; Mark, D.B.; Jaffer, F.A.; Leipsic, J.A.; Douglas, P.S.; et al. Noninvasive FFR Derived from Coronary CT Angiography. JACC Cardiovasc. Imaging 2017, 10, 1350–1358. [Google Scholar] [CrossRef]
- Curzen, N.; Nicholas, Z.; Stuart, B.; Wilding, S.; Hill, K.; Shambrook, J.; Eminton, Z.; Ball, D.; Barrett, C.; Johnson, L.; et al. Fractional flow reserve derived from computed tomography coronary angiography in the assessment and management of stable chest pain: The FORECAST randomized trial. Eur. Heart J. 2021, 42, 3844–3852. [Google Scholar] [CrossRef]
- Shaw, L.J.; Blankstein, R.; Bax, J.J.; Ferencik, M.; Bittencourt, M.S.; Min, J.K.; Berman, D.S.; Leipsic, J.; Villines, T.C.; Dey, D.; et al. Society of Cardiovascular Computed Tomography/North American Society of Cardiovascular Imaging—Expert Consensus Document on Coronary CT Imaging of Atherosclerotic Plaque. J. Cardiovasc. Comput. Tomogr. 2021, 15, 93–109. [Google Scholar] [CrossRef]
- Schlett, C.L.; Maurovich-Horvat, P.; Ferencik, M.; Alkadhi, H.; Stolzmann, P.; Scheffel, H.; Seifarth, H.; Nakano, M.; Do, S.; Vorpahl, M.; et al. Histogram Analysis of Lipid-Core Plaques in Coronary Computed Tomographic Angiography. Investig. Radiol. 2013, 48, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.C.; Kwiecinski, J.; Doris, M.; McElhinney, P.; D’Souza, M.S.; Cadet, S.; Adamson, P.D.; Moss, A.J.; Alam, S.; Hunter, A.; et al. Low-Attenuation Noncalcified Plaque on Coronary Computed Tomography Angiography Predicts Myocardial Infarction. Circulation 2020, 141, 1452–1462. [Google Scholar] [CrossRef] [PubMed]
- Cury, R.C.; Leipsic, J.; Abbara, S.; Achenbach, S.; Berman, D.; Bittencourt, M.; Budoff, M.; Chinnaiyan, K.; Choi, A.D.; Ghoshhajra, B.; et al. CAD-RADSTM 2.0—2022 Coronary Artery Disease—Reporting and Data System An Expert Consensus Document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Cardiology (ACC), the American College of Radiology (ACR) and the North America Society of Cardiovascular Imaging (NASCI). Radiol. Cardiothorac. Imaging 2022, 4, e220183. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Granillo, G.A. Coronary artery remodelling is related to plaque composition. Heart 2005, 92, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Jeong, M.H.; Choi, Y.H.; Ko, J.S.; Lee, M.G.; Kang, W.Y.; Lee, S.E.; Kim, S.H.; Park, K.H.; Sim, D.S.; et al. Positive remodeling is associated with more plaque vulnerability and higher frequency of plaque prolapse accompanied with post-procedural cardiac enzyme elevation compared with intermediate/negative remodeling in patients with acute myocardial infarction. J. Cardiol. 2009, 53, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Cilla, M.; Peña, E.; Martínez, M.A.; Kelly, D.J. Comparison of the vulnerability risk for positive versus negative atheroma plaque morphology. J. Biomech. 2013, 46, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, Y.; Puri, R.; Hammadah, M.; Duggal, B.; Uno, K.; Kapadia, S.R.; Tuzcu, E.M.; Nissen, S.E.; Nicholls, S.J. Spotty calcification and plaque vulnerability in vivo: Frequency-domain optical coherence tomography analysis. Cardiovasc. Diagn. Ther. 2014, 4, 460–469. [Google Scholar] [CrossRef]
- Schroeder, S.; Kopp, A.F.; Baumbach, A.; Meisner, C.; Kuettner, A.; Georg, C.; Ohnesorge, B.; Herdeg, C.; Claussen, C.D.; Karsch, K.R. Noninvasive detection and evaluation of atherosclerotic coronary plaques with multislice computed tomography. J. Am. Coll. Cardiol. 2001, 37, 1430–1435. [Google Scholar] [CrossRef]
- Leber, A.W.; Knez, A.; White, C.W.; Becker, A.; von Ziegler, F.; Muehling, O.; Becker, C.; Reiser, M.; Steinbeck, G.; Boekstegers, P. Composition of coronary atherosclerotic plaques in patients with acute myocardial infarction and stable angina pectoris determined by contrast-enhanced multislice computed tomography. Am. J. Cardiol. 2003, 91, 714–718. [Google Scholar] [CrossRef]
- Leber, A.W.; Knez, A.; Becker, A.; Becker, C.; von Ziegler, F.; Nikolaou, K.; Rist, C.; Reiser, M.; White, C.; Steinbeck, G.; et al. Accuracy of multidetector spiral computed tomography in identifying and differentiating the composition of coronary atherosclerotic plaques. J. Am. Coll. Cardiol. 2004, 43, 1241–1247. [Google Scholar] [CrossRef]
- Motoyama, S.; Kondo, T.; Anno, H.; Sugiura, A.; Ito, Y.; Mori, K.; Ishii, J.; Sato, T.; Inoue, K.; Sarai, M.; et al. Atherosclerotic Plaque Characterization by 0.5-mm-Slice Multislice Computed Tomographic Imaging Comparison with Intravascular Ultrasound. Circ. J. 2007, 71, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.J.; Kang, D.K.; Tahk, S.J.; Choi, S.Y.; Yoon, M.H.; Lim, H.S.; Kang, S.J.; Yang, H.M.; Park, J.S.; Zheng, M.; et al. Comparison of 64-Slice Multidetector Computed Tomography with Spectral Analysis of Intravascular Ultrasound Backscatter Signals for Characterizations of Noncalcified Coronary Arterial Plaques. Am. J. Cardiol. 2008, 102, 988–993. [Google Scholar] [CrossRef]
- Voros, S.; Rinehart, S.; Qian, Z.; Vazquez, G.; Anderson, H.; Murrieta, L.; Wilmer, C.; Carlson, H.; Taylor, K.; Ballard, W.; et al. Prospective Validation of Standardized, 3-Dimensional, Quantitative Coronary Computed Tomographic Plaque Measurements Using Radiofrequency Backscatter Intravascular Ultrasound as Reference Standard in Intermediate Coronary Arterial Lesions. JACC Cardiovasc. Interv. 2011, 4, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Obaid, D.R.; Calvert, P.A.; Gopalan, D.; Parker, R.A.; Hoole, S.P.; West, N.E.; Goddard, M.; Rudd, J.H.; Bennett, M.R. Atherosclerotic Plaque Composition and Classification Identified by Coronary Computed Tomography. Circ. Cardiovasc. Imaging 2013, 6, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Obaid, D.R.; Calvert, P.A.; Gopalan, D.; Parker, R.A.; West, N.E.; Goddard, M.; Rudd, J.H.; Bennett, M.R. Dual-energy computed tomography imaging to determine atherosclerotic plaque composition: A prospective study with tissue validation. J. Cardiovasc. Comput. Tomogr. 2014, 8, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Mintz, G.S.; Kim, S.Y.; Hong, Y.J.; Kim, S.W.; Okabe, T.; Pichard, A.D.; Satler, L.F.; Kent, K.M.; Suddath, W.O.; et al. Attenuated Plaque Detected by Intravascular Ultrasound. JACC Cardiovasc. Interv. 2009, 2, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Soeda, T.; Uemura, S.; Morikawa, Y.; Ishigami, K.; Okayama, S.; Hee, S.J.; Nishida, T.; Onoue, K.; Somekawa, S.; Takeda, Y.; et al. Diagnostic accuracy of dual-source computed tomography in the characterization of coronary atherosclerotic plaques: Comparison with intravascular optical coherence tomography. Int. J. Cardiol. 2011, 148, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.H.; Kang, S.J.; Koo, H.J.; Chang, M.; Kang, J.W.; Lim, T.H.; Baek, S.; Han, S.; Lee, P.H.; Roh, J.H.; et al. Coronary CT angiography characteristics of OCT-defined thin-cap fibroatheroma: A section-to-section comparison study. Eur. Radiol. 2018, 28, 833–843. [Google Scholar] [CrossRef]
- Tanisawa, H.; Matsumoto, H.; Cadet, S.; Higuchi, S.; Ohya, H.; Isodono, K.; Irie, D.; Kaneko, K.; Sumida, A.; Hirano, T.; et al. Quantification of Low-Attenuation Plaque Burden from Coronary CT Angiography: A Head-to-Head Comparison with Near-Infrared Spectroscopy Intravascular US. Radiol. Cardiothorac. Imaging 2023, 5, e230090. [Google Scholar] [CrossRef]
- Kashiwagi, M.; Tanaka, A.; Kitabata, H.; Tsujioka, H.; Kataiwa, H.; Komukai, K.; Tanimoto, T.; Takemoto, K.; Takarada, S.; Kubo, T.; et al. Feasibility of Noninvasive Assessment of Thin-Cap Fibroatheroma by Multidetector Computed Tomography. JACC Cardiovasc. Imaging 2009, 2, 1412–1419. [Google Scholar] [CrossRef]
- Maurovich-Horvat, P.; Schlett, C.L.; Alkadhi, H.; Nakano, M.; Otsuka, F.; Stolzmann, P.; Scheffel, H.; Ferencik, M.; Kriegel, M.F.; Seifarth, H.; et al. The Napkin-Ring Sign Indicates Advanced Atherosclerotic Lesions in Coronary CT Angiography. JACC Cardiovasc. Imaging 2012, 5, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Seifarth, H.; Schlett, C.L.; Nakano, M.; Otsuka, F.; Károlyi, M.; Liew, G.; Maurovich-Horvat, P.; Alkadhi, H.; Virmani, R.; Hoffmann, U. Histopathological correlates of the napkin-ring sign plaque in coronary CT angiography. Atherosclerosis 2012, 224, 90–96. [Google Scholar] [CrossRef]
- Van Velzen, J.E.; de Graaf, F.R.; de Graaf, M.A.; Schuijf, J.D.; Kroft, L.J.; de Roos, A.; Reiber, J.H.; Bax, J.J.; Jukema, J.W.; Boersma, E.; et al. Comprehensive assessment of spotty calcifications on computed tomography angiography: Comparison to plaque characteristics on intravascular ultrasound with radiofrequency backscatter analysis. J. Nucl. Cardiol. 2011, 18, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Pezel, T.; Sideris, G.; Dillinger, J.G.; Logeart, D.; Manzo-Silberman, S.; Cohen-Solal, A.; Beauvais, F.; Devasenapathy, N.; Laissy, J.P.; Henry, P. Coronary Computed Tomography Angiography Analysis of Calcium Content to Identify Non-culprit Vulnerable Plaques in Patients with Acute Coronary Syndrome. Front. Cardiovasc. Med. 2022, 9, 876730. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, S.; Ropers, D.; Hoffmann, U.; MacNeill, B.; Baum, U.; Pohle, K.; Brady, T.J.; Pomerantsev, E.; Ludwig, J.; Flachskampf, F.A.; et al. assessment of coronary remodeling in stenotic and nonstenotic coronary atherosclerotic lesions by multidetector spiral computed tomography. J. Am. Coll. Cardiol. 2004, 43, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Kröner, E.S.J.; van Velzen, J.E.; Boogers, M.J.; Siebelink, H.M.; Schalij, M.J.; Kroft, L.J.; de Roos, A.; van der Wall, E.E.; Jukema, J.W.; Reiber, J.H.; et al. Positive Remodeling on Coronary Computed Tomography as a Marker for Plaque Vulnerability on Virtual Histology Intravascular Ultrasound. Am. J. Cardiol. 2011, 107, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Del Val, D.; Berta, B.; Roleder, T.; Malinowski, K.; Bastante, T.; Hermanides, R.S.; Wojakowski, W.; Fabris, E.; Cuesta, J.; De Luca, G.; et al. Vulnerable plaque features and adverse events in patients with diabetes mellitus: A post hoc analysis of the COMBINE OCT-FFR trial. EuroIntervention 2024, 20, e707–e717. [Google Scholar] [CrossRef]
- Fabris, E.; Berta, B.; Hommels, T.; Roleder, T.; Hermanides, R.S.; Rivero, F.; von Birgelen, C.; Escaned, J.; Camaro, C.; Kennedy, M.W.; et al. Long-term outcomes of patients with normal fractional flow reserve and thin-cap fibroatheroma. EuroIntervention 2023, 18, e1099–e1107. [Google Scholar] [CrossRef]
- Motoyama, S.; Sarai, M.; Harigaya, H.; Anno, H.; Inoue, K.; Hara, T.; Naruse, H.; Ishii, J.; Hishida, H.; Wong, N.D.; et al. Computed Tomographic Angiography Characteristics of Atherosclerotic Plaques Subsequently Resulting in Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2009, 54, 49–57. [Google Scholar] [CrossRef]
- Motoyama, S.; Ito, H.; Sarai, M.; Kondo, T.; Kawai, H.; Nagahara, Y.; Harigaya, H.; Kan, S.; Anno, H.; Takahashi, H.; et al. Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. J. Am. Coll. Cardiol. 2015, 66, 337–346. [Google Scholar] [CrossRef]
- Yamamoto, H.; Kitagawa, T.; Ohashi, N.; Utsunomiya, H.; Kunita, E.; Oka, T.; Urabe, Y.; Tsushima, H.; Awai, K.; Kihara, Y. Noncalcified atherosclerotic lesions with vulnerable characteristics detected by coronary CT angiography and future coronary events. J. Cardiovasc. Comput. Tomogr. 2013, 7, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Fukuda, S.; Shimada, K.; Ehara, S.; Inanami, H.; Matsumoto, K.; Taguchi, H.; Muro, T.; Yoshikawa, J.; Yoshiyama, M. Non-obstructive low attenuation coronary plaque predicts three-year acute coronary syndrome events in patients with hypertension: Multidetector computed tomographic study. J. Cardiol. 2012, 59, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, K.; Fukuda, S.; Tanaka, A.; Nakanishi, K.; Taguchi, H.; Yoshikawa, J.; Shimada, K.; Yoshiyama, M. Napkin-Ring Sign on Coronary CT Angiography for the Prediction of Acute Coronary Syndrome. JACC Cardiovasc. Imaging 2013, 6, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.H.; Lu, B.; Gao, Y.; Jiang, S.L.; Wang, Y.; Li, W.; Budoff, M.J. Prognostic Value of Coronary CT Angiography and Calcium Score for Major Adverse Cardiac Events in Outpatients. JACC Cardiovasc. Imaging 2012, 5, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Ferencik, M.; Mayrhofer, T.; Bittner, D.O.; Emami, H.; Puchner, S.B.; Lu, M.T.; Meyersohn, N.M.; Ivanov, A.V.; Adami, E.C.; Patel, M.R.; et al. Use of High-Risk Coronary Atherosclerotic Plaque Detection for Risk Stratification of Patients with Stable Chest Pain. JAMA Cardiol. 2018, 3, 144. [Google Scholar] [CrossRef] [PubMed]
- Taron, J.; Foldyna, B.; Mayrhofer, T.; Osborne, M.T.; Meyersohn, N.; Bittner, D.O.; Puchner, S.B.; Emami, H.; Lu, M.T.; Ferencik, M.; et al. Risk Stratification with the Use of Coronary Computed Tomographic Angiography in Patients with Nonobstructive Coronary Artery Disease. JACC Cardiovasc. Imaging 2021, 14, 2186–2195. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.J.; Lin, F.Y.; Lee, S.E.; Andreini, D.; Bax, J.; Cademartiri, F.; Chinnaiyan, K.; Chow, B.J.W.; Conte, E.; Cury, R.C.; et al. Coronary Atherosclerotic Precursors of Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2018, 71, 2511–2522. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.C.; Moss, A.J.; Dweck, M.; Adamson, P.D.; Alam, S.; Hunter, A.; Shah, A.S.V.; Pawade, T.; Weir-McCall, J.R.; Roditi, G.; et al. Coronary Artery Plaque Characteristics Associated with Adverse Outcomes in the SCOT-HEART Study. J. Am. Coll. Cardiol. 2019, 73, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.C.; Kwiecinski, J.; Doris, M.; McElhinney, P.; D’Souza, M.S.; Cadet, S.; Adamson, P.D.; Moss, A.J.; Alam, S.; Hunter, A.; et al. Sex-Specific Computed Tomography Coronary Plaque Characterization and Risk of Myocardial Infarction. JACC Cardiovasc. Imaging 2021, 14, 1804–1814. [Google Scholar] [CrossRef]
- Feuchtner, G.; Kerber, J.; Burghard, P.; Dichtl, W.; Friedrich, G.; Bonaros, N.; Plank, F. The high-risk criteria low-attenuation plaque <60 HU and the napkin-ring sign are the most powerful predictors of MACE: A long-term follow-up study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 772–779. [Google Scholar] [CrossRef]
- Senoner, T.; Plank, F.; Barbieri, F.; Beyer, C.; Birkl, K.; Widmann, G.; Adukauskaite, A.; Friedrich, G.; Dichtl, W.; Feuchtner, G.M. Added value of high-risk plaque criteria by coronary CTA for prediction of long-term outcomes. Atherosclerosis 2020, 300, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Choi, G.; Chun, E.J.; Kim, H.J.; Park, J.; Jung, J.H.; Lee, M.H.; Otake, H.; Doh, J.H.; Nam, C.W.; et al. Computational fluid dynamic measures of wall shear stress are related to coronary lesion characteristics. Heart 2016, 102, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Choi, G.; Koo, B.K.; Hwang, D.; Park, J.; Zhang, J.; Kim, K.J.; Tong, Y.; Kim, H.J.; Grady, L.; et al. Identification of High-Risk Plaques Destined to Cause Acute Coronary Syndrome Using Coronary Computed Tomographic Angiography and Computational Fluid Dynamics. JACC Cardiovasc. Imaging 2019, 12, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Hoshino, M.; Koo, B.K.; Yonetsu, T.; Zhang, J.; Hwang, D.; Shin, E.S.; Doh, J.H.; Nam, C.W.; Wang, J.; et al. Relationship of Plaque Features at Coronary CT to Coronary Hemodynamics and Cardiovascular Events. Radiology 2022, 305, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Meah, M.N.; Tzolos, E.; Wang, K.L.; Bularga, A.; Dweck, M.R.; Curzen, N.; Kardos, A.; Keating, L.; Storey, R.F.; Mills, N.L.; et al. Plaque Burden and 1-Year Outcomes in Acute Chest Pain. JACC Cardiovasc. Imaging 2022, 15, 1916–1925. [Google Scholar] [CrossRef]
- Gallone, G.; Bellettini, M.; Gatti, M.; Tore, D.; Bruno, F.; Scudeler, L.; Cusenza, V.; Lanfranchi, A.; Angelini, A.; de Filippo, O.; et al. Coronary Plaque Characteristics Associated with Major Adverse Cardiovascular Events in Atherosclerotic Patients and Lesions. JACC Cardiovasc. Imaging 2023, 16, 1584–1604. [Google Scholar] [CrossRef] [PubMed]
- Jonas, R.A.; Weerakoon, S.; Fisher, R.; Griffin, W.F.; Kumar, V.; Rahban, H.; Marques, H.; Karlsberg, R.P.; Jennings, R.S.; Crabtree, T.R.; et al. Interobserver variability among expert readers quantifying plaque volume and plaque characteristics on coronary CT angiography: A CLARIFY trial sub-study. Clin. Imaging 2022, 91, 19–25. [Google Scholar] [CrossRef]
- Quintana, R.A.; von Knebel Doeberitz, P.; Vatsa, N.; Liu, C.; Ko, Y.A.; De Cecco, C.N.; van Assen, M.; Quyyumi, A.A. Intra- and inter-reader reproducibility in quantitative coronary plaque analysis on coronary computed tomography angiography. Curr. Probl. Cardiol. 2024, 49, 102585. [Google Scholar] [CrossRef]
- Masuda, T.; Nakaura, T.; Funama, Y.; Okimoto, T.; Sato, T.; Higaki, T.; Noda, N.; Imada, N.; Baba, Y.; Awai, K. Machine-learning integration of CT histogram analysis to evaluate the composition of atherosclerotic plaques: Validation with IB-IVUS. J. Cardiovasc. Comput. Tomogr. 2019, 13, 163–169. [Google Scholar] [CrossRef]
- Choi, A.D.; Marques, H.; Kumar, V.; Griffin, W.F.; Rahban, H.; Karlsberg, R.P.; Zeman, R.K.; Katz, R.J.; Earls, J.P. CT Evaluation by Artificial Intelligence for Atherosclerosis, Stenosis and Vascular Morphology (CLARIFY): A Multi-center, international study. J. Cardiovasc. Comput. Tomogr. 2021, 15, 470–476. [Google Scholar] [CrossRef]
- Lin, A.; Manral, N.; McElhinney, P.; Killekar, A.; Matsumoto, H.; Kwiecinski, J.; Pieszko, K.; Razipour, A.; Grodecki, K.; Park, C.; et al. Deep learning-enabled coronary CT angiography for plaque and stenosis quantification and cardiac risk prediction: An international multicentre study. Lancet Digit. Health 2022, 4, e256–e265. [Google Scholar] [CrossRef]
- Koo, B.K.; Yang, S.; Jung, J.W.; Zhang, J.; Lee, K.; Hwang, D.; Lee, K.S.; Doh, J.H.; Nam, C.W.; Kim, T.H.; et al. Artificial Intelligence–Enabled Quantitative Coronary Plaque and Hemodynamic Analysis for Predicting Acute Coronary Syndrome. JACC Cardiovasc. Imaging 2024. [Google Scholar] [CrossRef] [PubMed]
- Tzimas, G.; Gulsin, G.S.; Everett, R.J.; Akodad, M.; Meier, D.; Sewnarain, K.; Ally, Z.; Alnamasy, R.; Ng, N.; Mullen, S.; et al. Age- and Sex-Specific Nomographic CT Quantitative Plaque Data from a Large International Cohort. JACC Cardiovasc. Imaging 2024, 17, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in medical imaging—“how-to” guide and critical reflection. Insights Imaging 2020, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Kolossváry, M.; Karády, J.; Szilveszter, B.; Kitslaar, P.; Hoffmann, U.; Merkely, B.; Maurovich-Horvat, P. Radiomic Features Are Superior to Conventional Quantitative Computed Tomographic Metrics to Identify Coronary Plaques with Napkin-Ring Sign. Circ. Cardiovasc. Imaging 2017, 10, e006843. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Kolossváry, M.; Cadet, S.; McElhinney, P.; Goeller, M.; Han, D.; Yuvaraj, J.; Nerlekar, N.; Slomka, P.J.; Marwan, M.; et al. Radiomics-Based Precision Phenotyping Identifies Unstable Coronary Plaques from Computed Tomography Angiography. JACC Cardiovasc. Imaging 2022, 15, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Kolossváry, M.; Karády, J.; Kikuchi, Y.; Ivanov, A.; Schlett, C.L.; Lu, M.T.; Foldyna, B.; Merkely, B.; Aerts, H.J.; Hoffmann, U.; et al. Radiomics versus Visual and Histogram-based Assessment to Identify Atheromatous Lesions at Coronary CT Angiography: An Ex Vivo Study. Radiology 2019, 293, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xie, G.; Tang, C.X.; Yang, L.; Xu, P.; Gao, X.; Lu, M.; Fu, Y.; Huo, Y.; Zheng, S.; et al. Development and Validation of CCTA-based Radiomics Signature for Predicting Coronary Plaques with Rapid Progression. Circ. Cardiovasc. Imaging 2023, 16, e015340. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Pan, T.; Wang, Y.N.; Schoepf, U.J.; Bidwell, S.L.; Qiao, H.; Feng, Y.; Xu, C.; Xu, H.; Xie, G.; et al. A Coronary CT Angiography Radiomics Model to Identify Vulnerable Plaque and Predict Cardiovascular Events. Radiology 2023, 307, e221693. [Google Scholar] [CrossRef]
- Nissen, S.E.; Tuzcu, E.M.; Schoenhagen, P.; Brown, B.G.; Ganz, P.; Vogel, R.A.; Crowe, T.; Howard, G.; Cooper, C.J.; Brodie, B.; et al. Effect of Intensive Compared with Moderate Lipid-Lowering Therapy on Progression of Coronary Atherosclerosis. JAMA 2004, 291, 1071. [Google Scholar] [CrossRef]
- Okazaki, S.; Yokoyama, T.; Miyauchi, K.; Shimada, K.; Kurata, T.; Sato, H.; Daida, H. Early Statin Treatment in Patients with Acute Coronary Syndrome. Circulation 2004, 110, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Ballantyne, C.M.; Barter, P.J.; Chapman, M.J.; Erbel, R.M.; Libby, P.; Raichlen, J.S.; Uno, K.; Borgman, M.; Wolski, K.; et al. Effect of Two Intensive Statin Regimens on Progression of Coronary Disease. N. Engl. J. Med. 2011, 365, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Nicholls, S.J.; Sipahi, I.; Libby, P.; Raichlen, J.S.; Ballantyne, C.M.; Davignon, J.; Erbel, R.; Fruchart, J.C.; Tardif, J.C.; et al. Effect of Very High-Intensity Statin Therapy on Regression of Coronary Atherosclerosis. JAMA 2006, 295, 1556. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Chang, H.J.; Sung, J.M.; Park, H.B.; Heo, R.; Rizvi, A.; Lin, F.Y.; Kumar, A.; Hadamitzky, M.; Kim, Y.J.; et al. Effects of Statins on Coronary Atherosclerotic Plaques. JACC Cardiovasc. Imaging 2018, 11, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Van Rosendael, S.E.; van den Hoogen, I.J.; Lin, F.Y.; Andreini, D.; Al-Mallah, M.H.; Budoff, M.J.; Cademartiri, F.; Chinnaiyan, K.; Choi, J.H.; Conte, E.; et al. Clinical and Coronary Plaque Predictors of Atherosclerotic Nonresponse to Statin Therapy. JACC Cardiovasc. Imaging 2023, 16, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Smit, J.M.; van Rosendael, A.R.; El Mahdiui, M.; Neglia, D.; Knuuti, J.; Saraste, A.; Buechel, R.R.; Teresinska, A.; Pizzi, M.N.; Roque, A.; et al. Impact of Clinical Characteristics and Statins on Coronary Plaque Progression by Serial Computed Tomography Angiography. Circ. Cardiovasc. Imaging 2020, 13, e009750. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, K.; Theofilis, P.; Iliakis, P.; Pyrpyris, N.; Dri, E.; Sakalidis, A.; Soulaidopoulos, S.; Tsioufis, P.; Fragkoulis, C.; Chrysohoou, C.; et al. Management of dyslipidemia in coronary artery disease: The present and the future. Coron. Artery Dis. 2024, 35, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Puri, R.; Anderson, T.; Ballantyne, C.M.; Cho, L.; Kastelein, J.J.; Koenig, W.; Somaratne, R.; Kassahun, H.; Yang, J.; et al. Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients. JAMA 2016, 316, 2373. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Kataoka, Y.; Nissen, S.E.; Prati, F.; Windecker, S.; Puri, R.; Hucko, T.; Aradi, D.; Herrman, J.R.; Hermanides, R.S.; et al. Effect of Evolocumab on Coronary Plaque Phenotype and Burden in Statin-Treated Patients Following Myocardial Infarction. JACC Cardiovasc. Imaging 2022, 15, 1308–1321. [Google Scholar] [CrossRef]
- Räber, L.; Ueki, Y.; Otsuka, T.; Losdat, S.; Häner, J.D.; Lonborg, J.; Fahrni, G.; Iglesias, J.F.; van Geuns, R.J.; Ondracek, A.S.; et al. Effect of Alirocumab Added to High-Intensity Statin Therapy on Coronary Atherosclerosis in Patients with Acute Myocardial Infarction. JAMA 2022, 327, 1771. [Google Scholar] [CrossRef]
- Pérez de Isla, L.; Díaz-Díaz, J.L.; Romero, M.J.; Muñiz-Grijalvo, O.; Mediavilla, J.D.; Argüeso, R.; Sánchez Muñoz-Torrero, J.F.; Rubio, P.; Álvarez-Baños, P.; Ponte, P.; et al. Alirocumab and Coronary Atherosclerosis in Asymptomatic Patients with Familial Hypercholesterolemia: The ARCHITECT Study. Circulation 2023, 147, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Biccirè, F.G.; Häner, J.; Losdat, S.; Ueki, Y.; Shibutani, H.; Otsuka, T.; Kakizaki, R.; Hofbauer, T.M.; van Geuns, R.J.; Stortecky, S.; et al. Concomitant Coronary Atheroma Regression and Stabilization in Response to Lipid-Lowering Therapy. J. Am. Coll. Cardiol. 2023, 82, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, K.; Pyrpyris, N.; Tsioufis, K. The potential future role of extensive lipid lowering in ACS patients with the use of PCSK9 inhibitors: Early bird catches the worm. Eur. Heart J. Cardiovasc. Pharmacother. 2024, 10, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Maehara, A.; Ali, Z.A.; Held, C.; Matsumura, M.; Kjøller-Hansen, L.; Bøtker, H.E.; Maeng, M.; Engstrøm, T.; Wiseth, R.; et al. Percutaneous Coronary Intervention for Vulnerable Coronary Atherosclerotic Plaque. J. Am. Coll. Cardiol. 2020, 76, 2289–2301. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Ahn, J.M.; Kang, D.Y.; Yun, S.C.; Ahn, Y.K.; Kim, W.J.; Nam, C.W.; Jeong, J.O.; Chae, I.H.; Shiomi, H.; et al. Preventive percutaneous coronary intervention versus optimal medical therapy alone for the treatment of vulnerable atherosclerotic coronary plaques (PREVENT): A multicentre, open-label, randomised controlled trial. Lancet 2024, 403, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Van Veelen, A.; Küçük, I.T.; Garcia-Garcia, H.M.; Fuentes, F.H.; Kahsay, Y.; Delewi, R.; Beijk, M.A.M.; den Hartog, A.W.; Grundeken, M.J.; Vis, M.M.; et al. Paclitaxel-coated balloons for vulnerable lipid-rich plaques. EuroIntervention 2024, 20, e826–e830. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Morris, P.B.; Ballantyne, C.M.; Birtcher, K.K.; Covington, A.M.; DePalma, S.M.; Minissian, M.B.; Orringer, C.E.; Smith, S.C., Jr.; Waring, A.A.; et al. 2022 ACC Expert Consensus Decision Pathway on the Role of Nonstatin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk. J. Am. Coll. Cardiol. 2022, 80, 1366–1418. [Google Scholar] [CrossRef]
- Van der Bie, J.; van Straten, M.; Booij, R.; Bos, D.; Dijkshoorn, M.L.; Hirsch, A.; Sharma, S.P.; Oei, E.H.G.; Budde, R.P.J. Photon-counting CT: Review of initial clinical results. Eur. J. Radiol. 2023, 163, 110829. [Google Scholar] [CrossRef]
- Si-Mohamed, S.A.; Boccalini, S.; Lacombe, H.; Diaw, A.; Varasteh, M.; Rodesch, P.A.; Dessouky, R.; Villien, M.; Tatard-Leitman, V.; Bochaton, T.; et al. Coronary CT Angiography with Photon-counting CT: First-In-Human Results. Radiology 2022, 303, 303–313. [Google Scholar] [CrossRef]
- Mergen, V.; Sartoretti, T.; Baer-Beck, M.; Schmidt, B.; Petersilka, M.; Wildberger, J.E.; Euler, A.; Eberhard, M.; Alkadhi, H. Ultra-High-Resolution Coronary CT Angiography with Photon-Counting Detector CT. Investig. Radiol. 2022, 57, 780–788. [Google Scholar] [CrossRef]
- McCollough, C.H.; Winfree, T.N.; Melka, E.F.; Rajendran, K.; Carter, R.E.; Leng, S. Photon-Counting Detector Computed Tomography Versus Energy-Integrating Detector Computed Tomography for Coronary Artery Calcium Quantitation. J. Comput. Assist. Tomogr. 2024, 48, 212–216. [Google Scholar] [CrossRef]
- Mergen, V.; Eberhard, M.; Manka, R.; Euler, A.; Alkadhi, H. First in-human quantitative plaque characterization with ultra-high resolution coronary photon-counting CT angiography. Front. Cardiovasc. Med. 2022, 9, 981012. [Google Scholar] [CrossRef] [PubMed]
- Baturin, P.; Alivov, Y.; Molloi, S. Spectral CT imaging of vulnerable plaque with two independent biomarkers. Phys. Med. Biol. 2012, 57, 4117–4138. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; Trotta, M.C.; Sasso, F.C.; Sacra, C.; Carpinella, G.; Mauro, C.; Minicucci, F.; Calabrò, P.; D’Amico, M.; D’Ascenzo, F.; et al. SGLT2-inhibitors effects on the coronary fibrous cap thickness and MACEs in diabetic patients with inducible myocardial ischemia and multi vessels non-obstructive coronary artery stenosis. Cardiovasc. Diabetol. 2023, 22, 80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Gao, X.; Chen, T.; Zhang, H.; Zhang, X.; Xin, Y.; Shi, D.; Du, Y.; Xu, L.; Zhou, Y. Longitudinal assessment of coronary plaque regression related to sodium–glucose cotransporter-2 inhibitor using coronary computed tomography angiography. Cardiovasc. Diabetol. 2024, 23, 267. [Google Scholar] [CrossRef] [PubMed]
- Rakipovski, G.; Rolin, B.; Nøhr, J.; Klewe, I.; Frederiksen, K.S.; Augustin, R.; Hecksher-Sørensen, J.; Ingvorsen, C.; Polex-Wolf, J.; Knudsen, L.B. The GLP-1 Analogs Liraglutide and Semaglutide Reduce Atherosclerosis in ApoE−/− and LDLr−/− Mice by a Mechanism That Includes Inflammatory Pathways. JACC Basic Transl. Sci. 2018, 3, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Hamal, S.; Cherukuri, L.; Shaikh, K.; Kinninger, A.; Doshi, J.; Birudaraju, D.; Budoff, M.J. Effect of semaglutide on coronary atherosclerosis progression in patients with type II diabetes: Rationale and design of the semaglutide treatment on coronary progression trial. Coron. Artery Dis. 2020, 31, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Montarello, N.J.; Singh, K.; Sinhal, A.; Wong, D.T.L.; Alcock, R.; Rajendran, S.; Dautov, R.; Barlis, P.; Patel, S.; Nidorf, S.M.; et al. Assessing the Impact of Colchicine on Coronary Plaque Phenotype After Myocardial Infarction with Optical Coherence Tomography: Rationale and Design of the COCOMO-ACS Study. Cardiovasc. Drugs Ther. 2022, 36, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Nerlekar, N.; Brown, A.J.; Muthalaly, R.G.; Talman, A.; Hettige, T.; Cameron, J.D.; Wong, D.T.L. Association of Epicardial Adipose Tissue and High-Risk Plaque Characteristics: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2017, 6, e006379. [Google Scholar] [CrossRef]
- Park, S.S.; Jung, J.; Mintz, G.S.; Jin, U.; Park, J.S.; Park, B.; Shin, H.B.; Seo, K.W.; Yang, H.M.; Lim, H.S.; et al. Epicardial Adipose Tissue Thickness Is Related to Plaque Composition in Coronary Artery Disease. Diagnostics 2022, 12, 2836. [Google Scholar] [CrossRef]
- Guglielmo, M.; Penso, M.; Carerj, M.L.; Giacari, C.M.; Volpe, A.; Fusini, L.; Baggiano, A.; Mushtaq, S.; Annoni, A.; Cannata, F.; et al. DEep LearnIng-based QuaNtification of epicardial adipose tissue predicts MACE in patients undergoing stress CMR. Atherosclerosis 2024, 117549. [Google Scholar] [CrossRef] [PubMed]
- Walpot, J.; Van Herck, P.; Van de Heyning, C.M.; Bosmans, J.; Massalha, S.; Malbrain, L.N.G.; Heidbuchel, H.; Inácio, J.R. Computed tomography measured epicardial adipose tissue and psoas muscle attenuation: New biomarkers to predict major adverse cardiac events (MACE) and mortality in patients with heart disease and critically ill patients. Part I: Epicardial adipose tissue. Anaesthesiol. Intensive Ther. 2023, 55, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, L.; Pavon, A.G.; Angeli, F.; Tuttolomondo, D.; Belmonte, M.; Armillotta, M.; Sansonetti, A.; Foà, A.; Paolisso, P.; Baggiano, A.; et al. The Role of Non-Invasive Multimodality Imaging in Chronic Coronary Syndrome: Anatomical and Functional Pathways. Diagnostics 2023, 13, 2083. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitriadis, K.; Pyrpyris, N.; Theofilis, P.; Mantzouranis, E.; Beneki, E.; Kostakis, P.; Koutsopoulos, G.; Aznaouridis, K.; Aggeli, K.; Tsioufis, K. Computed Tomography Angiography Identified High-Risk Coronary Plaques: From Diagnosis to Prognosis and Future Management. Diagnostics 2024, 14, 1671. https://doi.org/10.3390/diagnostics14151671
Dimitriadis K, Pyrpyris N, Theofilis P, Mantzouranis E, Beneki E, Kostakis P, Koutsopoulos G, Aznaouridis K, Aggeli K, Tsioufis K. Computed Tomography Angiography Identified High-Risk Coronary Plaques: From Diagnosis to Prognosis and Future Management. Diagnostics. 2024; 14(15):1671. https://doi.org/10.3390/diagnostics14151671
Chicago/Turabian StyleDimitriadis, Kyriakos, Nikolaos Pyrpyris, Panagiotis Theofilis, Emmanouil Mantzouranis, Eirini Beneki, Panagiotis Kostakis, George Koutsopoulos, Konstantinos Aznaouridis, Konstantina Aggeli, and Konstantinos Tsioufis. 2024. "Computed Tomography Angiography Identified High-Risk Coronary Plaques: From Diagnosis to Prognosis and Future Management" Diagnostics 14, no. 15: 1671. https://doi.org/10.3390/diagnostics14151671






