Diagnostic Yield of Trio Whole-Genome Sequencing in Children with Undiagnosed Developmental Delay or Congenital Anomaly: A Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations and Patient Consents
2.2. Study Participants and Design
2.3. WGS and Data Analysis
3. Results
3.1. Clinical Characteristics of Patients
3.2. Diagnostic Yield of WGS after Chromosome Analysis, CES, and CMA
3.3. Analysis of the Variants Identified through WGS
3.4. Factors Contributing to Non-Diagnosis by Chromosome Analysis, CMA, and CES
3.5. Genetic Diagnosis and Impact on Medical Care (Treatment Impact)
3.6. Some Case Presentations on Variants Identified through WGS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shevell, M.; Ashwal, S.; Donley, D.; Flint, J.; Gingold, M.; Hirtz, D.; Majnemer, A.; Noetzel, M.; Sheth, R.D.; Quality Standards Subcommittee of the American Academy; et al. Practice parameter: Evaluation of the child with global developmental delay: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2003, 60, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, K.; Polke, J.; Hagelstrom, R.T.; Dolzhenko, E.; Pasko, D.; Thomas, E.R.A.; Daugherty, L.C.; Kasperaviciute, D.; Smith, K.R.; WGS for Neurological Diseases Group; et al. Whole genome sequencing for the diagnosis of neurological repeat expansion disorders in the UK: A retrospective diagnostic accuracy and prospective clinical validation study. Lancet Neurol. 2022, 21, 234–245. [Google Scholar] [CrossRef] [PubMed]
- De Cario, R.; Kura, A.; Suraci, S.; Magi, A.; Volta, A.; Marcucci, R.; Gori, A.M.; Pepe, G.; Giusti, B.; Sticchi, E. Sanger Validation of High-Throughput Sequencing in Genetic Diagnosis: Still the Best Practice? Front. Genet. 2020, 11, 592588. [Google Scholar] [CrossRef]
- Arteche-Lopez, A.; Avila-Fernandez, A.; Romero, R.; Riveiro-Alvarez, R.; Lopez-Martinez, M.A.; Gimenez-Pardo, A.; Velez-Monsalve, C.; Gallego-Merlo, J.; Garcia-Vara, I.; Almoguera, B.; et al. Sanger sequencing is no longer always necessary based on a single-center validation of 1109 NGS variants in 825 clinical exomes. Sci. Rep. 2021, 11, 5697. [Google Scholar] [CrossRef] [PubMed]
- Manickam, K.; McClain, M.R.; Demmer, L.A.; Biswas, S.; Kearney, H.M.; Malinowski, J.; Massingham, L.J.; Miller, D.; Yu, T.W.; Hisama, F.M. Exome and genome sequencing for pediatric patients with congenital anomalies or intellectual disability: An evidence-based clinical guideline of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2021, 23, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Cohen, J.S.; Vernon, H.; Barañano, K.; McClellan, R.; Jamal, L.; Naidu, S.; Fatemi, A. Clinical whole exome sequencing in child neurology practice. Ann. Neurol. 2014, 76, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.F.; Chi, C.S.; Tsai, C.R. Diagnostic yield and treatment impact of whole-genome sequencing in paediatric neurological disorders. Dev. Med. Child. Neurol. 2021, 63, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Deignan, J.L.; Dorrani, N.; Strom, S.P.; Kantarci, S.; Quintero-Rivera, F.; Das, K.; Toy, T.; Harry, B.; Yourshaw, M. Clinical exome sequencing for genetic identification of rare Mendelian disorders. JAMA 2014, 312, 1880–1887. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.; Jang, D.H. Combining chromosomal microarray and clinical exome sequencing for genetic diagnosis of intellectual disability. Sci. Rep. 2023, 13, 22807. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Peng, J.; Liang, D.; Ye, X.; Xu, N.; Chen, L.; Yan, D.; Zhang, H.; Xiao, B.; Qiu, W. Genome sequencing demonstrates high diagnostic yield in children with undiagnosed global developmental delay/intellectual disability: A prospective study. Hum. Mutat. 2022, 43, 568–581. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.M.; Stark, Z.; Farnaes, L.; Tan, T.Y.; White, S.M.; Dimmock, D.; Kingsmore, S.F. Meta-analysis of the diagnostic and clinical utility of genome and exome sequencing and chromosomal microarray in children with suspected genetic diseases. NPJ Genom. Med. 2018, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Devanna, P.; Van de Vorst, M.; Pfundt, R.; Gilissen, C.; Vernes, S.C. Genome-wide investigation of an ID cohort reveals de novo 3′ UTR variants affecting gene expression. Human. Genet. 2018, 137, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Wanke, K.A.; Devanna, P.; Vernes, S.C. Understanding neurodevelopmental disorders: The promise of regulatory variation in the 3′ UTRome. Biol. Psychiatry 2018, 83, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Stark, Z.; Schofield, D.; Martyn, M.; Rynehart, L.; Shrestha, R.; Alam, K.; Lunke, S.; Tan, T.Y.; Gaff, C.L.; White, S.M. Does genomic sequencing early in the diagnostic trajectory make a difference? A follow-up study of clinical outcomes and cost-effectiveness. Genet. Med. 2019, 21, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Schobers, G.; Schieving, J.H.; Yntema, H.G.; Pennings, M.; Pfundt, R.; Derks, R.; Hofste, T.; de Wijs, I.; Wieskamp, N.; van den Heuvel, S.; et al. Reanalysis of exome negative patients with rare disease: A pragmatic workflow for diagnostic applications. Genome Med. 2022, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Honda, A.; Ewans, L.; McGaughran, J.; Burnett, L.; Law, M.; Phan, T.G. Recommendations for next generation sequencing data reanalysis of unsolved cases with suspected Mendelian disorders: A systematic review and meta-analysis. Genet. Med. 2022, 24, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Nambot, S.; Thevenon, J.; Kuentz, P.; Duffourd, Y.; Tisserant, E.; Bruel, A.-L.; Mosca-Boidron, A.-L.; Masurel-Paulet, A.; Lehalle, D.; Jean-Marçais, N. Clinical whole-exome sequencing for the diagnosis of rare disorders with congenital anomalies and/or intellectual disability: Substantial interest of prospective annual reanalysis. Genet. Med. 2018, 20, 645–654. [Google Scholar] [CrossRef] [PubMed]

| N (Total = 52) | Whole Genome Sequencing | ||
|---|---|---|---|
| Diagnosis (+) | Diagnosis (−) | ||
| Age (mean), yr | 6.5 | ||
| Sex | |||
| Male | 33 | 6 | 27 |
| Female | 19 | 4 | 15 |
| Developmental delay | 51 | ||
| Intellectual disability | 42 | 7 | 35 |
| Gross motor delay | 25 | 8 | 17 |
| Congenital anomaly | 12 | 3 | 9 |
| Brain MRI abnormality (N = 48) | 8 | 3 | 5 |
| Combined phenotype * | |||
| Skeletal | 10 | 2 | 8 |
| Ophthalmology | 7 | 3 | 4 |
| Gastrointestinal | 3 | 2 | 1 |
| Cardiac | 2 | 1 | 1 |
| Epilepsy | 2 | 0 | 2 |
| Gynecology | 1 | 1 | 0 |
| Patient No. | Sex/Age | Genotype | Inheritance (Origin) | Disorder | Phenotypes | Reasons for Non-Detection in Previous Tests * |
|---|---|---|---|---|---|---|
| 1 | F/18 | ASXL3 NC_000018.10: g.33740509_33743845delins AGAAGCCTAGGTGTAC, 3.3 kb | AD (De novo) | Bainbridge-Ropers syndrome | ID, gross motor delay, hypotonia, speech delay, dysmorphic face | Small structural variant |
| 2 | M/3 | SPEN chr1:g.15928344C>T, NM_015001.3:c.2104C>T, (p.Arg702Ter) | AD (De novo) | Radio-Tartaglia syndrome | Gross motor delay, pes cavus, 4th toe underlapping | Identified in genes not included in the CES panel |
| 3 | M/4 | WBP11 chr12:g.14787469G>GC, NM_016312.3:c.1521dup, (p.Arg508AlafsTer41) | AD (De novo) | VCTERL (Vertebral, cardiac, tracheoesophageal, renal, and limb defects) syndrome | Cervical spine fusion (C2–C3), spina bifida (C3, C4, C6), Sprengel deformity, urachal cyst | |
| 4 | M/2 | GRIN2B chr12:g.13571953A> AGGTCTCTGGAACT, NM_000834.5:c.2011-2_2021dup, (p.ASn675ValfsTer5) | AD (De novo) | GRIN2B-Related Neurodevelopmental Disorder | ID, gross motor delay, hypotonia, ptosis, microcephaly (Brain MRI) mild brain atrophy, hypomyelination | |
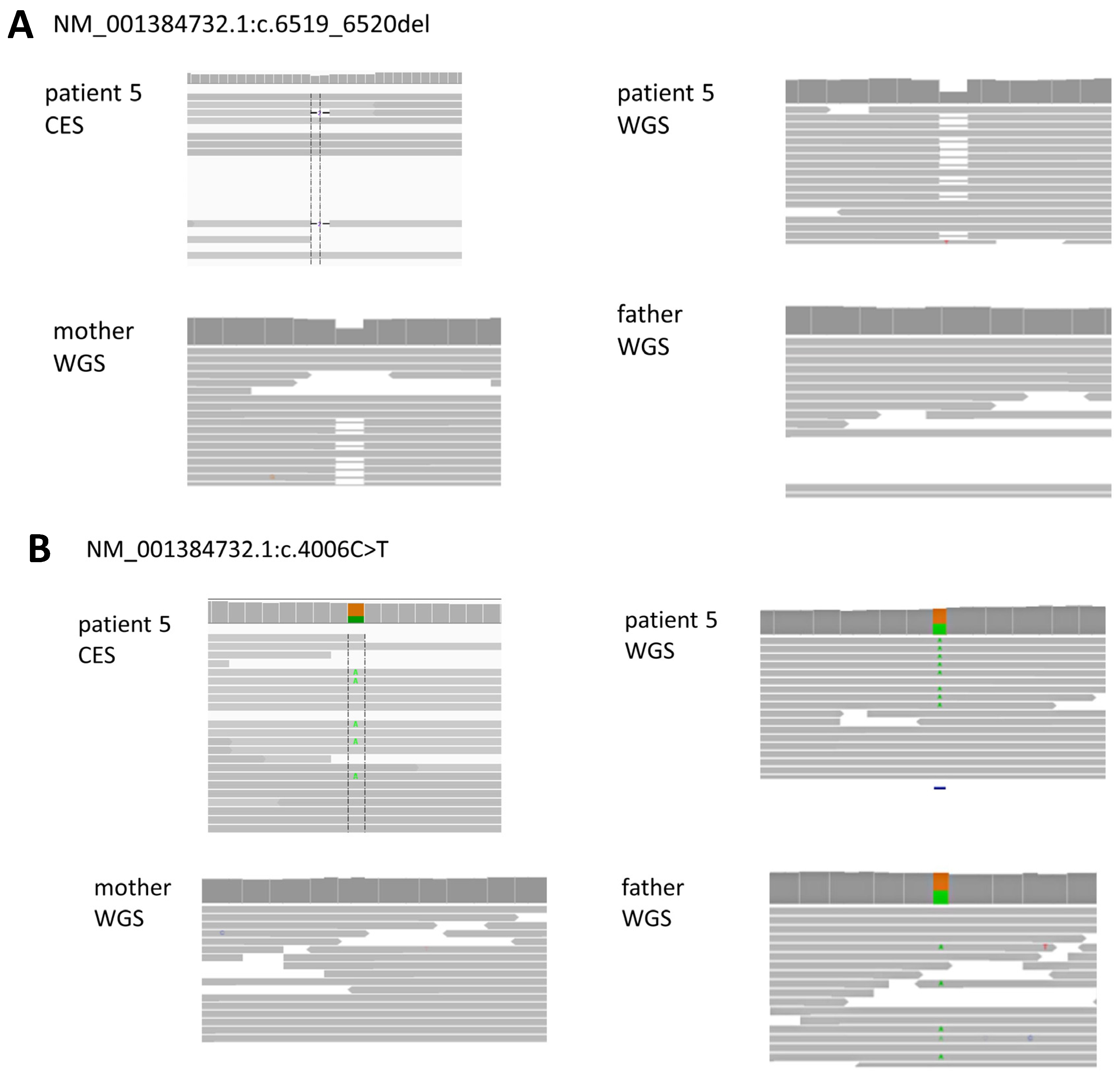
| 5 | F/27 | CPLANE1 chr5:g.37187488G>A NM_001384732.1:c.4006C>T, (p.Arg1336Trp)pat CPLANE1 chr5:g.37169503CCT>C, NM_001384732.1:c.6519_6520del, (p.Gly2174ThrfsTer37)mat | AR (Parental) | Joubert syndrome 17 | ID, gross motor delay, ataxia, dizziness, disconnected speech, severe articulation disorder (Brain MRI) cerebellum (superior vermis) hypoplasia | Insufficient depth |
| 6 | M/3 | TRIP12 chr2:g.229771526G>A, NM_001348323.3:c.5801C>T, (p.Pro1934Leu) | AD (unknown) | Clark-Baraitser syndrome (Intellectual developmental disorder, autosomal dominant 49) | ID, autism spectrum disorder | Low VAF (11.2%, 11/98 depth) |
| 7 | M/3 | MEIS2 chr15:g.36950363GCTAA>G, NM_170675.5:c.934_937del, (p.Leu312ArgfsTer11) | AD (De novo) | Cleft palate, cardiac defects, and impaired intellectual development | ID, gross motor delay, hypotonia, speech delay, dysmorphic face, VSD, scoliosis, wide 1–2 toe web | Low VAF (6.3%, 4/64 depth) |
| 8 | M/12 | H1-4 chr6:g.26156798G>GA, NM_005321.3:c.410dup, (p.Pro138AlafsTer58) | AD (De novo) | Rahmman syndrome | ID, gross motor delay, hypotonia, congenital megacolon | Newly identified gene |
| 9 | F/12 | SOX2 chr3:g.181712670G>T, NM_003106.4:c.310G>T, (p.Glu104Ter) | AD (De novo) | SOX2 Disorder | ID, gross motor delay, dyskinesia, spasticity, myopia, retinal disorder, hypoplastic uterus, ovarian agenesis | Variant interpretation issues (reported phenotype differs from proband’s clinical symptoms) |
| 10 | F/4 | NT5C2 chr10:g.103093137A>C, NM_001351169.2:c.1159+2T>G p.? pat NT5C2 chr:10:g.103093192A>G, NM_001351169.2:c.1106T>C, (p.Phe369Ser)mat | AR (Parental) | Spastic paraplegia 45 | Gross motor delay, spasticity (Brain MRI) corpus callosum hypoplasia | Differing interpretations of VUS among clinicians |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Lee, J.; Kim, M.; Jang, D.-H. Diagnostic Yield of Trio Whole-Genome Sequencing in Children with Undiagnosed Developmental Delay or Congenital Anomaly: A Prospective Cohort Study. Diagnostics 2024, 14, 1680. https://doi.org/10.3390/diagnostics14151680
Kim J, Lee J, Kim M, Jang D-H. Diagnostic Yield of Trio Whole-Genome Sequencing in Children with Undiagnosed Developmental Delay or Congenital Anomaly: A Prospective Cohort Study. Diagnostics. 2024; 14(15):1680. https://doi.org/10.3390/diagnostics14151680
Chicago/Turabian StyleKim, Jaewon, Jaewoong Lee, Myungshin Kim, and Dae-Hyun Jang. 2024. "Diagnostic Yield of Trio Whole-Genome Sequencing in Children with Undiagnosed Developmental Delay or Congenital Anomaly: A Prospective Cohort Study" Diagnostics 14, no. 15: 1680. https://doi.org/10.3390/diagnostics14151680





