The Importance of Topographical Recognition of Pulmonary Arteries in Diagnostics and Treatment of CTEPH, Based on an Analysis of a Dissected Case Model—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Dissection Procedure
2.3. Evaluation of the Arterial Branching, Measurements and Comparisons
2.4. Statistical Methods
3. Results
3.1. Branching Variant of the Right Lung
3.2. Branching Variant of the Left Lung
3.3. Morphological Parameters of the Dissected Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aggarwal, V.; Giri, J.; Visovatti, S.H.; Mahmud, E.; Matsubara, H.; Madani, M.; Rogers, F.; Gopalan, D.; Rosenfield, K.; McLaughlin, V.V.; et al. Status and Future Directions for Balloon Pulmonary Angioplasty in Chronic Thromboembolic Pulmonary Disease With and Without Pulmonary Hypertension: A Scientific Statement From the American Heart Association. Circulation 2024, 149, e1090–e1107. [Google Scholar] [CrossRef] [PubMed]
- Cory, R.A.S.; Valentine, E.J. Varying Patterns of the Lobar Branches of the Pulmonary Artery: A Study of 524 Lungs and Lobes Seen at Operation on 426 Patients. Thorax 1959, 14, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Wąsik, J.; Tubbs, R.S.; Zielinska, N.; Karauda, P.; Olewnik, Ł. Lung Segments from Anatomy to Surgery. Folia. Morphol. 2024, 83, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Armitage, M.N.; Mughal, A.Z.; Huntley, C.C.; Lasserson, D.; Newnham, M. A Multicentre Observational Study of the Prevalence, Management, and Outcomes of Subsegmental Pulmonary Embolism. J. Thromb. Thrombolysis 2023, 55, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Rahaghi, F.N.; Ross, J.C.; Agarwal, M.; González, G.; Come, C.E.; Diaz, A.A.; Vegas-Sánchez-Ferrero, G.; Hunsaker, A.; Estépar, R.S.J.; Waxman, A.B.; et al. Pulmonary Vascular Morphology as an Imaging Biomarker in Chronic Thromboembolic Pulmonary Hypertension. Pulm. Circ. 2016, 6, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Leber, L.; Beaudet, A.; Muller, A. Epidemiology of Pulmonary Arterial Hypertension and Chronic Thromboembolic Pulmonary Hypertension: Identification of the Most Accurate Estimates from a Systematic Literature Review. Pulm. Circ. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rose-Jones, L.J.; Mclaughlin, V.V. Pulmonary Hypertension: Types and Treatments. Curr. Cardiol. Rev. 2015, 11, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Madani, M.M.; Nakanishi, N.; Meyer, B.; Cebotari, S.; Rubin, L.J. Chronic Thromboembolic Pulmonary Hypertension. Lancet Respir. Med. 2014, 2, 573–582. [Google Scholar] [CrossRef]
- Brenot, P.; Jaïs, X.; Taniguchi, Y.; Garcia Alonso, C.; Gerardin, B.; Mussot, S.; Mercier, O.; Fabre, D.; Parent, F.; Jevnikar, M.; et al. French Experience of Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension. Eur. Respir. J. 2019, 53, 1802095. [Google Scholar] [CrossRef]
- Wiedenroth, C.B.; Deissner, H.; Adameit, M.S.D.; Kriechbaum, S.D.; Ghofrani, H.-A.; Breithecker, A.; Haas, M.; Roller, F.; Rolf, A.; Hamm, C.W.; et al. Complications of Balloon Pulmonary Angioplasty for Inoperable Chronic Thromboembolic Pulmonary Hypertension: Impact on the Outcome. J. Heart Lung Transplant. 2022, 41, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, N.; Kawakami, T.; Satoh, T.; Matsubara, H.; Nakanishi, N.; Ogino, H.; Tamura, Y.; Tsujino, I.; Ogawa, A.; Sakao, S.; et al. Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension: A Systematic Review. Respir. Investig. 2018, 56, 332–341. [Google Scholar] [CrossRef]
- Pereira, A.R.; Cale, R.; Ferreira, F.; Alegria, S.; Sebaiti, D.; Martinho, M.; Repolho, D.; Vitorino, S.; Santos, P.; Loureiro, M.J.; et al. Complications of Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Disease According to the Classification Proposed by the 6th World Symposium on Pulmonary Hypertension. Eur. Heart J. 2021, 42, ehab724-1916. [Google Scholar] [CrossRef]
- Lang, I.; Meyer, B.C.; Ogo, T.; Matsubara, H.; Kurzyna, M.; Ghofrani, H.-A.; Mayer, E.; Brenot, P. Balloon Pulmonary Angioplasty in Chronic Thromboembolic Pulmonary Hypertension. Eur. Respir. Rev. 2017, 26, 160119. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Yamashita, J.; Ikeda, S.; Nakajima, Y.; Kasahara, T.; Sasaki, Y.; Suzuki, S.; Takahashi, L.; Komatsu, I.; Murata, N.; et al. Predictors of Procedural Complications in Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension. J. Cardiol. 2023, 82, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Barberini, F.; Brunone, F.A. A “conservative” method of thoracic wall dissection: A proposal for teaching human anatomy. Ital. J. Anat. Embryol. 2008, 113, 187–195. [Google Scholar]
- Michaud, E.; Pan, M.; Lakhter, V.; Reddy Maligireddy, A.; Hyder, S.N.; Patel, N.; Moles, V.M.; McLaughlin, V.V.; Agarwal, P.P.; Visovatti, S.H.; et al. Anatomical Variations in Pulmonary Arterial Branches in Patients Undergoing Evaluation for Chronic Thromboembolic Pulmonary Hypertension. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 101108. [Google Scholar] [CrossRef]
- Zhou, Q.; Tan, W.; Li, Q.; Li, B.; Zhou, L.; Liu, X.; Yang, J.; Zhao, D. A New Segment Method for Pulmonary Artery and Vein. Health Inf. Sci. Syst. 2023, 11, 47. [Google Scholar] [CrossRef]
- Tellapuri, S.; Park, H.S.; Kalva, S.P. Pulmonary Arteriovenous Malformations. Int. J. Cardiovasc. Imaging 2019, 35, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.; Tay, E.; Sia, C.; Poh, K. Chronic Thromboembolic Pulmonary Hypertension: A Review. Singap. Med. J. 2021, 62, 318–325. [Google Scholar] [CrossRef]
- Zafar, H.; Neelam-Naganathan, D.; Middleton, J.T.; Binmahfooz, S.K.; Battersby, C.; Rogers, D.; Swift, A.J.; Rothman, A.M.K. Anatomical Characterization of Pulmonary Artery and Implications to Pulmonary Artery Pressure Monitor Implantation. Sci. Rep. 2023, 13, 20528. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Madani, M.M.; Mahmud, E.; Kim, N.H. Evaluation and Management of Chronic Thromboembolic Pulmonary Hypertension. Chest 2023, 164, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Nishimura, T.; Morikawa, M. Quantitative Evaluation of Pulmonary Hypertension Using 4D Flow MRI: A Retrospective Study. Heliyon 2024, 10, e31177. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.D.; Alpard, S.K.; Deyo, D.J.; Lick, S.D.; Traber, D.L.; Zwischenberger, J.B. Anatomic Study of the Pulmonary Artery as a Conduit for an Artificial Lung. ASAIO J. 2001, 47, 34–36. [Google Scholar] [CrossRef]
- Zhai, Z.; Boon, G.J.A.M.; Staring, M.; Van Dam, L.F.; Kroft, L.J.M.; Hernández Girón, I.; Ninaber, M.K.; Bogaard, H.J.; Meijboom, L.J.; Vonk Noordegraaf, A.; et al. Automated Quantification of the Pulmonary Vasculature in Pulmonary Embolism and Chronic Thromboembolic Pulmonary Hypertension. Pulm. Circ. 2023, 13, e12223. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, N. Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension. Cardiovasc. Interv. Ther. 2020, 35, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Stricker, H. Chronic Thromboembolic Pulmonary Hypertension—A Diagnostic and Therapeutic Update. Vasa 2016, 45, 195–199. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, J.Z.; Poch, D.S.; Ang, L.; Mahmud, E.; Kim, N.H. Balloon Pulmonary Angioplasty in the Current Era of CTEPH Treatment: How Did We Get Here? Pulm. Circ. 2023, 13, e12312. [Google Scholar] [CrossRef]
- Mahammedi, A.; Oshmyansky, A.; Hassoun, P.M.; Thiemann, D.R.; Siegelman, S.S. Pulmonary Artery Measurements in Pulmonary Hypertension: The Role of Computed Tomography. J. Thorac. Imaging 2013, 28, 96–103. [Google Scholar] [CrossRef]
- Robbins, I.M.; Pugh, M.E.; Hemnes, A.R. Update on Chronic Thromboembolic Pulmonary Hypertension. Trends Cardiovasc. Med. 2017, 27, 29–37. [Google Scholar] [CrossRef]
- Tunariu, N.; Gibbs, S.J.R.; Win, Z.; Gin-Sing, W.; Graham, A.; Gishen, P.; AL-Nahhas, A. Ventilation–Perfusion Scintigraphy Is More Sensitive than Multidetector CTPA in Detecting Chronic Thromboembolic Pulmonary Disease as a Treatable Cause of Pulmonary Hypertension. J. Nucl. Med. 2007, 48, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic Definitions and Updated Clinical Classification of Pulmonary Hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef] [PubMed]
- Teerapuncharoen, K.; Bag, R. Chronic Thromboembolic Pulmonary Hypertension. Lung 2022, 200, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; King, C.S.; Brown, A.W.; Albano, M.C.; Atkins, M.; Sheridan, M.J.; Ahmad, S.; Newton, K.M.; Weir, N.; Shlobin, O.A.; et al. Pulmonary Artery Size as a Predictor of Pulmonary Hypertension and Outcomes in Patients with Chronic Obstructive Pulmonary Disease. Respir. Med. 2014, 108, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Cortopassi, I.O.; Gosangi, B.; Asch, D.; Bader, A.S.; Gange, C.P.; Rubinowitz, A.N. Diseases of the Pulmonary Arteries: Imaging Appearances and Pearls. Clin. Imaging 2022, 91, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Olsson, K.M.; Meyer, B.; Hinrichs, J.; Vogel-Claussen, J.; Hoeper, M.M.; Cebotari, S. Chronic Thromboembolic Pulmonary Hypertension. Dtsch. Arztebl. Int. 2014, 111, 856. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.W.; Lichtenberger, J.P.; Wu, C.C. Acquired Abnormalities of the Pulmonary Arteries. Am. J. Roentgenol. 2014, 202, W415–W421. [Google Scholar] [CrossRef] [PubMed]
- Fourdrain, A.; De Dominicis, F.; Blanchard, C.; Iquille, J.; Lafitte, S.; Beuvry, P.-L.; Michel, D.; Merlusca, G.; Havet, E.; Berna, P. Three-Dimensional CT Angiography of Anatomic Variations in the Pulmonary Arterial Tree. Surg. Radiol. Anat. 2018, 40, 45–53. [Google Scholar] [CrossRef]
- Kandathil, A.; Chamarthy, M. Pulmonary Vascular Anatomy & Anatomical Variants. Cardiovasc. Diagn. Ther. 2018, 8, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Liu, C. Demarcation of Arteriopulmonary Segments: A Novel and Effective Method for the Identification of Pulmonary Segments. J. Int. Med. Res. 2021, 49, 030006052110143. [Google Scholar] [CrossRef]
- Martín-Ruiz, S.; Gutiérrez-Collar, C.; Forcén Vicente De Vera, E.; Bernabé-Barrios, M.J.; De Blas, C.S.; Konschake, M.; Ramón Sañudo, J.; Maranillo, E. The Bronchial Segmentation and Its Anatomical Variations. A Clinical-Anatomic and Bronchoscopy Study. Ann. Anat.-Anat. Anz. 2021, 235, 151677. [Google Scholar] [CrossRef]
- Chen, Z.; Chu, X.; Zhang, J.; Fu, R.; Kang, J.; Chen, J.; Jiang, B.; Wu, Y.; Zhong, W.; Nie, Q. The Regularity of Anatomical Variations of Dominant Pulmonary Segments in the Right Upper Lobe. Thorac. Cancer 2023, 14, 462–469. [Google Scholar] [CrossRef]
- Akdeniz, A.; Kara, E. Determining Tracheobronchial Tree with Anatomical Dissection: 204 Cases. Turk. Thorac. J. 2021, 22, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Murlimanju, B.V.; Massand, A.; Madhyastha, S.; Pai, M.M.; Prabhu, L.V.; Saralaya, V.V. Anatomical Variations of the Arrangement of Structures at the Pulmonary Hilum: A Cadaveric Study. Surg. Radiol. Anat. 2017, 39, 51–56. [Google Scholar] [CrossRef] [PubMed]
- George, B.M.; Nayak, S.B.; Marpalli, S. Morphological Variations of the Lungs: A Study Conducted on Indian Cadavers. Anat. Cell Biol. 2014, 47, 253. [Google Scholar] [CrossRef]
- Nagashima, T.; Shimizu, K.; Ohtaki, Y.; Obayashi, K.; Kakegawa, S.; Nakazawa, S.; Kamiyoshihara, M.; Igai, H.; Takeyoshi, I. An Analysis of Variations in the Bronchovascular Pattern of the Right Upper Lobe Using Three-Dimensional CT Angiography and Bronchography. Gen. Thorac. Cardiovasc. Surg. 2015, 63, 354–360. [Google Scholar] [CrossRef]
- Fan, K.; Feng, J.; Li, Y.; Liu, B.; Tao, R.; Wang, Z.; Zhao, H.; Zhang, Y.; Wang, J.; Zhang, G. Application of Three-dimensional Reconstruction of Left Upper Lung Lobes in Anatomical Segmental Resection. Thorac. Cancer 2022, 13, 1176–1183. [Google Scholar] [CrossRef]
- Barrett, R.J.; Day, J.C.; Tuttle, W.M. The Arterial Distribution to the Left Upper Pulmonary Lobe. J. Thorac. Surg. 1956, 32, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Henderson, R.; Horsfield, K.; Harding, K.; Cumming, G. Morphometry of the Human Pulmonary Arterial Tree. Circ. Res. 1973, 33, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Horsfield, K. Axial Pathways Compared with Complete Data in Morphological Studies of the Lung. Respir. Physiol. 1984, 55, 317–324. [Google Scholar] [CrossRef]
- Huang, W.; Yen, R.T.; McLaurine, M.; Bledsoe, G. Morphometry of the Human Pulmonary Vasculature. J. Appl. Physiol. 1996, 81, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Amore, D.; Casazza, D.; Caterino, U.; Imitazione, P.; Saglia, A.; Izzo, A.; Rispoli, M.; Curcio, C. Variations in the Branching Patterns of Pulmonary Artery during Thoracoscopic Pulmonary Resection. Surg. Radiol. Anat. 2021, 43, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Salibe-Filho, W.; Araujo, T.L.S.; Melo, E.G.; Coimbra, L.B.C.T.; Lapa, M.S.; Acencio, M.M.P.; Freitas-Filho, O.; Capelozzi, V.L.; Teixeira, L.R.; Fernandes, C.J.C.S.; et al. Shear Stress-Exposed Pulmonary Artery Endothelial Cells Fail to Upregulate HSP70 in Chronic Thromboembolic Pulmonary Hypertension. PLoS ONE 2020, 15, e0242960. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.; Darvish, A.; Akhavan, R.; Rad, M.P.; Farrokh, D.; Emadzadeh, M.; Dehghani, S. Decreased Pulmonary Artery Bifurcation Angle: A Novel Imaging Criterion for the Diagnosis of Chronic Pulmonary Thromboembolism. Iran. J. Med. Sci. 2022, 47, 360–366. [Google Scholar] [CrossRef]
- Velázquez, M.; Maneiro, N.; Albarrán, A.; Sarnago, F.; Huertas, S.; Cruz-Utrilla, A.; Hinojosa, W.; López-Gude, M.J.; Alonso, S.; Revilla, Y.; et al. Balloon Pulmonary Angioplasty Can Be an Effective and Safe Therapeutic Option in Non-Surgical Elderly Patients. Front. Cardiovasc. Med. 2022, 9, 1001518. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, M.; Maneiro, N.; Lareo, A.; Albarrán, A.; Huertas, S.; Olazábal, A.P.; Delgado, J.F.; Alonso, S.; Sarnago, F.; García Tejada, J.; et al. Selective Segmental Pulmonary Angiography: Anatomical, Technical and Safety Aspects of a Must-Learn Technique in Times of Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension. J. Clin. Med. 2021, 10, 3358. [Google Scholar] [CrossRef]
- Xie, Z.; Zhu, X.; Li, F.; Zhao, J.; Li, C. Pulmonary Arterial Anatomical Patterns: A Classification Scheme Based on Lobectomy and 3D-CTBA. Thorac. Cardiovasc. Surg. 2024. [Google Scholar] [CrossRef]
- Narechania, S.; Renapurkar, R.; Heresi, G.A. Mimickers of Chronic Thromboembolic Pulmonary Hypertension on Imaging Tests: A Review. Pulm. Circ. 2020, 10, 2045894019882620. [Google Scholar] [CrossRef]

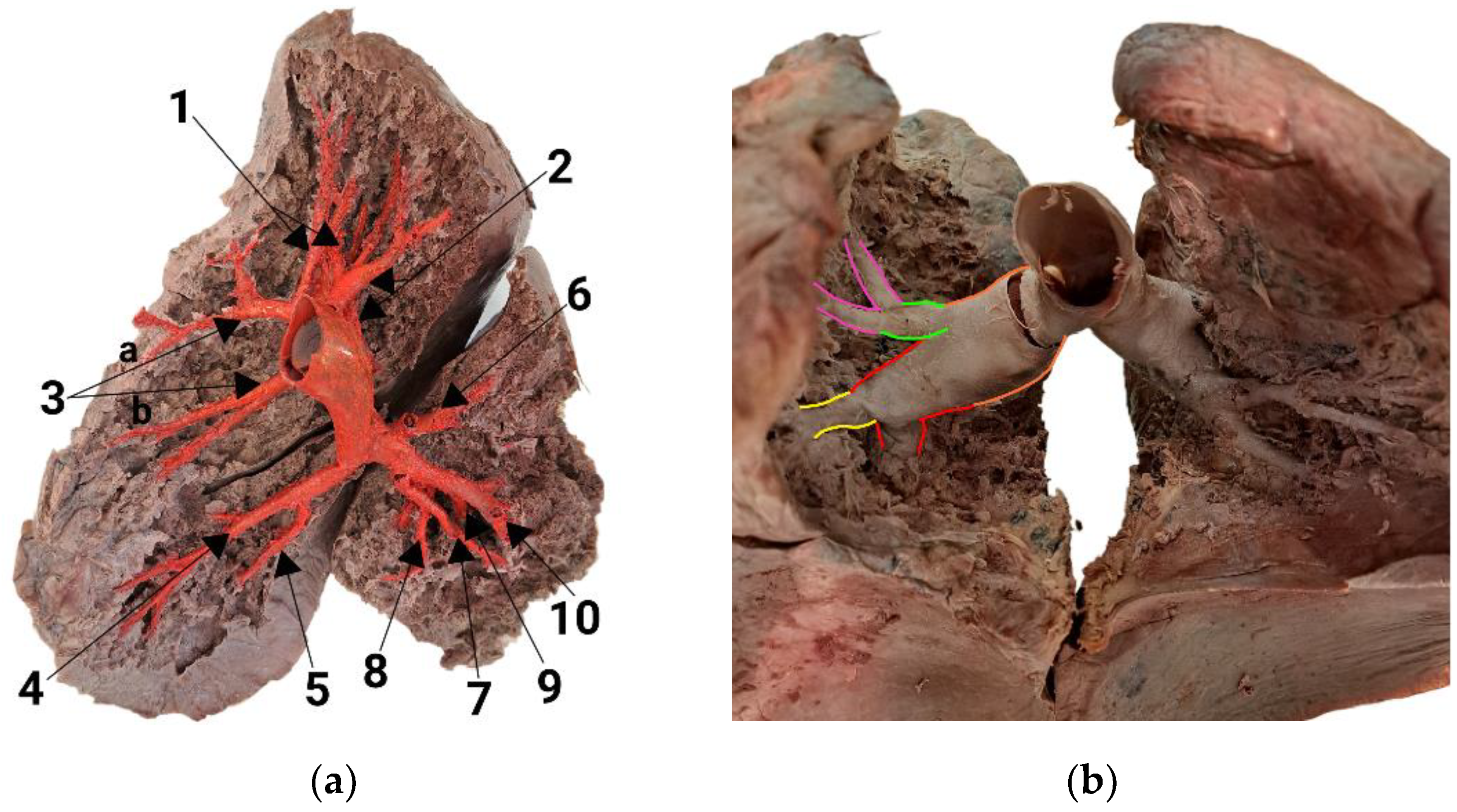
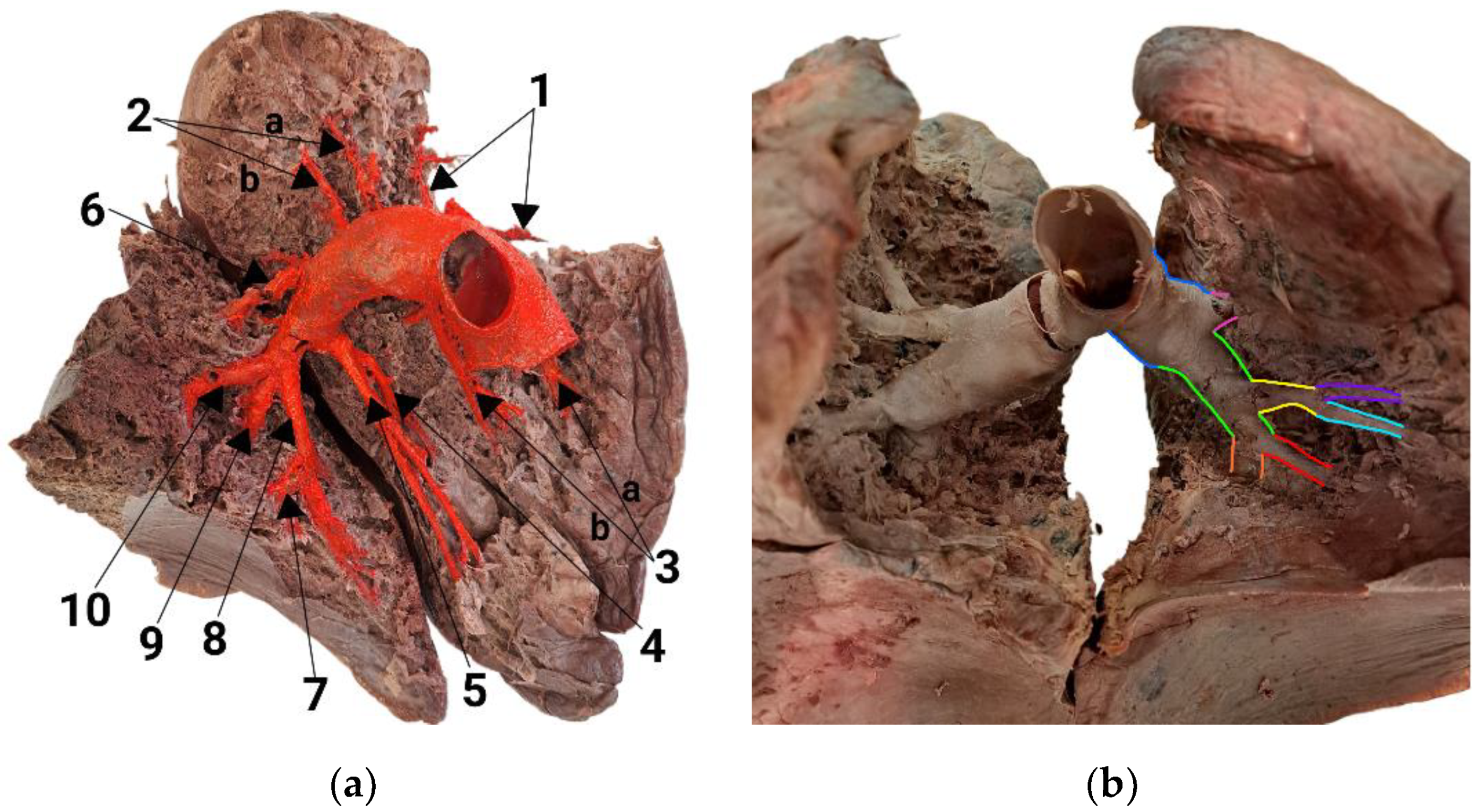
| Artery | Generation | Length, mm | Diameter, mm |
|---|---|---|---|
| Pulmonary trunk | 1 | 48.51 | 29.70 |
| Right pulmonary artery | 2 | 33.15 | 25.95 |
| A1, apical | 3 | 18.20 | 9.29 |
| A2, posterior | 3 | - 2 | 5.87 |
| A3, anterior 1 | 3 | 29.82 | 7.81 |
| A4, lateral | 4 | 53.91 | 5.63 |
| A5, medial | 4 | 36.84 | 4.84 |
| A6, superior | 3 | 17.87 | 7.43 |
| A7, medial basal | 4 | 15.93 | 4.75 |
| A8, anterior basal | 4 | 16.81 | 7.20 |
| A9, lateral basal | 5 | 37.68 | 4.34 |
| A10, posterior basal | 5 | 20.46 | 6.92 |
| Artery | Generation | Length, mm | Diameter, mm |
|---|---|---|---|
| Pulmonary trunk | 1 | 48.51 | 29.70 |
| Left pulmonary artery | 2 | 29.46 | 25.87 |
| A1, apical | 3 | 15.98 | 4.48 |
| A2, posterior 1 | 3 | 14.77 | 3.64 |
| A3, anterior 1 | 3 | 9.09 | 7.48 |
| A4, superior lingular | 4 | 19.34 | 4.79 |
| A5, inferior lingular | 4 | 15.87 | 4.76 |
| A6, superior | 3 | 19.09 | 6.65 |
| A7, medial basal | 5 | 12.02 | 5.25 |
| A8, anterior basal | 5 | 15.78 | 3.04 |
| A9, lateral basal | 4 | 11.26 | 6.33 |
| A10, posterior basal | 4 | 28.94 | 6.42 |
| Generation | Mean, mm | SD, mm | CV, % |
|---|---|---|---|
| 2 | 31.31 | 1.85 | 5.89 |
| 3 | 17.83 | 5.79 | 32.47 |
| 4 | 24.86 | 13.44 | 54.06 |
| 5 | 21.46 | 9.82 | 45.69 |
| Generation | Mean, mm | SD, mm | CV, % |
|---|---|---|---|
| 2 | 25.91 | 0.04 | 0.15 |
| 3 | 6.58 | 1.73 | 26.29 |
| 4 | 5.59 | 0.90 | 16.03 |
| 5 | 4.89 | 1.41 | 28.88 |
| Branching Angle Group | Mean Angle, Right Lung | Mean Angle, Left Lung |
|---|---|---|
| Off the interlobar artery | 85.0° | 77.0° |
| Between the segmental arteries | 57.5° | 38.0° |
| Between the subsegmental arteries | 28.0° | 33.6° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zicans, M.; Kazoka, D.; Pilmane, M.; Skride, A. The Importance of Topographical Recognition of Pulmonary Arteries in Diagnostics and Treatment of CTEPH, Based on an Analysis of a Dissected Case Model—A Pilot Study. Diagnostics 2024, 14, 1684. https://doi.org/10.3390/diagnostics14151684
Zicans M, Kazoka D, Pilmane M, Skride A. The Importance of Topographical Recognition of Pulmonary Arteries in Diagnostics and Treatment of CTEPH, Based on an Analysis of a Dissected Case Model—A Pilot Study. Diagnostics. 2024; 14(15):1684. https://doi.org/10.3390/diagnostics14151684
Chicago/Turabian StyleZicans, Matiss, Dzintra Kazoka, Mara Pilmane, and Andris Skride. 2024. "The Importance of Topographical Recognition of Pulmonary Arteries in Diagnostics and Treatment of CTEPH, Based on an Analysis of a Dissected Case Model—A Pilot Study" Diagnostics 14, no. 15: 1684. https://doi.org/10.3390/diagnostics14151684
APA StyleZicans, M., Kazoka, D., Pilmane, M., & Skride, A. (2024). The Importance of Topographical Recognition of Pulmonary Arteries in Diagnostics and Treatment of CTEPH, Based on an Analysis of a Dissected Case Model—A Pilot Study. Diagnostics, 14(15), 1684. https://doi.org/10.3390/diagnostics14151684







