A 10-Year Retrospective Cohort Study of Endometrial Cancer Outcomes and Associations with Lymphovascular Invasion: A Single-Center Study from Germany
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Patient Selection and Definitions
2.3. Data Collection
2.4. Statistical Analysis
2.5. Follow-Up
3. Results
4. Discussion
4.1. Analysis of Findings
4.2. Study Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bassette, E.; Ducie, J.A. Endometrial Cancer in Reproductive-Aged Females: Etiology and Pathogenesis. Biomedicines 2024, 12, 886. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Staples, J.N.; Rauh, L.; Peach, M.S.; Baker, W.D.; Modesitt, S.C. Endometrial cancer in an increasingly obese population: Exploring alternative options when surgery may not cut it. Gynecol. Oncol. Rep. 2018, 25, 30–34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Constantine, G.D.; Kessler, G.; Graham, S.; Goldstein, S.R. Increased Incidence of Endometrial Cancer Following the Women’s Health Initiative: An Assessment of Risk Factors. J. Womens Health 2019, 28, 237–243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuhn, T.M.; Dhanani, S.; Ahmad, S. An Overview of Endometrial Cancer with Novel Therapeutic Strategies. Curr. Oncol. 2023, 30, 7904–7919. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Restaino, S.; Paglietti, C.; Arcieri, M.; Biasioli, A.; Della Martina, M.; Mariuzzi, L.; Andreetta, C.; Titone, F.; Bogani, G.; Raimondo, D.; et al. Management of Patients Diagnosed with Endometrial Cancer: Comparison of Guidelines. Cancers 2023, 15, 1091. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, W. Molecular Classification of Endometrial Cancer and the 2023 FIGO Staging: Exploring the Challenges and Opportunities for Pathologists. Cancers 2023, 15, 4101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ogunmuyiwa, J.; Williams, V. Emerging Advances in Endometrial Cancer: Integration of Molecular Classification into Staging for Enhanced Prognostic Accuracy and Implications for Racial Disparities. Cancers 2024, 16, 1172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Broaddus, R.R.; Zhang, W. Identifying aggressive forms of endometrioid-type endometrial cancer: New insights into molecular subtyping. Expert Rev. Anticancer Ther. 2015, 15, 1–3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Corr, B.; Cosgrove, C.; Spinosa, D.; Guntupalli, S. Endometrial cancer: Molecular classification and future treatments. BMJ Med. 2022, 1, e000152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Addante, F.; d’Amati, A.; Santoro, A.; Angelico, G.; Inzani, F.; Arciuolo, D.; Travaglino, A.; Raffone, A.; D’Alessandris, N.; Scaglione, G.; et al. Mismatch Repair Deficiency as a Predictive and Prognostic Biomarker in Endometrial Cancer: A Review on Immunohistochemistry Staining Patterns and Clinical Implications. Int. J. Mol. Sci. 2024, 25, 1056. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anderson, C.; Bae-Jump, V.L.; Broaddus, R.R.; Olshan, A.F.; Nichols, H.B. Long-term Patterns of Excess Mortality among Endometrial Cancer Survivors. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1079–1088. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gottwald, L.; Pluta, P.; Piekarski, J.; Spych, M.; Hendzel, K.; Topczewska-Tylinska, K.; Nejc, D.; Bibik, R.; Korczyński, J.; Ciałkowska-Rysz, A. Long-term survival of endometrioid endometrial cancer patients. Arch. Med. Sci. 2010, 6, 937–944. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shin, D.W.; Jung, K.W.; Ha, J.; Bae, J. Conditional relative survival of patients with endometrial cancer: A Korean National Cancer Registry study. J. Gynecol. Oncol. 2022, 33, e23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kasius, J.C.; Pijnenborg, J.M.A.; Lindemann, K.; Forsse, D.; van Zwol, J.; Kristensen, G.B.; Krakstad, C.; Werner, H.M.J.; Amant, F. Risk Stratification of Endometrial Cancer Patients: FIGO Stage, Biomarkers and Molecular Classification. Cancers 2021, 13, 5848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Njoku, K.; Barr, C.E.; Crosbie, E.J. Current and Emerging Prognostic Biomarkers in Endometrial Cancer. Front. Oncol. 2022, 12, 890908. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sait, K.H.; Anfinan, N.; Sait, H.; Shamrani, H.; Sait, M. Overall and progression-free survival in endometrial carcinoma: A single-center retrospective study of patients treated between 2000–2018. Ann. Saudi Med. 2023, 43, 315–328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arslan, S.A.; Avcı, G.G.; Akkas, E.A.; Guney, Y. Improved disease-free survival with adjuvant radiotherapy in early-stage endometrial cancer: 10-year outcome analysis. J. Contemp. Brachytherapy 2020, 12, 572–578. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Radojević, M.Ž.; Mujković, S.; Zoran, P.; Lončar, D.; Nedović, N.; Nedović, J.; Vojinović, R.; Dimitrijević, A.; Vulović, T.; Milosavljević, N. Five-year disease-free survival in FIGO IA stage endometrial cancer patients: Tertiary institution experience in a developing country. J. Contemp. Brachytherapy 2023, 15, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Tong, N.; Kumar, A.; Gelowitz, G.; Tinker, A.; Holloway, C.; Ko, J. Impact of the adjuvant management and risk factors on survival in FIGO stage 3 endometrial cancer patients. Front. Oncol. 2023, 13, 1035511. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smart, A.; Buscariollo, D.; Alban, G.; Buzurovic, I.; Cheng, T.; Pretz, J.; Krechmer, B.; King, M.; Lee, L. Low-dose adjuvant vaginal cylinder brachytherapy for early-stage non-endometrioid endometrial cancer: Recurrence risk and survival outcomes. Int. J. Gynecol. Cancer 2020, 30, 1908–1914. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Perez, M.R.; Padilla-Iserte, P.; Arencibia-Sanchez, O.; Martin-Arriscado, C.; Muruzabal, J.C.; Diaz-Feijóo, B.; Cabrera, S.; Coronado, P.; Martín-Salamanca, M.B.; Pantoja-Garrido, M.; et al. Lymphovascular Space Invasion in Early-Stage Endometrial Cancer (LySEC): Patterns of Recurrence and Predictors. A Multicentre Retrospective Cohort Study of the Spain Gynecologic Oncology Group. Cancers 2023, 15, 2612. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.; Xu, P.; Yang, X.; Yu, Q.; Xu, X.; Zou, G.; Zhang, X. Association of Myometrial Invasion with Lymphovascular Space Invasion, Lymph Node Metastasis, Recurrence, and Overall Survival in Endometrial Cancer: A Meta-Analysis of 79 Studies with 68,870 Patients. Front. Oncol. 2021, 11, 762329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cuylan, Z.F.; Oz, M.; Ozkan, N.T.; Comert, G.K.; Sahin, H.; Turan, T.; Akbayir, O.; Kuscu, E.; Celik, H.; Dede, M.; et al. Prognostic factors and patterns of recurrence in lymphovascular space invasion positive women with stage IIIC endometriod endometrial cancer. J. Obstet. Gynaecol. Res. 2018, 44, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Stålberg, K.; Bjurberg, M.; Borgfeldt, C.; Carlson, J.; Dahm-Kähler, P.; Flöter-Rådestad, A.; Hellman, K.; Hjerpe, E.; Holmberg, E.; Kjølhede, P.; et al. Lymphovascular space invasion as a predictive factor for lymph node metastases and survival in endometrioid endometrial cancer—A Swedish Gynecologic Cancer Group (SweGCG) study. Acta Oncol. 2019, 58, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- de Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; Colombo, A.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): Final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 295–309, Erratum in Lancet Oncol. 2018, 19, e184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Variables | No LV Invasion (n = 261) | LV Invasion (n = 50) | p-Value |
|---|---|---|---|
| Age, years (mean ± SD) | 67.0 ± 11.1 | 68.2 ± 13.1 | 0.726 |
| Age category | 0.185 | ||
| 18–40 years | 1.1% | 4.0% | |
| 40–60 years | 26.1% | 18.0% | |
| >60 years | 72.8% | 78.0% | |
| Comorbidities | 0.729 | ||
| Cardiovascular disease | 8.1% | 2.0% | |
| Diabetes | 6.5% | 4.0% | |
| Obesity | 10.3% | 6.0% |
| Variables | No LV Invasion (n = 261) | LV Invasion (n = 50) | p-Value |
|---|---|---|---|
| Secondary carcinoma (%) | 9.58% | 10.0% | 0.916 |
| Histology (%) | 0.772 | ||
| Endometrioid | 79.3% | 76.0% | |
| Serous | 8.0% | 12.0% | |
| Carcinosarcoma | 6.9% | 8.0% | |
| Other | 5.7% | 4.0% | |
| MSI (%) | 8.4% | 18.0% | 0.038 |
| p53 (%) | 3.4% | 14.0% | 0.002 |
| Time from diagnosis to surgery (mean ± SD) | 7.79 ± 6.35 | 9.01 ± 7.28 | 0.211 |
| Type of surgery | 0.001 | ||
| Total abdominal hysterectomy | 25.29% | 36.00% | |
| Laparoscopic hysterectomy | 64.37% | 44.00% | |
| Vaginal hysterectomy | 1.15% | 8.00% | |
| Robot-assisted hysterectomy | 1.92% | 6.00% | |
| Surgical access | <0.001 | ||
| Abdominal access | 17.24% | 44.00% | |
| Vaginal access | 69.73% | 42.00% | |
| Laparoscopic access | 3.45% | 2.00% | |
| Combined access | 0.38% | 4.00% | |
| Residual tumor after surgery | <0.001 | ||
| R0 (No residual tumor) | 89.27% | 62.00% | |
| R1 (Microscopic residual tumor) | 1.15% | 18.00% | |
| R2 (Macroscopic residual tumor) | 0.77% | 14.00% |
| Variables | No LV Invasion (n = 261) | LV Invasion (n = 50) | p-Value |
|---|---|---|---|
| Pathological node status pN (%) | <0.001 | ||
| 0 | 48.00% | 0.00% | |
| 1 | 0.00% | 69.73% | |
| 2 | 0.00% | 30.27% | |
| x | 52.00% | 0.00% | |
| Grade (%) | <0.001 | ||
| 1–2 | 73.46% | 34.00% | |
| 3–4 | 25.77% | 64.00% | |
| Venous invasion (%) | <0.001 | ||
| Yes | 1.53% | 64.00% | |
| No | 98.47% | 36.00% | |
| Distant metastasis (%) | <0.001 | ||
| Yes | 1.92% | 22.00% | |
| No | 95.77% | 74.00% | |
| Number of removed lymph nodes (mean ± SD) | 9.39 ± 16.55 | 14.43 ± 18.35 | 0.320 |
| Variables | No LV Invasion (n = 261) | LV Invasion (n = 50) | p-Value |
|---|---|---|---|
| Second surgery (%) | 78.95% No | 66.67% No | 0.047 |
| Intraoperative and immediate postoperative complications (%) | 39.08% Yes | 60.00% Yes | 0.014 |
| Radiotherapy (%) | 49.43% No, 39.08% Yes | 26.00% No, 60.00% Yes | 0.003 |
| Chemotherapy (%) | 72.94% No, 22.94% Yes | 28.57% No, 57.14% Yes | <0.001 |
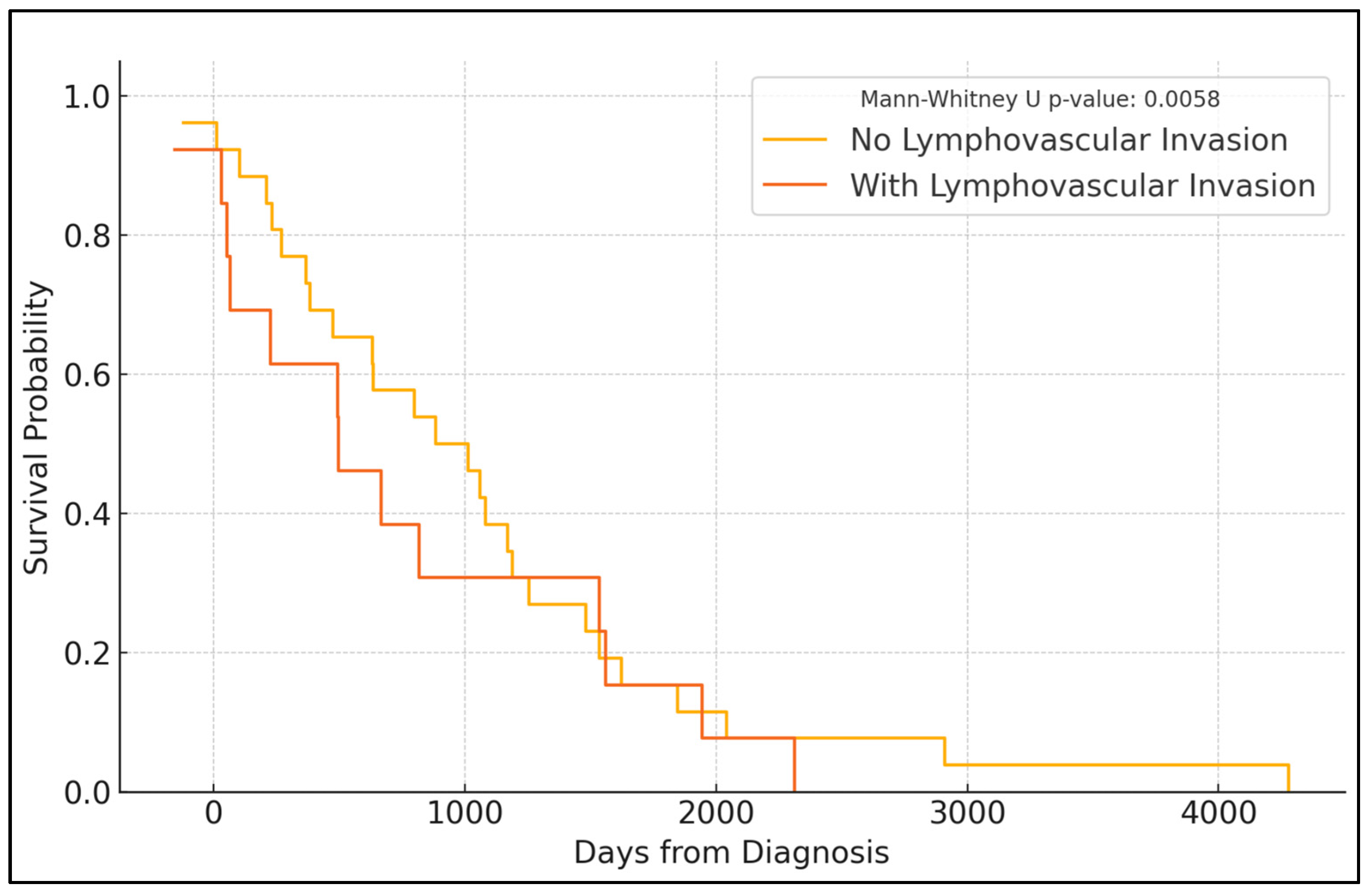
| Follow-up PFS (%) | 0.001 | ||
| Year 1 | 95.1% | 84.6% | |
| Year 2 | 90.6% | 73.3% | |
| Year 3 | 85.9% | 66.0% | |
| Year 4 | 81.7% | 57.4% | |
| Year 5 | 74.3% | 50.8% | |
| Recurrence (%) | 14.78% Yes | 17.95% Yes | 0.738 |
| Deceased (%) | 17.59% Yes | 40.48% Yes | <0.001 |
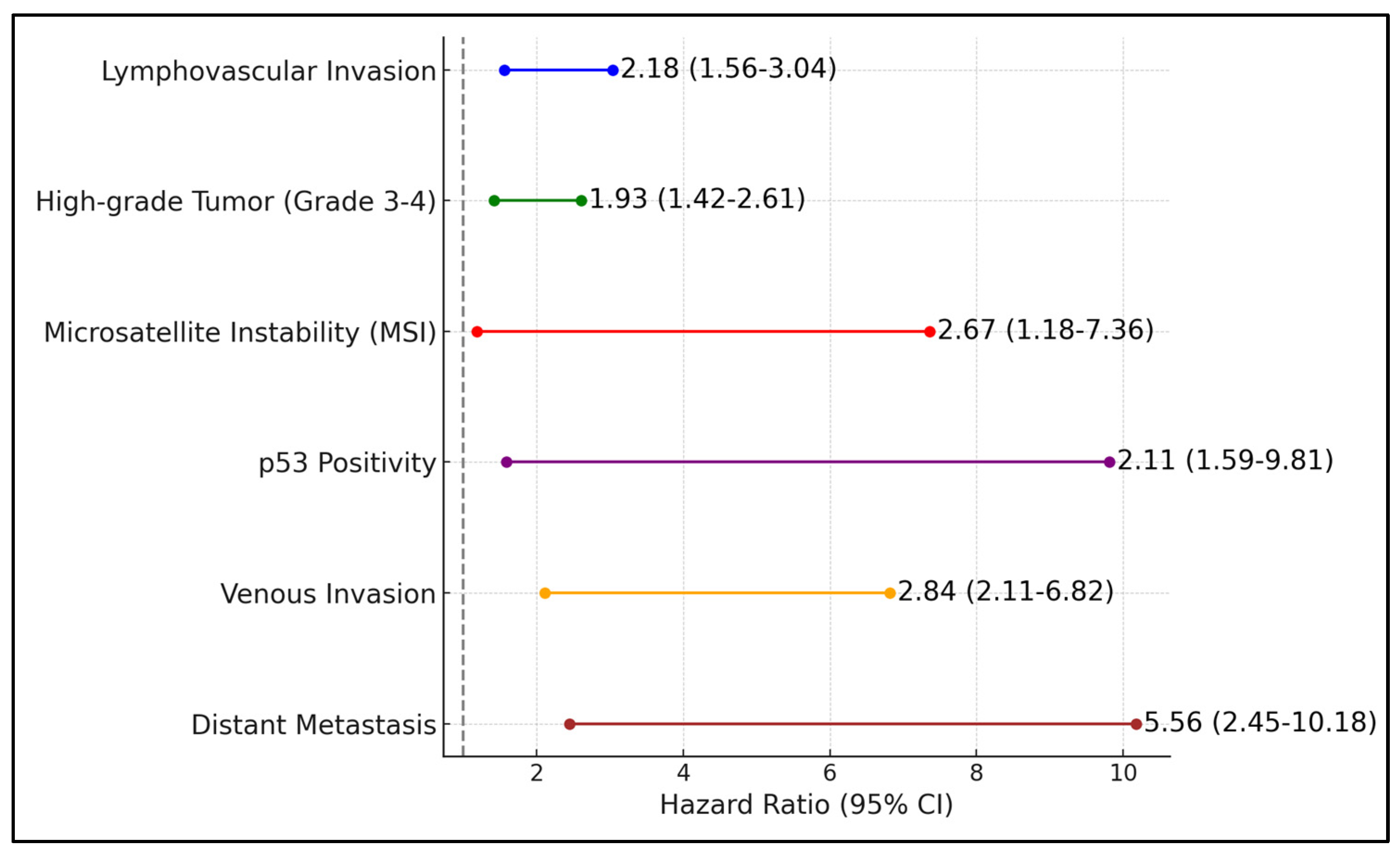
| Negative Factors | PFS Median | Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|---|
| Lymphovascular invasion | 3.2 | 2.18 | 1.56–3.04 | <0.001 |
| High-grade tumor (grade 3–4) | 2.8 | 1.93 | 1.42–2.61 | 0.001 |
| Microsatellite instability (MSI) | 2.9 | 2.67 | 1.18–7.36 | 0.004 |
| p53 positivity | 2.5 | 2.11 | 1.59–9.81 | <0.001 |
| Venous invasion | 2.3 | 2.84 | 2.11–6.82 | <0.001 |
| Distant metastasis | 1.6 | 5.56 | 2.45–10.18 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nienhaus, A.; Rajakulendran, R.; Bernad, E. A 10-Year Retrospective Cohort Study of Endometrial Cancer Outcomes and Associations with Lymphovascular Invasion: A Single-Center Study from Germany. Diagnostics 2024, 14, 1686. https://doi.org/10.3390/diagnostics14151686
Nienhaus A, Rajakulendran R, Bernad E. A 10-Year Retrospective Cohort Study of Endometrial Cancer Outcomes and Associations with Lymphovascular Invasion: A Single-Center Study from Germany. Diagnostics. 2024; 14(15):1686. https://doi.org/10.3390/diagnostics14151686
Chicago/Turabian StyleNienhaus, Alexandra, Rahavie Rajakulendran, and Elena Bernad. 2024. "A 10-Year Retrospective Cohort Study of Endometrial Cancer Outcomes and Associations with Lymphovascular Invasion: A Single-Center Study from Germany" Diagnostics 14, no. 15: 1686. https://doi.org/10.3390/diagnostics14151686
APA StyleNienhaus, A., Rajakulendran, R., & Bernad, E. (2024). A 10-Year Retrospective Cohort Study of Endometrial Cancer Outcomes and Associations with Lymphovascular Invasion: A Single-Center Study from Germany. Diagnostics, 14(15), 1686. https://doi.org/10.3390/diagnostics14151686







