An Immunohistochemical Study of MAGE Proteins in Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tumor Samples
2.2. Ethical Considerations
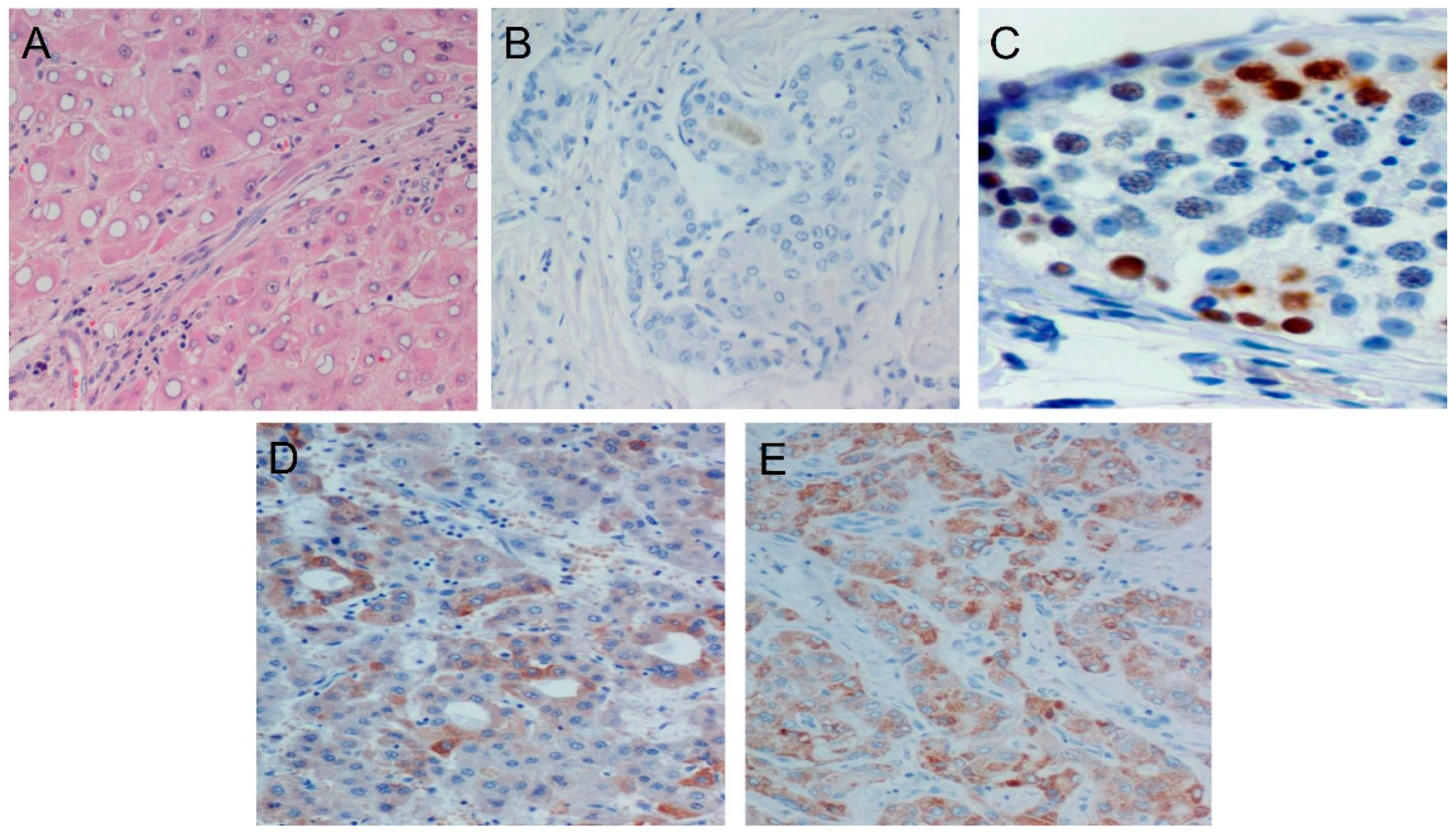
2.3. Immunohistochemical Staining
2.4. Statistical Analysis
3. Results
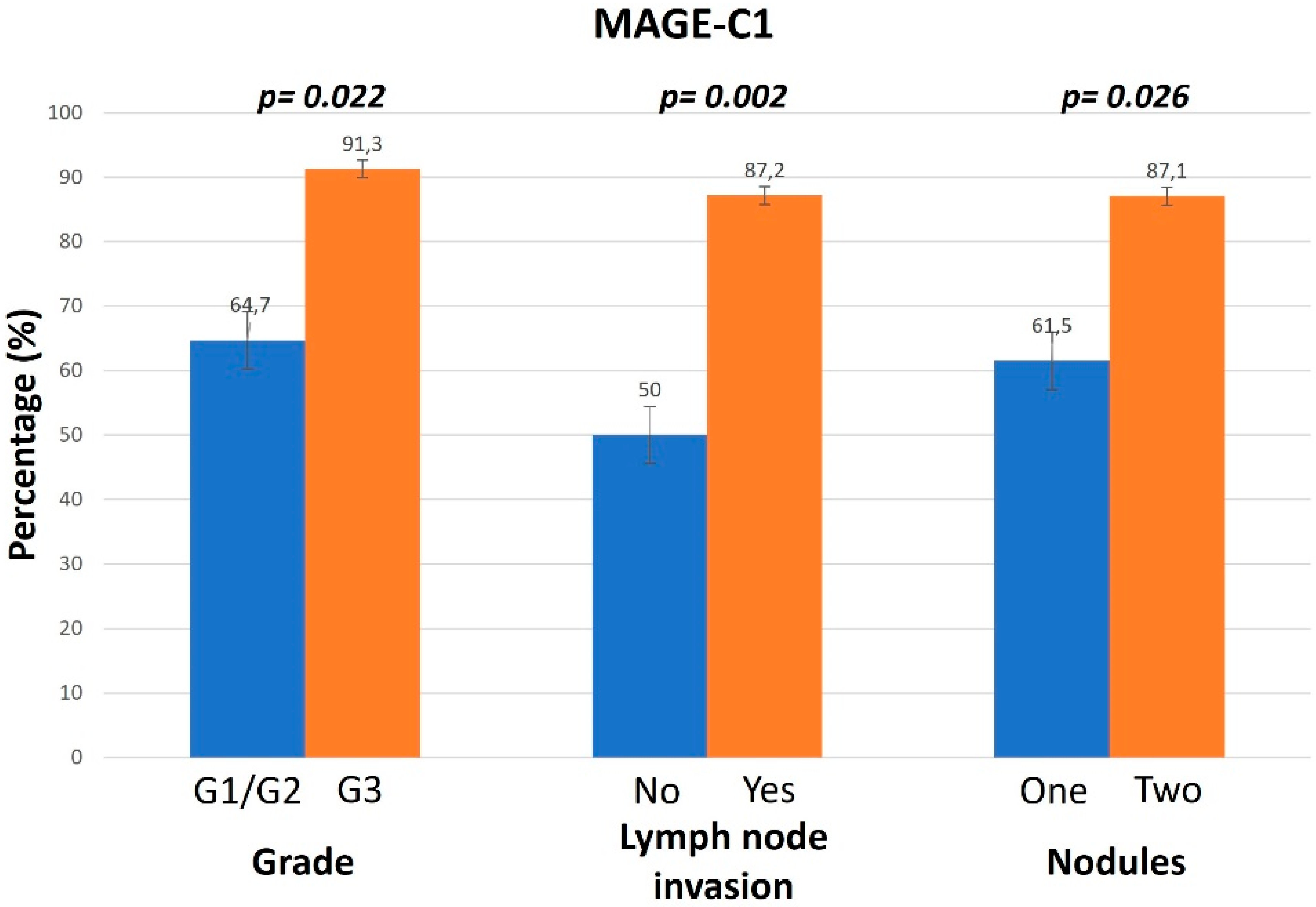
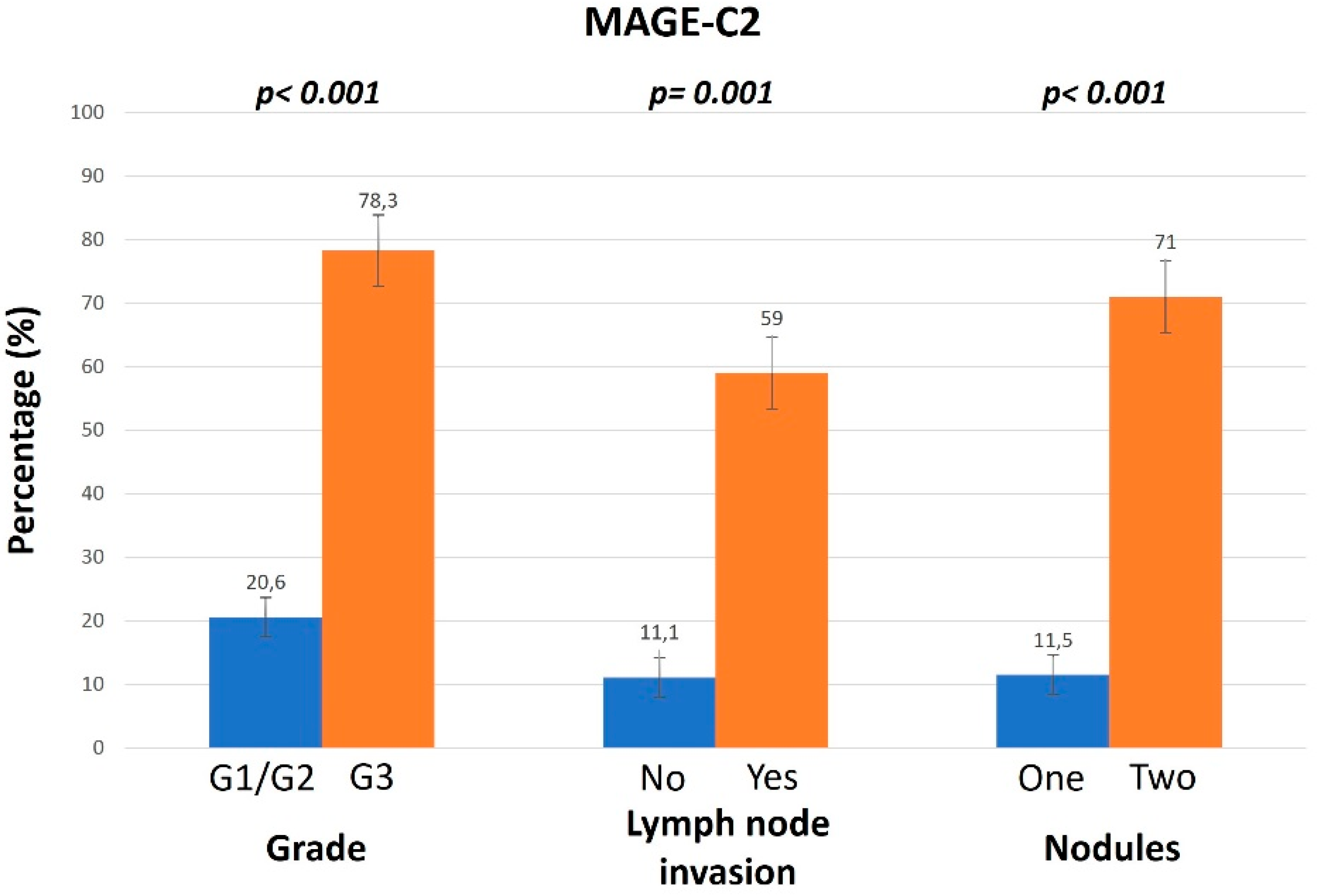
3.1. Association of MAGE Expression Levels and Different Clinicopathological Parameters
3.2. Association of MAGE Expression Levels and Overall Survival Rates of Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, J.U.; Andersen, J.B.; Thorgeirsson, S.S. Functional and genetic deconstruction of the cellular origin in liver cancer. Nat. Rev. Cancer 2015, 15, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Gu, X.; Ni, H.; Zhang, W.; Chang, F.; Gong, L.; Chen, X.; Li, J.; Qiu, L.; Shi, C.; et al. High expression of inositol polyphosphate phosphatase-like 1 associates with unfavorable survival in hepatocellular carcinoma. Int. J. Clin. Exp. Pathol. 2013, 6, 2515–2522. [Google Scholar] [PubMed]
- Xie, C.; Xie, D.Y.; Lin, B.L.; Zhang, G.L.; Wang, P.P.; Peng, L.; Gao, Z.L. Interferon-β gene-modified human bone marrow mesenchymal stem cells attenuate hepatocellular carcinoma through inhibiting AKT/FOXO3a pathway. Br. J. Cancer 2013, 109, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Poon, D.; Anderson, B.O.; Chen, L.T.; Tanaka, K.; Lau, W.Y.; Van Cutsem, E.; Singh, H.; Chow, W.C.; Ooi, L.L.; Chow, P.; et al. Management of hepatocellular carcinoma in Asia: Consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009, 10, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Yuen, M.F.; Lai, C.L. Serological markers of liver cancer. Best Pract. Research. Clin. Gastroenterol. 2005, 19, 91–99. [Google Scholar] [CrossRef]
- Franssen, B.; Alshebeeb, K.; Tabrizian, P.; Marti, J.; Pierobon, E.S.; Lubezky, N.; Roayaie, S.; Florman, S.; Schwartz, M.E. Differences in surgical outcomes between hepatitis B- and hepatitis C-related hepatocellular carcinoma: A retrospective analysis of a single North American center. Ann. Surg. 2014, 260, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Schwartz, M.; Mazzaferro, V. Resection and liver transplantation for hepatocellular carcinoma. Semin. Liver Dis. 2005, 25, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Sang, M.; Lian, Y.; Zhou, X.; Shan, B. MAGE-A family: Attractive targets for cancer immunotherapy. Vaccine 2011, 29, 8496–8500. [Google Scholar] [CrossRef]
- Chung, F.Y.; Cheng, T.L.; Chang, H.J.; Chiu, H.H.; Huang, M.Y.; Chang, M.S.; Chen, C.C.; Yang, M.J.; Wang, J.Y.; Lin, S.R. Differential gene expression profile of MAGE family in taiwanese patients with colorectal cancer. J. Surg. Oncol. 2010, 102, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Chomez, P.; De Backer, O.; Bertrand, M.; De Plaen, E.; Boon, T.; Lucas, S. An overview of the MAGE gene family with the identification of all human members of the family. Cancer Res. 2001, 61, 5544–5551. [Google Scholar]
- Weon, J.L.; Potts, P.R. The MAGE protein family and cancer. Curr. Opin. Cell Biol. 2015, 37, 1–8. [Google Scholar] [CrossRef]
- Lian, Y.; Meng, L.; Ding, P.; Sang, M. Epigenetic regulation of MAGE family in human cancer progression-DNA methylation, histone modification, and non-coding RNAs. Clin. Epigenetics 2018, 10, 115. [Google Scholar] [CrossRef]
- Barker, P.A.; Salehi, A. The MAGE proteins: Emerging roles in cell cycle progression, apoptosis, and neurogenetic disease. J. Neurosci. Res. 2002, 67, 705–712. [Google Scholar] [CrossRef]
- De Donato, M.; Peters, S.O.; Hussain, T.; Rodulfo, H.; Thomas, B.N.; Babar, M.E.; Imumorin, I.G. Molecular evolution of type II MAGE genes from ancestral MAGED2 gene and their phylogenetic resolution of basal mammalian clades. Mamm. Genome 2017, 28, 443–454. [Google Scholar] [CrossRef]
- Karpf, A.R.; Bai, S.; James, S.R.; Mohler, J.L.; Wilson, E.M. Increased expression of androgen receptor coregulator MAGE-11 in prostate cancer by DNA hypomethylation and cyclic AMP. Mol. Cancer Res. 2009, 7, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Kawada, J.; Wada, H.; Isobe, M.; Gnjatic, S.; Nishikawa, H.; Jungbluth, A.A.; Okazaki, N.; Uenaka, A.; Nakamura, Y.; Fujiwara, S.; et al. Heteroclitic serological response in esophageal and prostate cancer patients after NY-ESO-1 protein vaccination. Int. J. Cancer 2012, 130, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Zhao, D.; Sun, D.P.; Wang, Y.; Li, Y.; Qiu, F.Q.; Ma, P. Adenovirus-mediated delivery of CALR and MAGE-A3 inhibits invasion and angiogenesis of glioblastoma cell line U87. J. Exp. Clin. Cancer Res 2012, 31, 8. [Google Scholar] [CrossRef]
- Weeraratne, S.D.; Amani, V.; Neiss, A.; Teider, N.; Scott, D.K.; Pomeroy, S.L.; Cho, Y.J. miR-34a confers chemosensitivity through modulation of MAGE-A and p53 in medulloblastoma. Neuro-Oncology 2011, 13, 165–175. [Google Scholar] [CrossRef]
- Takahashi, N.; Ohkuri, T.; Homma, S.; Ohtake, J.; Wakita, D.; Togashi, Y.; Kitamura, H.; Todo, S.; Nishimura, T. First clinical trial of cancer vaccine therapy with artificially synthesized helper/killer-hybrid epitope long peptide of MAGE-A4 cancer antigen. Cancer Sci. 2012, 103, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.L.; Wu, W.J.; Yang, J.; Tang, Z.J.; Chen, D.L.; Qiu, M.Z.; Luo, H.Y.; Wang, Z.Q.; Jin, Y.; Wang, D.S.; et al. Prognostic relevance of melanoma antigen D1 expression in colorectal carcinoma. J. Transl. Med. 2012, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Vansteenkiste, J.F.; Cho, B.C.; Vanakesa, T.; De Pas, T.; Zielinski, M.; Kim, M.S.; Jassem, J.; Yoshimura, M.; Dahabreh, J.; Nakayama, H.; et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. Oncol. 2016, 17, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Duperret, E.K.; Liu, S.; Paik, M.; Trautz, A.; Stoltz, R.; Liu, X.; Ze, K.; Perales-Puchalt, A.; Reed, C.; Yan, J.; et al. A Designer Cross-reactive DNA Immunotherapeutic Vaccine that Targets Multiple MAGE-A Family Members Simultaneously for Cancer Therapy. Clin. Cancer Res. 2018, 24, 6015–6027. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Xie, H.Q.; Xie, Y.Q.; Lv, X.D. Expression and Clinical Significance of MAGE-A Proteins and mRNA in Lung Cancer Patients: A Retrospective Study. Altern. Ther. Health Med. 2024, 30, 131–135. [Google Scholar] [PubMed]
- Zhao, J.; Wang, Y.; Mu, C.; Xu, Y.; Sang, J. MAGEA1 interacts with FBXW7 and regulates ubiquitin ligase-mediated turnover of NICD1 in breast and ovarian cancer cells. Oncogene 2017, 36, 5023–5034. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, A.; Kalita-de Croft, P.; Vargas, A.C.; Smart, C.E.; Simpson, P.T.; Saunus, J.M.; Lakhani, S.R. Expression of MAGE-A and NY-ESO-1 cancer/testis antigens is enriched in triple-negative invasive breast cancers. Histopathology 2018, 73, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Huo, J.; Su, H.; Xu, Y.; Zhang, F. The Association of Melanoma-Associated Antigen-C Gene With Clinicopatho-logical Characteristics and Prognosis in Breast Cancer: A Systematic Review and Meta-Analysis. Clin. Breast Cancer 2024, 24, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.W.; Yu, Q.Y.; Xu, M.J.; Zhou, C.Y.; Li, J.P.; Liao, X.H. MAGE-A11 is a potential prognostic biomarker and immunotherapeutic target in gastric cancer. Aging 2024, 16, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Brišar, N.; Šuster, K.; Brezar, S.K.; Vidmar, R.; Fonović, M.; Cör, A. An Engineered M13 Filamentous Nanoparticle as an Antigen Carrier for a Malignant Melanoma Immunotherapeutic Strategy. Viruses 2024, 16, 232. [Google Scholar] [CrossRef]
- Zhang, B.; Ren, Z.; Zhao, J.; Zhu, Y.; Huang, B.; Xiao, C.; Zhang, Y.; Deng, J.; Mao, L.; Tang, L.; et al. Global analysis of HLA-A2 restricted MAGE-A3 tumor antigen epitopes and corresponding TCRs in non-small cell lung cancer. Theranostics 2023, 13, 4449–4468. [Google Scholar] [CrossRef] [PubMed]
- Alsalloum, A.; Shevchenko, J.A.; Sennikov, S. The Melanoma-Associated Antigen Family A (MAGE-A): A Promising Target for Cancer Immunotherapy? Cancers 2023, 15, 1779. [Google Scholar] [CrossRef] [PubMed]
- Nasrah, S.; Radi, A.; Daberkow, J.K.; Hummler, H.; Weber, S.; Seaayfan, E.; Kömhoff, M. MAGED2 Depletion Promotes Stress-Induced Autophagy by Impairing the cAMP/PKA Pathway. Int. J. Mol. Sci. 2023, 24, 13433. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Sang, M.; Zhao, L.; Hou, R.; Shan, B. The expression of MAGE-C1 and MAGE-C2 in breast cancer and their clinical significance. Am. J. Surg. 2016, 211, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Melo, D.H.; Mamede, R.C.M.; Neder, L.; Silva, W.A.; Barros-Filho, M.C., Jr.; Kowalski, L.P.; Pinto, C.A.L.; Zago, M.A.; Figueiredo, D.L.A.; Jungbluth, A.A. Expression of cancer/testis antigens MAGE-A, MAGE-C1, GAGE and CTAG1B in benign and malignant thyroid diseases. Oncol. Lett. 2017, 14, 6485–6496. [Google Scholar] [PubMed]
- Curioni-Fontecedro, A.; Nuber, N.; Mihic-Probst, D.; Seifert, B.; Soldini, D.; Dummer, R.; Knuth, A.; van den Broek, M.; Moch, H. Expression of MAGE-C1/CT7 and MAGE-C2/CT10 predicts lymph node metastasis in melanoma patients. PLoS ONE 2011, 6, e21418. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.R.; Chen, H.S.; Mou, D.C.; Cao, J.; Cong, X.; Qin, L.L.; Wei, L.; Leng, X.S.; Wang, Y.; Chen, W.F. Expression of cancer/testis (CT) antigens in Chinese hepatocellular carcinoma and its correlation with clinical parameters. Cancer Lett. 2005, 219, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Sideras, K.; Bots, S.J.; Biermann, K.; Sprengers, D.; Polak, W.G.; IJzermans, J.N.; de Man, R.A.; Pan, Q.; Sleijfer, S.; Bruno, M.J.; et al. Tumour antigen expression in hepatocellular carcinoma in a low-endemic western area. Br. J. Cancer 2015, 112, 1911–1920. [Google Scholar] [CrossRef]


| Total Sample | |
|---|---|
| Sex | |
| Female | 17 (29.8) |
| Male | 40 (70.2) |
| Age | |
| <70 years | 23 (40.4) |
| ≥70 years | 34 (59.6) |
| HBV or HCV | |
| Negative | 45 (78.9) |
| Positive | 12 (21.1) |
| Grade | |
| G1/G2 | 34 (59.6) |
| G3 | 23 (40.4) |
| Lymph Node Invasion | |
| No | 18 (31.6) |
| Yes | 39 (68.4) |
| Νodules | |
| 1 | 26 (45.6) |
| 2 | 31 (54.5) |
| AFP | |
| Low | 25 (43.9) |
| High | 32 (56.1) |
| Cirrhosis | |
| No | 43 (75.4) |
| Yes | 14 (24.6) |
| Surgery | |
| No | 12 (21.1) |
| Yes | 45 (78.9) |
| Status | |
| Alive | 16 (28.1) |
| Dead | 41 (71.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tologkos, S.; Papadatou, V.; Mitrakas, A.G.; Pagonopoulou, O.; Tripsianis, G.; Alexiadis, T.; Alexiadi, C.-A.; Panagiotopoulos, A.-P.; Nikolaidou, C.; Lambropoulou, M. An Immunohistochemical Study of MAGE Proteins in Hepatocellular Carcinoma. Diagnostics 2024, 14, 1692. https://doi.org/10.3390/diagnostics14151692
Tologkos S, Papadatou V, Mitrakas AG, Pagonopoulou O, Tripsianis G, Alexiadis T, Alexiadi C-A, Panagiotopoulos A-P, Nikolaidou C, Lambropoulou M. An Immunohistochemical Study of MAGE Proteins in Hepatocellular Carcinoma. Diagnostics. 2024; 14(15):1692. https://doi.org/10.3390/diagnostics14151692
Chicago/Turabian StyleTologkos, Stylianos, Vasiliki Papadatou, Achilleas G. Mitrakas, Olga Pagonopoulou, Grigorios Tripsianis, Triantafyllos Alexiadis, Christina-Angelika Alexiadi, Antonios-Periklis Panagiotopoulos, Christina Nikolaidou, and Maria Lambropoulou. 2024. "An Immunohistochemical Study of MAGE Proteins in Hepatocellular Carcinoma" Diagnostics 14, no. 15: 1692. https://doi.org/10.3390/diagnostics14151692





