Imaging of Carotid Stenosis: Where Are We Standing? Comparison of Multiparametric Ultrasound, CT Angiography, and MRI Angiography, with Recent Developments
Abstract
1. Introduction
2. Multiparametric Ultrasound
2.1. Standard Carotid US
- -
- North American Symptomatic Carotid Endarterectomy Trial (NASCET): comparison of the stenotic segment with the normal distal diameter of the post-stenotic ICA [13];
- -
- European Carotid Surgery Trial (ECST): comparison of the diameter of the stenotic area with the normal diameter of the carotid bulb [14];
- -
- Common Carotid (CC): measurement of the residual lumen diameter in the most stenotic portion of the artery and subsequent comparison with the lumen diameter of the proximal common carotid artery (CCA) [15].
2.2. CEUS
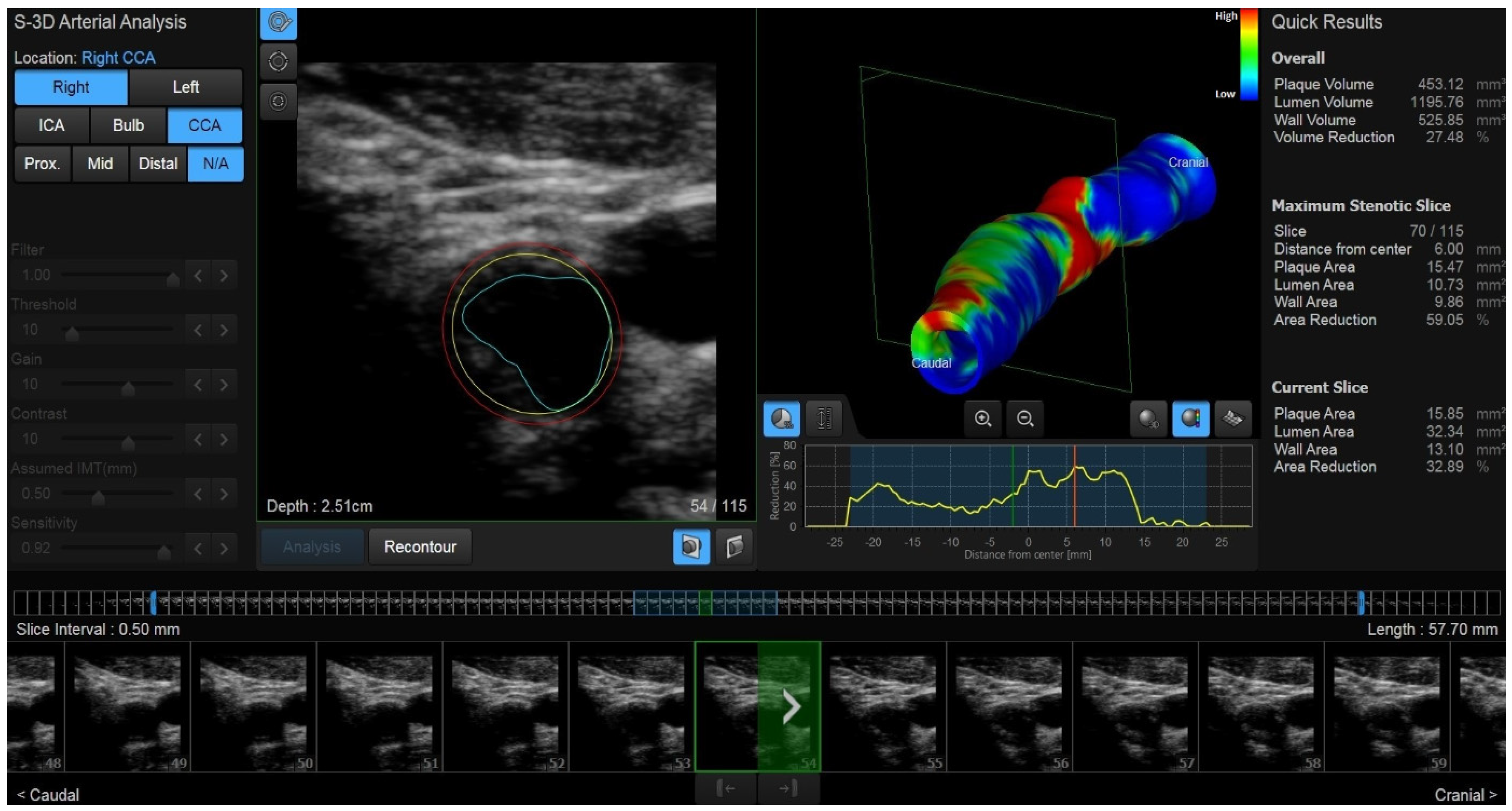
2.3. New Ultrasound Developments: 3D US and Vector Flow
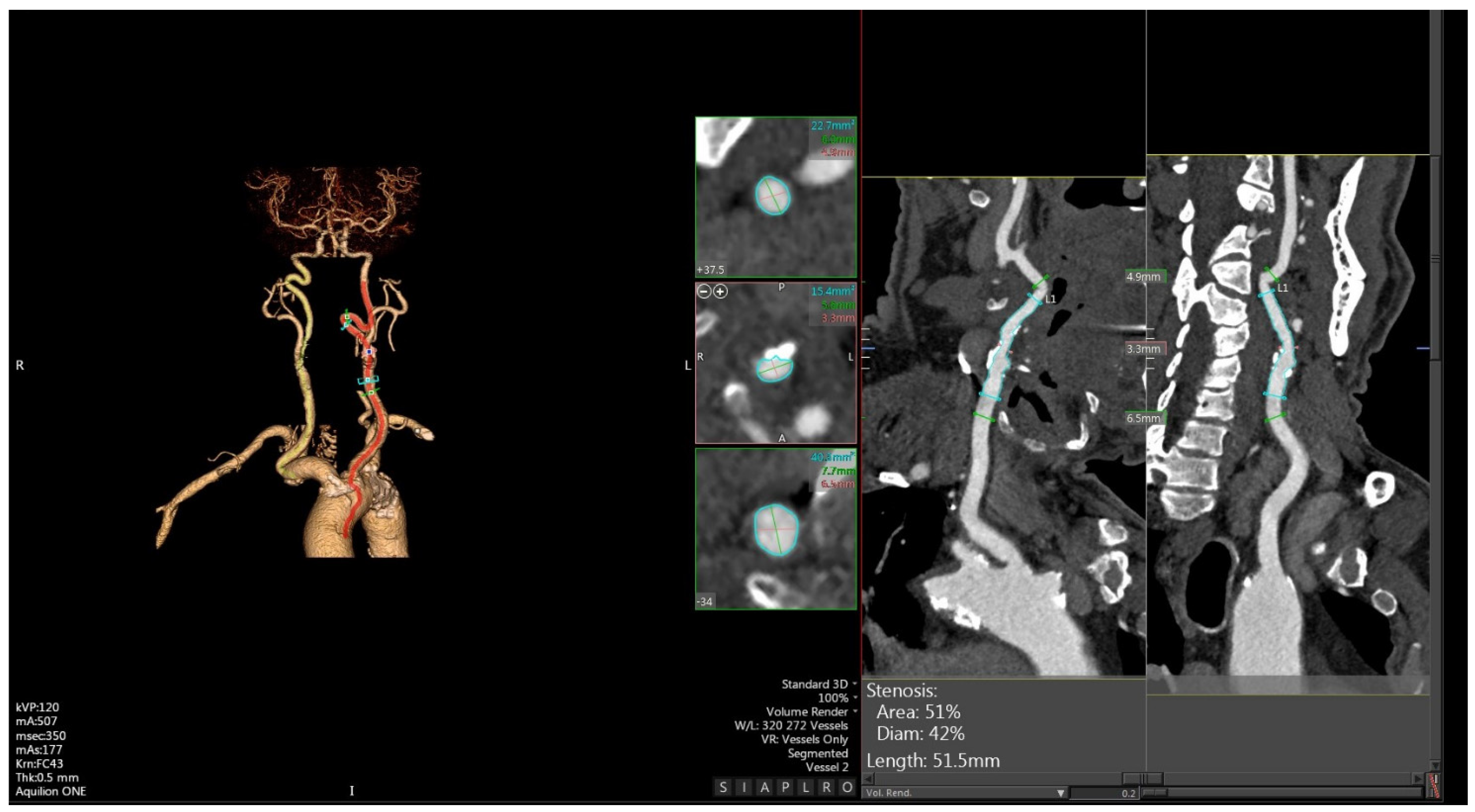
3. CTA
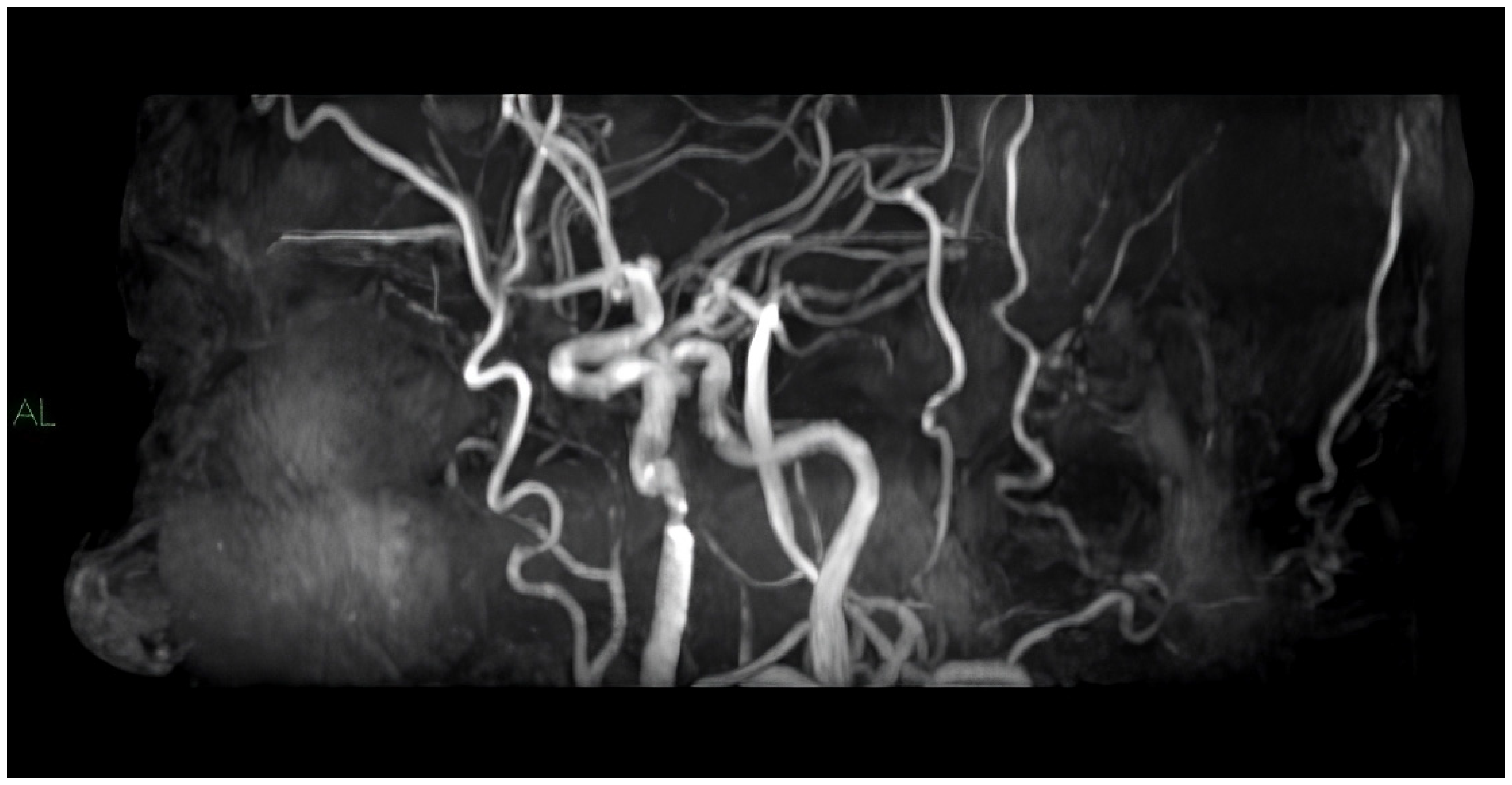
4. MRI
5. Discussion
Funding
Conflicts of Interest
References
- Mechtouff, L.; Rascle, L.; Crespy, V.; Canet-Soulas, E.; Nighoghossian, N.; Millon, A. A narrative review of the pathophysiology of ischemic stroke in carotid plaques: A distinction versus a compromise between hemodynamic and embolic mechanism. Ann. Transl. Med. 2021, 9, 1208. [Google Scholar] [CrossRef]
- Singh, N.; Moody, A.R.; Roifman, I.; Bluemke, D.A.; Zavodni, A.E. Advanced MRI for carotid plaque imaging. Int. J. Cardiovasc. Imaging. 2016, 32, 83–89. [Google Scholar] [CrossRef]
- Murgia, A.; Erta, M.; Suri, J.S.; Gupta, A.; Wintermark, M.; Saba, L. CT imaging features of carotid artery plaque vulnerability. Ann. Transl. Med. 2020, 8, 1261. [Google Scholar] [CrossRef]
- Goudot, G.; Khider, L.; Pedreira, O.; Poree, J.; Julia, P.; Alsac, J.M.; Amemiya, K.; Bruneval, P.; Messas, E.; Pernot, M.; et al. Innovative Multiparametric Characterization of Carotid Plaque Vulnerability by Ultrasound. Front. Physiol. 2020, 11, 157. [Google Scholar] [CrossRef]
- Pacini, P.; Polti, G.; Faggiano, A.; Giannetta, E.; Tarsitano, M.G.; Cantisani, V. Multiparametric ultrasound evaluation of a case of bilateral carotid body tumor. J. Ultrasound. 2021, 24, 311–315. [Google Scholar] [CrossRef]
- Chappell, F.M.; Wardlaw, J.M.; Young, G.R.; Gillard, J.H.; Roditi, G.H.; Yip, B.; Pell, J.P.; Rothwell, P.M.; Brown, M.M.; Gough, M.J.; et al. Carotid artery stenosis: Accuracy of noninvasive tests--individual patient data meta-analysis. Radiology 2009, 251, 493–502. [Google Scholar] [CrossRef]
- Rafailidis, V.; Li, X.; Sidhu, P.S.; Partovi, S.; Staub, D. Contrast imaging ultrasound for the detection and characterization of carotid vulnerable plaque. Cardiovasc. Diagn. Ther. 2020, 10, 965–981. [Google Scholar] [CrossRef]
- Brinjikji, W.; Huston, J., III; Rabinstein, A.A.; Kim, G.; Lerman, A.; Lanzino, G. Contemporary carotid imaging: From degree of stenosis to plaque vulnerability. J. Neurosurg. JNS 2016, 124, 27–42. [Google Scholar] [CrossRef]
- Murray, C.S.G.; Nahar, T.; Kalashyan, H.; Becher, H.; Nanda, N.C. Ultrasound assessment of carotid arteries: Current concepts, methodologies, diagnostic criteria, and technological advancements. Echocardiography 2018, 35, 2079–2091. [Google Scholar] [CrossRef]
- Makris, G.C.; Lavida, A.; Griffin, M.; Geroulakos, G.; Nicolaides, A.N. Three-dimensional ultrasound imaging for the evaluation of carotid atherosclerosis. Atherosclerosis 2011, 219, 377–383. [Google Scholar] [CrossRef]
- Calogero, E.; Fabiani, I.; Pugliese, N.R.; Santini, V.; Ghiadoni, L.; Di Stefano, R.; Galetta, F.; Sartucci, F.; Penno, G.; Berchiolli, R.; et al. Three-Dimensional Echographic Evaluation of Carotid Artery Disease. J. Cardiovasc. Echogr. 2018, 28, 218–227. [Google Scholar] [PubMed]
- Di Leo, N.; Venturini, L.; De Soccio, V.; Forte, V.; Lucchetti, P.; Cerone, G.; Alagna, G.; Caratozzolo, M.; Messineo, D.; Di Gioia, C.; et al. Multiparametric ultrasound evaluation with CEUS and shear wave elastography for carotid plaque risk stratification. J. Ultrasound. 2018, 21, 293–300. [Google Scholar] [CrossRef] [PubMed]
- North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N. Engl. J. Med. 1991, 325, 445–453. [Google Scholar] [CrossRef] [PubMed]
- European Carotid Surgery Trialists’ Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: Final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998, 351, 1379–1387. [Google Scholar] [CrossRef]
- Williams, M.A.; Nicolaides, A.N. Predicting the normal dimensions of the internal and external carotid arteries from the diameter of the common carotid. Eur. J. Vasc. Surg. 1987, 1, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Ottakath, N.; Al-Maadeed, S.; Zughaier, S.M.; Elharrouss, O.; Mohammed, H.H.; Chowdhury, M.E.H.; Bouridane, A. Ultrasound-Based Image Analysis for Predicting Carotid Artery Stenosis Risk: A Comprehensive Review of the Problem, Techniques, Datasets, and Future Directions. Diagnostics 2023, 13, 2614. [Google Scholar] [CrossRef] [PubMed]
- Aly, I.; Rizvi, A.; Roberts, W.; Khalid, S.; Kassem, M.W.; Salandy, S.; Du Plessis, M.; Tubbs, R.S.; Loukas, M. Cardiac ultrasound: An anatomical and clinical review. Transl. Res. Anat. 2021, 22, 100083. [Google Scholar] [CrossRef]
- Aziz, M.U.; Eisenbrey, J.R.; Deganello, A.; Zahid, M.; Sharbidre, K.; Sidhu, P.; Robbin, M.L. Microvascular Flow Imaging: A State-of-the-Art Review of Clinical Use and Promise. Radiology 2022, 305, 250–264. [Google Scholar] [CrossRef]
- Jiang, Z.Z.; Huang, Y.H.; Shen, H.L.; Liu, X.T. Clinical Applications of Superb Microvascular Imaging in the Liver, Breast, Thyroid, Skeletal Muscle, and Carotid Plaques. J. Ultrasound Med. 2019, 38, 2811–2820. [Google Scholar] [CrossRef]
- Cutler, J.J.; Campo, N.; Koch, S. B-Flow and B-Mode Ultrasound Imaging in Carotid Fibromuscular Dysplasia. J. Neuroimaging 2018, 28, 269–272. [Google Scholar] [CrossRef]
- Ten Kate, G.L.; Van Dijk, A.C.; Van Den Oord, S.C.; Hussain, B.; Verhagen, H.J.; Sijbrands, E.J.; Van Der Steen, A.F.; Van Der Lugt, A.; Schinkel, A.F. Usefulness of contrast-enhanced ultrasound for detection of carotid plaque ulceration in patients with symptomatic carotid atherosclerosis. Am. J. Cardiol. 2013, 112, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Abdelmoneim, S.S.; Ball, C.A.; Nhola, L.F.; Farrell, A.M.; Feinstein, S.; Mulvagh, S.L. Detection of Carotid Atherosclerotic Plaque Neovascularization Using Contrast Enhanced Ultrasound: A Systematic Review and Meta-Analysis of Diagnostic Accuracy Studies. J. Am. Soc. Echocardiogr. 2016, 29, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Rafailidis, V.; Huang, D.Y.; Yusuf, G.T.; Sidhu, P.S. General principles and overview of vascular contrast-enhanced ultrasonography. Ultrasonography 2020, 39, 22–42. [Google Scholar] [CrossRef] [PubMed]
- Greis, C. Technical aspects of contrast-enhanced ultrasound (CEUS) examinations: Tips and tricks. Clin. Hemorheol. Microcirc. 2014, 58, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Fürst, H.; Hartl, W.H.; Jansen, I.; Liepsch, D.; Lauterjung, L.; Schildberg, F.W. Color-flow Doppler sonography in the identification of ulcerative plaques in patients with high-grade carotid artery stenosis. AJNR Am. J. Neuroradiol. 1992, 13, 1581–1587. [Google Scholar] [PubMed]
- Schinkel, A.F.; Kaspar, M.; Staub, D. Contrast-enhanced ultrasound: Clinical applications in patients with atherosclerosis. Int. J. Cardiovasc. Imaging 2016, 32, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, P.S.; Cantisani, V.; Dietrich, C.F.; Gilja, O.H.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.A.; Cosgrove, D.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med. 2018, 39, e2–e44. [Google Scholar] [PubMed]
- Shah, F.; Balan, P.; Weinberg, M.; Reddy, V.; Neems, R.; Feinstein, M.; Dainauskas, J.; Meyer, P.; Goldin, M.; Feinstein, S.B. Contrast-enhanced ultrasound imaging of atherosclerotic carotid plaque neovascularization: A new surrogate marker of atherosclerosis? Vasc. Med. 2007, 12, 291–297. [Google Scholar] [CrossRef]
- Rafailidis, V.; Charitanti, A.; Tegos, T.; Destanis, E.; Chryssogonidis, I. Contrast-enhanced ultrasound of the carotid system: A review of the current literature. J. Ultrasound. 2017, 20, 97–109. [Google Scholar] [CrossRef]
- Clevert, D.A.; Sommer, W.H.; Helck, A.; Saam, T.; Reiser, M. Improved carotid atherosclerotic plaques imaging with contrast-enhanced ultrasound (CEUS). Clin. Hemorheol. Microcirc. 2011, 48, 141–148. [Google Scholar] [CrossRef]
- Goudot, G.; Poree, J.; Pedreira, O.; Khider, L.; Julia, P.; Alsac, J.M.; Laborie, E.; Mirault, T.; Tanter, M.; Messas, E.; et al. Wall Shear Stress Measurement by Ultrafast Vector Flow Imaging for Atherosclerotic Carotid Stenosis. Ultraschall Med. 2021, 42, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Sillesen, H.; Muntendam, P.; Adourian, A.; Entrekin, R.; Garcia, M.; Falk, E.; Fuster, V. Carotid plaque burden as a measure of subclinical atherosclerosis: Comparison with other tests for subclinical arterial disease in the high-risk plaque BioImage study. JACC Cardiovasc. Imaging 2012, 5, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, M.; Zielinski, T.; Schremmer, D.; Stumpe, K.O. Reproducibility of 3-dimensional ultrasound readings of volume of carotid atherosclerotic plaque. Cardiovasc. Ultrasound. 2008, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Heliopoulos, J.; Vadikolias, K.; Piperidou, C.; Mitsias, P. Detection of carotid artery plaque ulceration using 3-dimensional ultrasound. J. Neuroimaging 2011, 21, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.D. Approaching Automated 3-Dimensional Measurement of Atherosclerotic Plaque Volume. J. Am. Coll. Cardiol. 2017, 70, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Goddi, A.; Bortolotto, C.; Fiorina, I.; Raciti, M.V.; Fanizza, M.; Turpini, E.; Boffelli, G.; Calliada, F. High-frame rate vector flow imaging of the carotid bifurcation. Insights Imaging 2017, 8, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Tuenter, A.; Selwaness, M.; Arias Lorza, A.; Schuurbiers, J.C.H.; Speelman, L.; Cibis, M.; Van Der Lugt, A.; De Bruijne, M.; Van Der Steen, A.F.W.; Franco, O.H.; et al. High shear stress relates to intraplaque haemorrhage in asymptomatic carotid plaques. Atherosclerosis 2016, 251, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Barlinn, K.; Alexandrov, A.V. Vascular imaging in stroke: Comparative analysis. Neurotherapeutics 2011, 8, 340–348. [Google Scholar] [CrossRef]
- Baradan, H.; Gupta, A. Carotid Vessel Wall Imaging on CTA. AJNR Am. J. Neuroradiol. 2020, 41, 380–386. [Google Scholar] [CrossRef]
- Yuan, J.; Usman, A.; Das, T.; Patterson, A.J.; Gillard, J.H.; Graves, M.J. Imaging Carotid Atherosclerosis Plaque Ulceration: Comparison of Advanced Imaging Modalities and Recent Developments. AJNR Am. J. Neuroradiol. 2017, 38, 664–671. [Google Scholar] [CrossRef]
- Wannarong, T.; Parraga, G.; Buchanan, D.; Fenster, A.; House, A.A.; Hackam, D.G.; Spence, J.D. Progression of carotid plaque volume predicts cardiovascular events. Stroke 2013, 44, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Micheletti, G.; Brinjikji, W.; Garofalo, P.; Montisci, R.; Balestrieri, A.; Suri, J.S.; DeMarco, J.K.; Lanzino, G.; Sanfilippo, R. Carotid Intraplaque-Hemorrhage Volume and Its Association with cerebrovascular Events. AJNR Am. J. Neuroradiol. 2019, 40, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Chrencik, M.T.; Khan, A.A.; Luther, L.; Anthony, L.; Yokemick, J.; Patel, J.; Sorkin, J.D.; Sikdar, S.; Lal, B.K. Quantitative assessment of carotid plaque morphology (geometry and tissue composition) using computed tomography angiography. J. Vasc. Surg. 2019, 70, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Benson, J.C.; Cheek, H.; Aubry, M.C.; Lanzino, G.; Huston, J., III; Rabinstein, A.; Brinjikji, W. Cervical Carotid Plaque MRI: Review of Atherosclerosis Imaging Features and their Histologic Underpinnings. Clin. Neuroradiol. 2021, 31, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.M.; Hatsukami, T.S.; Ferguson, M.S.; Small, R.; Polissar, N.L.; Yuan, C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 2002, 106, 1368–1373. [Google Scholar] [CrossRef]
- Underhill, H.R.; Yamykh, V.L.; Hatsukami, T.S.; Wang, J.; Balu, N.; Hayes, C.E.; Oikawa, M.; Yu, W.; Xu, D.; Chu, B.; et al. Carotid plaque morphology and composition: Initial comparison between 1.5- and 3.0-T magnetic field strengths. Radiology 2008, 248, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Yarnykh, V.L.; Terashima, M.; Hayes, C.E.; Shimakawa, A.; Takaya, N.; Nguyen, P.K.; Brittain, J.H.; McConnell, M.V.; Yuan, C. Multicontrast black-blood MRI of carotid arteries: Comparison between 1.5 and 3 tesla magnetic field strengths. J. Magn. Reason. Imaging 2006, 23, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Koktzoglou, I.; Chung, Y.C.; Mani, V.; Carroll, T.J.; Morasch, M.D.; Mizsei, G.; Simonetti, O.P.; Fayad, Z.A.; Li, D. Multislice dark-blood carotid artery wall imaging: A 1.5 T and 3.0 T comparison. J. Magn. Reason. Imaging 2006, 23, 699–705. [Google Scholar] [CrossRef]
- Saam, T.; Ferguson, M.S.; Yarnykh, V.L.; Takaya, N.; Xu, D.; Polissar, N.L.; Hatsukami, T.S.; Yuan, C. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 234–239. [Google Scholar] [CrossRef]
- Bitar, R.; Moody, A.R.; Leung, G.; Symons, S.; Crisp, S.; Butany, J.; Rowsell, C.; Kiss, A.; Nelson, A.; Maggisano, R. In vivo 3D high-spatial-resolution MR imaging of intraplaque hemorrhage. Radiology 2008, 249, 259–267. [Google Scholar] [CrossRef]
- Du, J.; Corbeil, J.; Znamirowski, R.; Angle, N.; Peterson, M.; Bydder, G.M.; Kahn, A.M. Direct imaging and quantification of carotid plaque calcification. Magn. Reason. Med. 2011, 65, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Speelman, L.; Teng, Z.; Nederveen, A.J.; van der Lugt, A.; Gillard, J.H. MRI-based biomechanical parameters for carotid artery plaque vulnerability assessment. Thromb Haemost. 2016, 115, 493–500. [Google Scholar] [PubMed]
- Puppini, G.; Furlan, F.; Cirota, N.; Veraldi, G.; Piubello, Q.; Montemezzi, S.; Gortenuti, G. Characterisation of carotid atherosclerotic plaque: Comparison between magnetic resonance imaging and histology. Radiol. Med. 2006, 111, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Mitsumori, L.M.; Ferguson, M.S.; Polissar, N.L.; Echelard, D.; Ortiz, G.; Small, R.; Davies, J.W.; Kerwin, W.S.; Hatsukami, T.S. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation 2001, 104, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Malek, A.M.; Alper, S.L.; Izumo, S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 1999, 282, 2035–2042. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Tempel, D.; van Haperen, R.; de Boer, H.C.; Segers, D.; Huisman, M.; van Zonneveld, A.J.; Leenen, P.J.; van der Steen, A.; Serruys, P.M.; et al. Shear stress-induced changes in atherosclerotic plaque composition are modulated by chemokines. J. Clin. Investig. 2007, 117, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Matlung, H.L.; Neele, A.E.; Groen, H.C.; van Gaalen, K.; Tuna, B.G.; van Weert, A.; de Vos, J.; Wentzel, J.J.; Hoogenboezem, M.; van Buul, J.D.; et al. Transglutaminase activity regulates atherosclerotic plaque composition at locations exposed to oscillatory shear stress. Atherosclerosis 2012, 224, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Teng, Z.; Canton, G.; Yang, C.; Ferguson, M.; Huang, X.; Zheng, J.; Woodard, P.K.; Yuan, C. Sites of rupture in human atherosclerotic carotid plaques are associated with high structural stresses: An in vivo MRI-based 3D fluid-structure interaction study. Stroke 2009, 40, 3258–3263. [Google Scholar] [CrossRef] [PubMed]
- Cibis, M.; Potters, W.V.; Gijsen, F.J.; Marquering, H.; vanBavel, E.; van der Steen, A.F.; Nederveen, A.J.; Wentzel, J.J. Wall shear stress calculations based on 3D cine phase contrast MRI and computational fluid dynamics: A comparison study in healthy carotid arteries. NMR Biomed. 2014, 27, 826–834. [Google Scholar] [CrossRef]
- Papathanasopoulou, P.; Zhao, S.; Köhler, U.; Robertson, M.B.; Long, Q.; Hoskins, P.; Xu, X.Y.; Marshall, I. MRI measurement of time-resolved wall shear stress vectors in a carotid bifurcation model, and comparison with CFD predictions. J. Magn. Reason. Imaging 2003, 17, 153–162. [Google Scholar] [CrossRef]
- Stalder, A.F.; Russe, M.F.; Frydrychowicz, A.; Bock, J.; Hennig, J.; Markl, M. Quantitative 2D and 3D phase contrast MRI: Optimized analysis of blood flow and vessel wall parameters. Magn. Reason. Med. 2008, 60, 1218–1231. [Google Scholar] [CrossRef] [PubMed]
- Petersson, S.; Dyverfeldt, P.; Ebbers, T. Assessment of the accuracy of MRI wall shear stress estimation using numerical simulations. J. Magn. Reason. Imaging 2012, 36, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Potters, W.V.; van Ooij, P.; Marquering, H.; vanBavel, E.; Nederveen, A.J. Volumetric arterial wall shear stress calculation based on cine phase contrast MRI. J. Magn. Reson. Imaging 2015, 41, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.P.; Bennett, E.; Wisk, L.E.; Gharib, M.; Fraser, S.E.; Wen, H. Circumferential strain in the wall of the common carotid artery: Comparing displacement-encoded and cine MRI in volunteers. Magn. Reason. Med. 2008, 60, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Kampschulte, A.; Ferguson, M.S.; Kerwin, W.S.; Polissar, N.L.; Chu, B.; Saam, T.; Hatsukami, T.S.; Yuan, C. Differentiation of intraplaque versus juxtaluminal hemorrhage/thrombus in advanced human carotid atherosclerotic lesions by in vivo magnetic resonance imaging. Circulation 2004, 110, 3239–3244. [Google Scholar] [CrossRef] [PubMed]
- Gallo, D.; Steinman, D.A.; Bijari, P.B.; Morbiducci, U. Helical flow in carotid bifurcation as surrogate marker of exposure to disturbed shear. J. Biomech. 2012, 45, 2398–2404. [Google Scholar] [CrossRef]
- Peiffer, V.; Sherwin, S.J.; Weinberg, P.D. Computation in the rabbit aorta of a new metric—The transverse wall shear stress—To quantify the multidirectional character of disturbed blood flow. J. Biomech. 2013, 46, 2651–2658. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.; Guillén-Grima, F.; Morales, G.; Muñoz, S.; Aguinaga-Ontoso, I.; Fuentes-Aspe, R. Prevalence and incidence of ictus in Europe: Systematic review and meta-analysis. An Sist. Sanit. Navar. 2022, 45, e0979. [Google Scholar] [CrossRef] [PubMed]
- Saini, V.; Guada, L.; Yavagal, D.R. Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology 2021, 97, S6–S16. [Google Scholar] [CrossRef]
- Song, P.; Fang, Z.; Wang, H.; Cai, Y.; Rahimi, K.; Zhu, Y.; Fowkes, F.G.R.; Fowkes, F.J.I.; Rudan, I. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: A systematic review, meta-analysis, and modelling study. Lancet Glob. Health 2020, 8, e721–e729. [Google Scholar] [CrossRef]
- Lukanova, D.V.; Nikolov, N.K.; Genova, K.Z.; Stankev, M.D.; Georgieva, E.V. The Accuracy of Noninvasive Imaging Techniques in Diagnosis of Carotid Plaque Morphology. Open Access Maced. J. Med. Sci. 2015, 3, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Alexandratou, M.; Papachristodoulou, A.; Li, X.; Partovi, S.; Davidhi, A.; Rafailidis, V.; Prassopoulos, P.; Kamperidis, V.; Koutroulou, I.; Tsivgoulis, G.; et al. Advances in Noninvasive Carotid Wall Imaging with Ultrasound: A Narrative Review. J. Clin. Med. 2022, 11, 6196. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, E.; Grazhdani, H.; Aliotta, L.; Gavazzi, L.M.; Foti, P.V.; Palmucci, S.; Inì, C.; Tiralongo, F.; Castiglione, D.; Renda, M.; et al. Imaging of Carotid Stenosis: Where Are We Standing? Comparison of Multiparametric Ultrasound, CT Angiography, and MRI Angiography, with Recent Developments. Diagnostics 2024, 14, 1708. https://doi.org/10.3390/diagnostics14161708
David E, Grazhdani H, Aliotta L, Gavazzi LM, Foti PV, Palmucci S, Inì C, Tiralongo F, Castiglione D, Renda M, et al. Imaging of Carotid Stenosis: Where Are We Standing? Comparison of Multiparametric Ultrasound, CT Angiography, and MRI Angiography, with Recent Developments. Diagnostics. 2024; 14(16):1708. https://doi.org/10.3390/diagnostics14161708
Chicago/Turabian StyleDavid, Emanuele, Hektor Grazhdani, Lorenzo Aliotta, Livio Maria Gavazzi, Pietro Valerio Foti, Stefano Palmucci, Corrado Inì, Francesco Tiralongo, Davide Castiglione, Maurizio Renda, and et al. 2024. "Imaging of Carotid Stenosis: Where Are We Standing? Comparison of Multiparametric Ultrasound, CT Angiography, and MRI Angiography, with Recent Developments" Diagnostics 14, no. 16: 1708. https://doi.org/10.3390/diagnostics14161708
APA StyleDavid, E., Grazhdani, H., Aliotta, L., Gavazzi, L. M., Foti, P. V., Palmucci, S., Inì, C., Tiralongo, F., Castiglione, D., Renda, M., Pacini, P., Di Bella, C., Solito, C., Gigli, S., Fazio, A., Bella, R., Basile, A., & Cantisani, V. (2024). Imaging of Carotid Stenosis: Where Are We Standing? Comparison of Multiparametric Ultrasound, CT Angiography, and MRI Angiography, with Recent Developments. Diagnostics, 14(16), 1708. https://doi.org/10.3390/diagnostics14161708





