Optimizing Image Quality with High-Resolution, Deep-Learning-Based Diffusion-Weighted Imaging in Breast Cancer Patients at 1.5 T
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Image Acquisition
2.3. Image Reconstruction
2.4. Image Analysis
2.5. Statistical Analysis
3. Results
3.1. Subsections
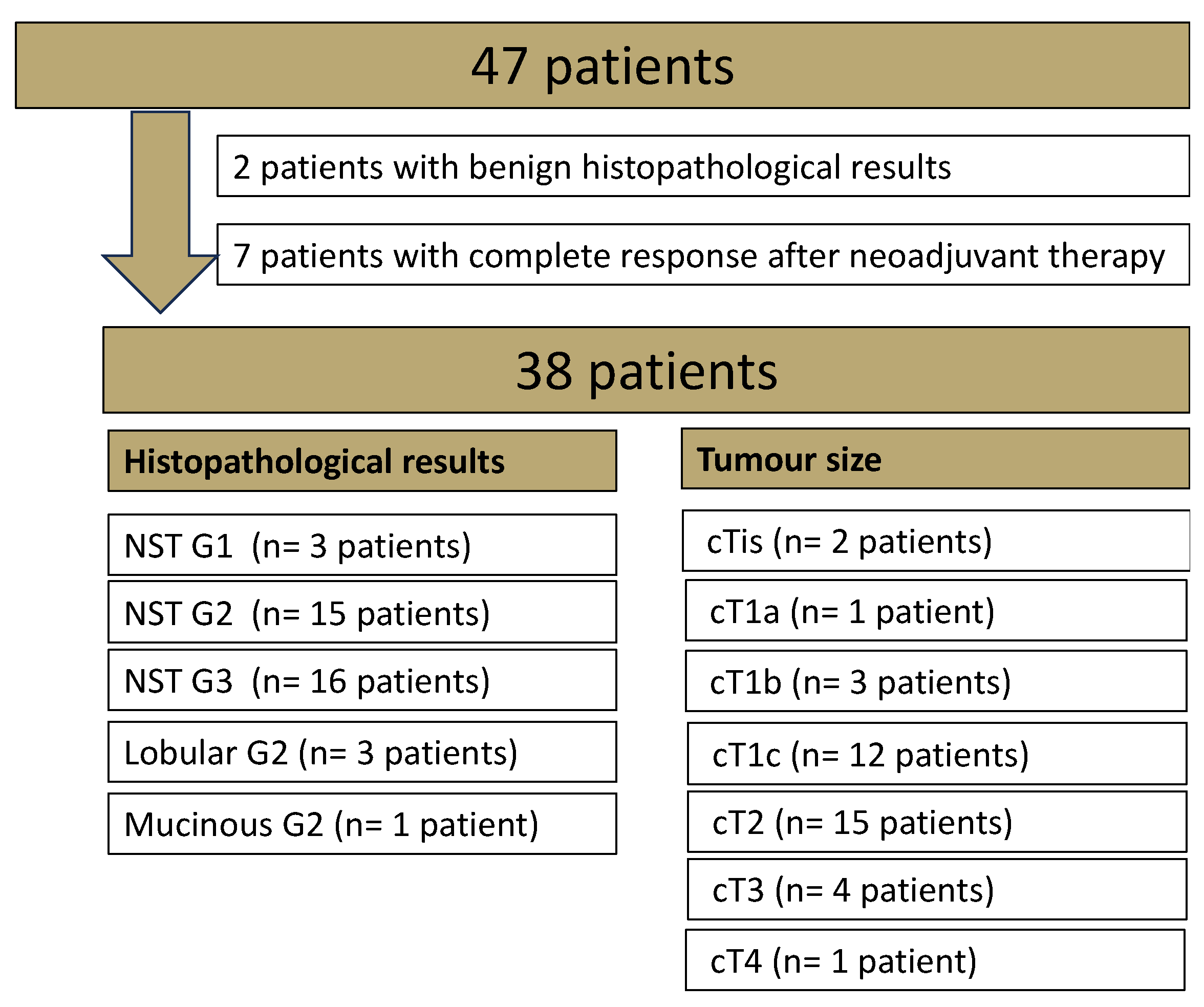
3.1.1. Patients
3.1.2. Qualitative Image Evaluation
Qualitative Image Evaluation for DWI
Qualitative Image Evaluation for ADC
3.1.3. Quantitative Image Evaluation
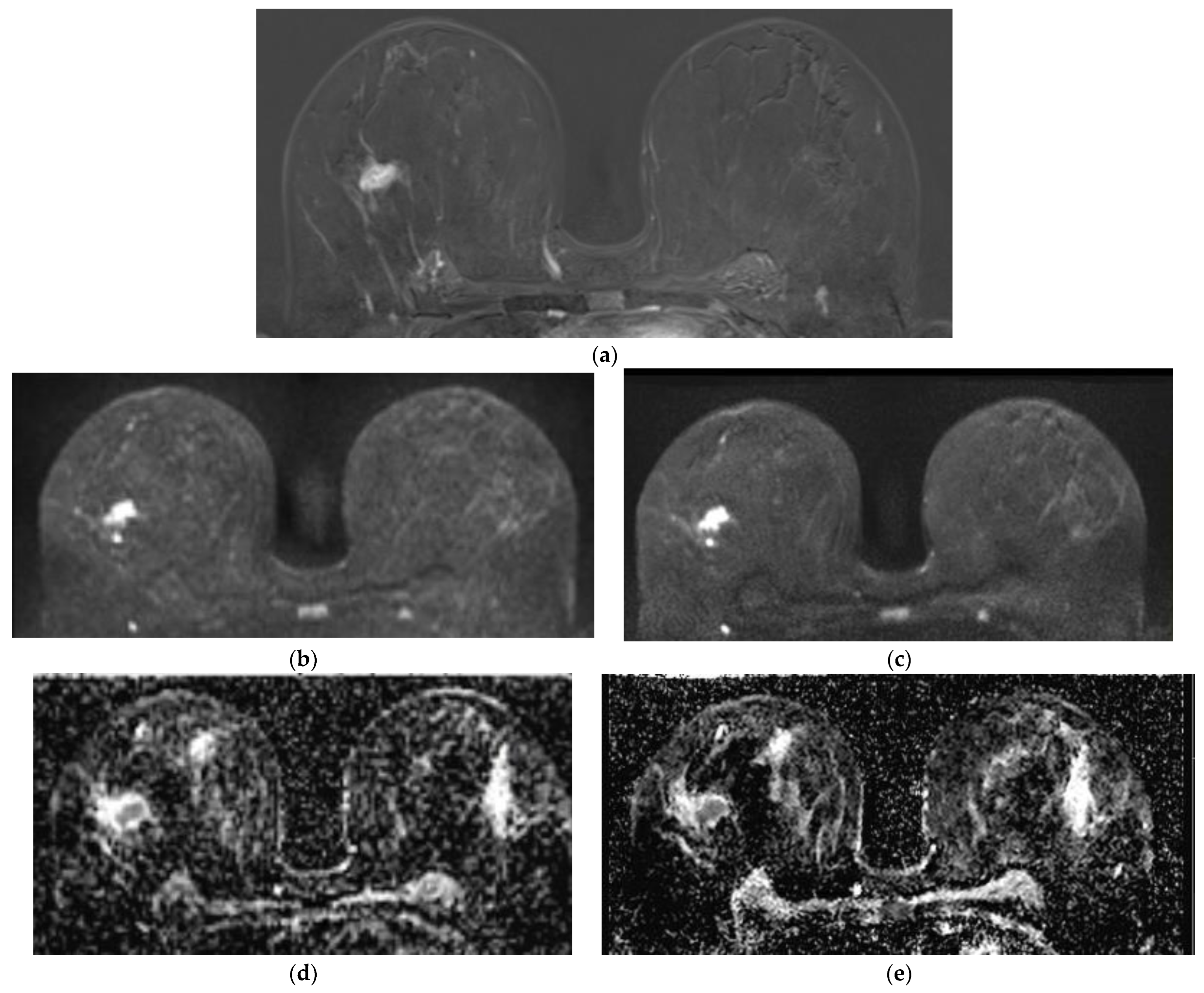
Lesion Visibility and Diameter
SNR
Image Acquisition Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mann, R.M.; Balleyguier, C.; Baltzer, P.A.; Bick, U.; Colin, C.; Cornford, E.; Evans, A.; Fallenberg, E.; Forrai, G.; Fuchsjager, M.H.; et al. Breast MRI: EUSOBI recommendations for women’s information. Eur. Radiol. 2015, 25, 3669–3678. [Google Scholar] [CrossRef] [PubMed]
- van der Molen, A.J.; Quattrocchi, C.C.; Mallio, C.A.; Dekkers, I.A.; European Society of Magnetic Resonance in Medicine, Biology Gadolinium Research, Educational Committee (ESMRMB-GREC). Ten years of gadolinium retention and deposition: ESMRMB-GREC looks backward and forward. Eur. Radiol. 2024, 34, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, C.K.; Schrading, S.; Strobel, K.; Schild, H.H.; Hilgers, R.D.; Bieling, H.B. Abbreviated breast magnetic resonance imaging (MRI): First postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 2304–2310. [Google Scholar] [CrossRef] [PubMed]
- Sardanelli, F.; Carbonaro, L.A.; Montemezzi, S.; Cavedon, C.; Trimboli, R.M. Clinical Breast MR Using MRS or DWI: Who Is the Winner? Front. Oncol. 2016, 6, 217. [Google Scholar] [CrossRef] [PubMed]
- Obara, M.; Kwon, J.; Yoneyama, M.; Ueda, Y.; Cauteren, M.V. Technical Advancements in Abdominal Diffusion-weighted Imaging. Magn. Reson. Med. Sci. 2023, 22, 191–208. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, M.; Min, Z.; Lu, J.; Lei, X.; Zhang, X. Accuracy of combined dynamic contrast-enhanced magnetic resonance imaging and diffusion-weighted imaging for breast cancer detection: A meta-analysis. Acta Radiol. 2016, 57, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Baltzer, P.; Mann, R.M.; Iima, M.; Sigmund, E.E.; Clauser, P.; Gilbert, F.J.; Martincich, L.; Partridge, S.C.; Patterson, A.; Pinker, K.; et al. Diffusion-weighted imaging of the breast-a consensus and mission statement from the EUSOBI International Breast Diffusion-Weighted Imaging working group. Eur. Radiol. 2020, 30, 1436–1450. [Google Scholar] [CrossRef] [PubMed]
- Wessling, D.; Gassenmaier, S.; Olthof, S.C.; Benkert, T.; Weiland, E.; Afat, S.; Preibsch, H. Novel deep-learning-based diffusion weighted imaging sequence in 1.5 T breast MRI. Eur. J. Radiol. 2023, 166, 110948. [Google Scholar] [CrossRef]
- Sauer, S.T.; Christner, S.A.; Lois, A.M.; Woznicki, P.; Curtaz, C.; Kunz, A.S.; Weiland, E.; Benkert, T.; Bley, T.A.; Baessler, B.; et al. Deep Learning k-Space-to-Image Reconstruction Facilitates High Spatial Resolution and Scan Time Reduction in Diffusion-Weighted Imaging Breast MRI. J. Magn. Reson. Imaging 2023, 60, 1190–1200. [Google Scholar] [CrossRef]
- Wilpert, C.; Neubauer, C.; Rau, A.; Schneider, H.; Benkert, T.; Weiland, E.; Strecker, R.; Reisert, M.; Benndorf, M.; Weiss, J.; et al. Accelerated Diffusion-Weighted Imaging in 3 T Breast MRI Using a Deep Learning Reconstruction Algorithm With Superresolution Processing: A Prospective Comparative Study. Investig. Radiol. 2023, 58, 842–852. [Google Scholar] [CrossRef]
- Kiryu, S.; Akai, H.; Yasaka, K.; Tajima, T.; Kunimatsu, A.; Yoshioka, N.; Akahane, M.; Abe, O.; Ohtomo, K. Clinical Impact of Deep Learning Reconstruction in MRI. Radiographics 2023, 43, e220133. [Google Scholar] [CrossRef] [PubMed]
- Chaika, M.; Afat, S.; Wessling, D.; Afat, C.; Nickel, D.; Kannengiesser, S.; Herrmann, J.; Almansour, H.; Mannlin, S.; Othman, A.E.; et al. Deep learning-based super-resolution gradient echo imaging of the pancreas: Improvement of image quality and reduction of acquisition time. Diagn. Interv. Imaging 2022, 104, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Feng, Y.S.; Gassenmaier, S.; Grunz, J.P.; Koerzdoerfer, G.; Lingg, A.; Almansour, H.; Nickel, D.; Othman, A.E.; Afat, S. Fast 5-minute shoulder MRI protocol with accelerated TSE-sequences and deep learning image reconstruction for the assessment of shoulder pain at 1.5 and 3 Tesla. Eur. J. Radiol. Open 2024, 12, 100557. [Google Scholar] [CrossRef] [PubMed]
- Gassenmaier, S.; Warm, V.; Nickel, D.; Weiland, E.; Herrmann, J.; Almansour, H.; Wessling, D.; Afat, S. Thin-Slice Prostate MRI Enabled by Deep Learning Image Reconstruction. Cancers 2023, 15, 578. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Darwish, O.; Benkert, T.; Weiland, E.; Nickel, M.D. Improved Readout-Segmented EPI Using Deep Learning Reconstruction. 2024. Available online: https://submissions.mirasmart.com/ISMRM2024/Itinerary/Login.aspx (accessed on 7 August 2024).
- Hammernik, K.; Klatzer, T.; Kobler, E.; Recht, M.P.; Sodickson, D.K.; Pock, T.; Knoll, F. Learning a variational network for reconstruction of accelerated MRI data. Magn. Reson. Med. 2018, 79, 3055–3071. [Google Scholar] [CrossRef]
- Tao, W.; Pan, Z.; Wu, G.; Tao, Q. The Strength of Nesterov’s Extrapolation in the Individual Convergence of Nonsmooth Optimization. IEEE Trans. Neural Netw. Learn. Syst. 2020, 31, 2557–2568. [Google Scholar] [CrossRef]
- Shi, W.; Caballero, J.; Huszár, F. Real-time single image and video super-resolution using an efficient sub-pixel convolutional neural net-work. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; pp. 1874–1883. [Google Scholar] [CrossRef]
- Song, S.E.; Woo, O.H.; Cho, K.R.; Seo, B.K.; Son, Y.H.; Grimm, R.; Liu, W.; Moon, W.K. Simultaneous Multislice Readout-Segmented Echo Planar Imaging for Diffusion-Weighted MRI in Patients With Invasive Breast Cancers. J. Magn. Reson. Imaging 2021, 53, 1108–1115. [Google Scholar] [CrossRef]
- Sheikh, A.; Hussain, S.A.; Ghori, Q.; Naeem, N.; Fazil, A.; Giri, S.; Sathian, B.; Mainali, P.; Al Tamimi, D.M. The spectrum of genetic mutations in breast cancer. Asian Pac. J. Cancer Prev. 2015, 16, 2177–2185. [Google Scholar] [CrossRef]
- Partridge, S.C.; Nissan, N.; Rahbar, H.; Kitsch, A.E.; Sigmund, E.E. Diffusion-weighted breast MRI: Clinical applications and emerging techniques. J. Magn. Reson. Imaging 2017, 45, 337–355. [Google Scholar] [CrossRef]
- Wekking, D.; Porcu, M.; De Silva, P.; Saba, L.; Scartozzi, M.; Solinas, C. Breast MRI: Clinical Indications, Recommendations, and Future Applications in Breast Cancer Diagnosis. Curr. Oncol. Rep. 2023, 25, 257–267. [Google Scholar] [CrossRef]
- Messina, C.; Bignone, R.; Bruno, A.; Bruno, A.; Bruno, F.; Calandri, M.; Caruso, D.; Coppolino, P.; Robertis, R.; Gentili, F.; et al. Diffusion-Weighted Imaging in Oncology: An Update. Cancers 2020, 12, 1493. [Google Scholar] [CrossRef] [PubMed]

| Protocol Parameter | DWIStd | DWIDL |
|---|---|---|
| Resolution | 2.2 × 2.2 × 3.0 mm | 0.8 (i) × 0.8 (i) × 3.0 mm |
| Acquisition time (TA) | 4:54 min | 3:49 min |
| Repitition time (TR)/ Echo time (TE) | 11,700/58 ms | 12,900/63 ms |
| Fat Saturation | Spectral attenuated inversion recovery (SPAIR) | SPAIR |
| Parallel imaging factor | 2 | 2 |
| b-values (averages) | 50 (4)/800 (16) s/mm2 | 50 (3)/800 (12) s/mm2 |
| Diffusion mode | 3D Diagonal | 3D Diagonal |
| Partial Fourier | None | 6/8 |
| Deep Learning (DL) | None | DL reconstruction, DL super resolution |
| Reader 1 | Reader 2 | Interreader Reliability (r) | ||||||
|---|---|---|---|---|---|---|---|---|
| Image Parameters DWI Sequence | DWIStd Mean (SD) | DWIDL Mean (SD) | p-Value | DWIStd Mean (SD) | DWIDL Mean (SD) | p-Value | DWIStd | DWIDL |
| Overall Image Quality | ||||||||
| IQ 1 | 3.86 (0.58) | 4.49 (0.65) | <0.001 | 3.92 (0.54) | 4.70 (0.46) | <0.001 | 0.746 | 0.585 |
| Sharpness | 3.86 (0.67) | 4.68 (0.58) | <0.001 | 3.78 (0.41) | 4.76 (0.49) | <0.001 | 0.684 | 0.782 |
| Noise | 4.00 (0.68) | 4.65 (0.63) | <0.001 | 4.08 (0.49) | 4.78 (0.47) | <0.001 | 0.403 | 0.567 |
| Contrast | 4.49 (0.76) | 4.65 (0.67) | 0.010 | 4.36 (0.68) | 4.62 (0.54) | 0.010 | 0.730 | 0.609 |
| Artifacts | 4.16 (0.72) | 4.43 (0.76) | 0.020 | 4.46 (0.55) | 4.16 (0.60) | 0.010 | 0.702 | 0.387 |
| DC 2 | 4.49 (0.98) | 4.59 (0.89) | 0.100 | 4.59 (0.83) | 4.73 (0.60) | 0.020 | 0.955 | 0.915 |
| Reader 1 | Reader 2 | Interreader Reliability (r) | ||||||
|---|---|---|---|---|---|---|---|---|
| Image Parameters ADC | ADCStd Mean (SD) | ADCIDL Mean (SD) | p-Value | ADCStd Mean (SD) | ADCDL Mean (SD) | p-Value | ADCStd | ADCDL |
| Overall Image Quality | ||||||||
| IQ 1 | 3.41 (0.59) | 3.95 (0.91) | <0.001 | 3.46 (0.50) | 4.11 (0.51) | <0.001 | 0.377 | 0.486 |
| Sharpness | 3.41 (0.68) | 4.05 (0.91 | <0.001 | 3.41 (0.59) | 4.16 (0.72) | <0.001 | 0.671 | 0.615 |
| Noise | 3.24 (0.64) | 3.62 (0.89) | <0.001 | 3.24 (0.49) | 3.73 (0.69) | <0.001 | 0.596 | 0.548 |
| Contrast | 3.51 (0.83) | 3.76 (0.89) | <0.001 | 3.86 (0.48) | 3.62 (0.54) | 0.020 | 0.522 | 0.376 |
| Artifacts | 3.41 (0.68) | 3.81 (0.93) | <0.001 | 3.78 (0.53) | 3.73 (0.69) | 0.660 | 0.474 | 0.30 |
| DC 2 | 3.62 (1.06) | 3.97 (1.04) | <0.001 | 3.62 (1.01) | 3.78 (1.03) | 0.030 | 0.743 | 0.668 |
| Lesion Size in mm | Std | DL |
|---|---|---|
| DWI b 800 | 24.13 (−4.90%) | 23.94 (−5.7%) |
| ADC (mm2/s) | 24.00 (−5.20%) | 23.48 (−7.2%) |
| 2nd SUB | 25.3 | |
| Quantitative Image Parameters (Lesion) | DWIStd Mean (SD 1) | DWIDL Mean (SD 1) | p-Value |
|---|---|---|---|
| ADC (mm2/s) | 936.22 (262.47) | 980.78 (274.00) | 0.02 |
| Noise | DWIStd SD 1 | DWIDL SD 1 | p-Value | SNR 2 | DWIStd SNR 2 | DWIDL SNR 2 | p-Value |
|---|---|---|---|---|---|---|---|
| b 50 | 96.78 | 88.61 | 0.001 | b 50 | 4.17 | 4.36 | 0.073 |
| b 800 | 29.72 | 27.59 | <0.001 | b 800 | 7.27 | 7.29 | 0.925 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olthof, S.-C.; Weiland, E.; Benkert, T.; Wessling, D.; Leyhr, D.; Afat, S.; Nikolaou, K.; Preibsch, H. Optimizing Image Quality with High-Resolution, Deep-Learning-Based Diffusion-Weighted Imaging in Breast Cancer Patients at 1.5 T. Diagnostics 2024, 14, 1742. https://doi.org/10.3390/diagnostics14161742
Olthof S-C, Weiland E, Benkert T, Wessling D, Leyhr D, Afat S, Nikolaou K, Preibsch H. Optimizing Image Quality with High-Resolution, Deep-Learning-Based Diffusion-Weighted Imaging in Breast Cancer Patients at 1.5 T. Diagnostics. 2024; 14(16):1742. https://doi.org/10.3390/diagnostics14161742
Chicago/Turabian StyleOlthof, Susann-Cathrin, Elisabeth Weiland, Thomas Benkert, Daniel Wessling, Daniel Leyhr, Saif Afat, Konstantin Nikolaou, and Heike Preibsch. 2024. "Optimizing Image Quality with High-Resolution, Deep-Learning-Based Diffusion-Weighted Imaging in Breast Cancer Patients at 1.5 T" Diagnostics 14, no. 16: 1742. https://doi.org/10.3390/diagnostics14161742





