Performance Evaluation of Open Channel Buhlmann Fecal Calprotectin Turbo Assay on Abbott Alinity C Analyzer
Abstract
1. Introduction
2. Materials and Methods
2.1. Precision Testing
2.2. Determination of Analytical Measurement Range (AMR) and Clinical Reportable Range (CRR)
2.3. Method Comparison
3. Data Analysis
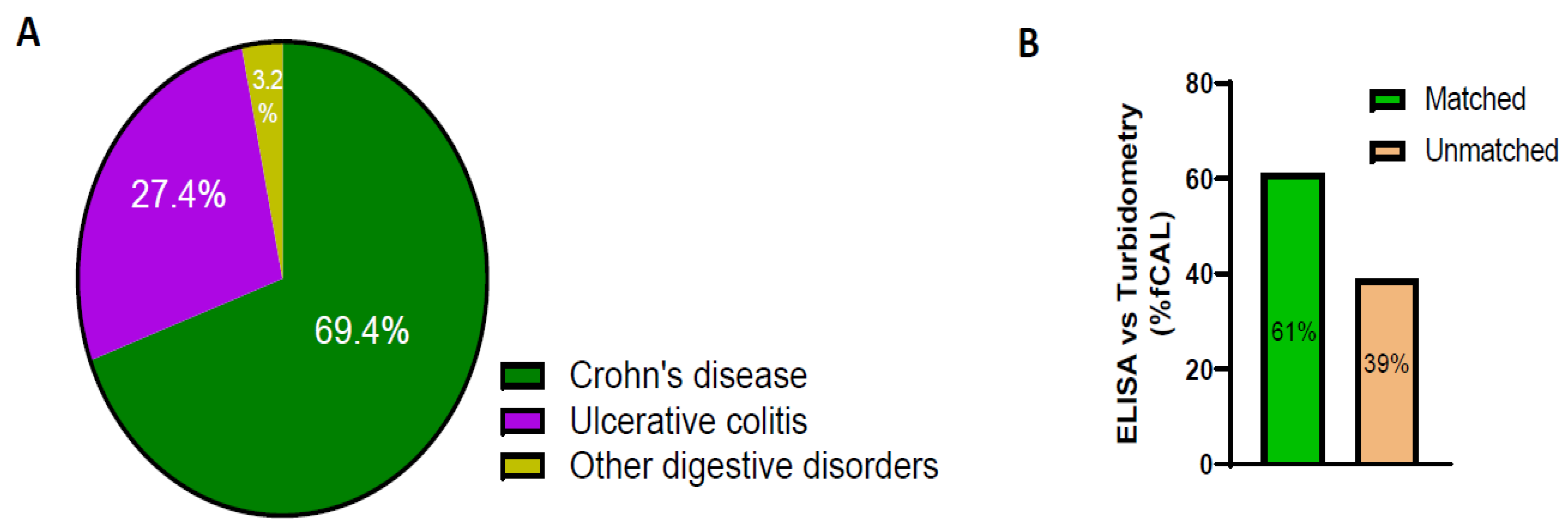
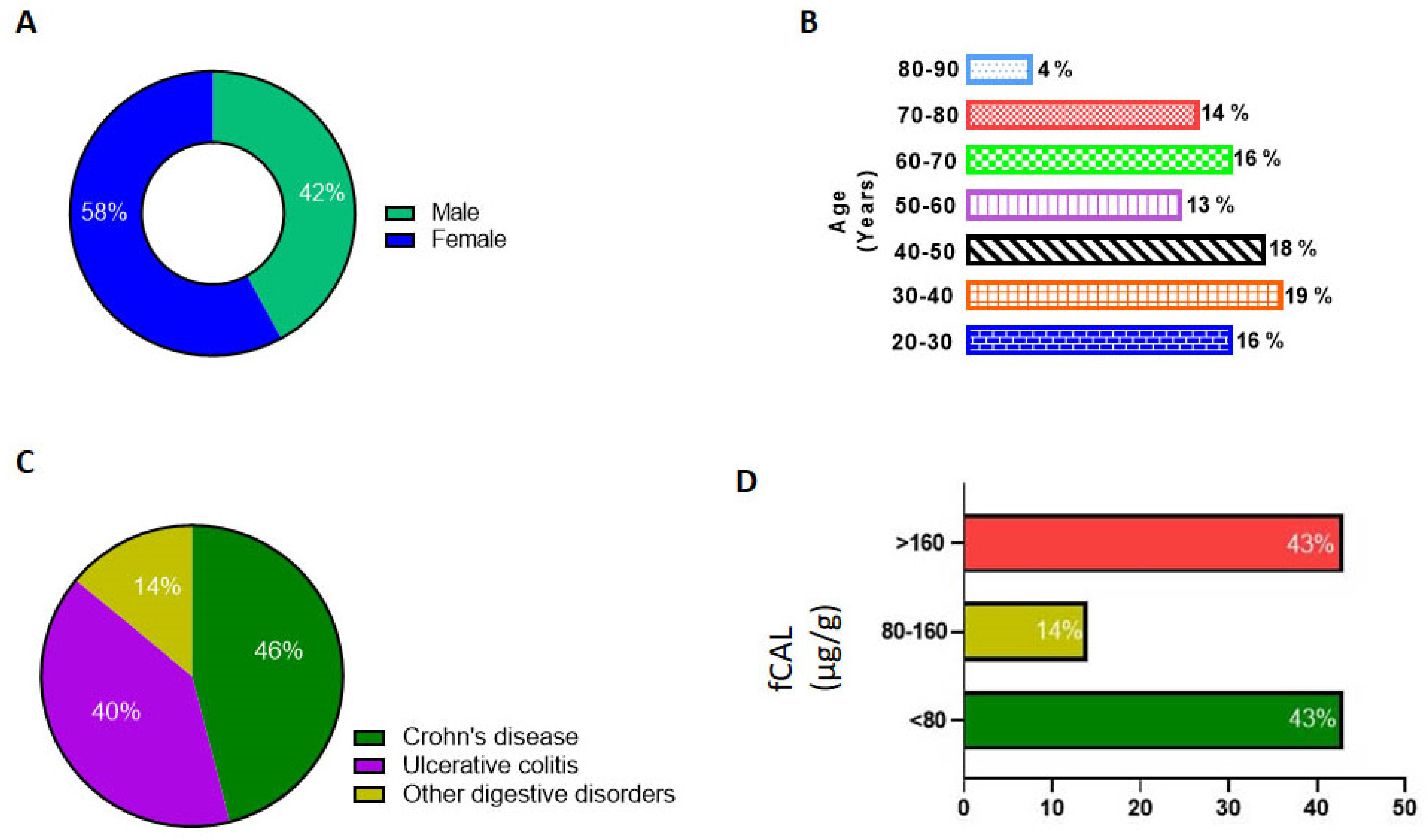
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spiceland, C.M.; Lodhia, N. Endoscopy in inflammatory bowel disease: Role in diagnosis, management, and treatment. World J. Gastroenterol. 2018, 24, 4014–4020. [Google Scholar] [CrossRef] [PubMed]
- Reghefaoui, M.; Peresuodei, T.S.; Saavedra Palacios, M.S.; Gill, A.; Orji, C.; Reghefaoui, T.; Mostafa, J. The Role of Serological Markers in the Prediction of Disease Course and Response to Therapy in Inflammatory Bowel Disease. Cureus 2023, 15, e48442. [Google Scholar] [CrossRef] [PubMed]
- Stallhofer, J.; Friedrich, M.; Konrad-Zerna, A.; Wetzke, M.; Lohse, P.; Glas, J.; Tillack-Schreiber, C.; Schnitzler, F.; Beigel, F.; Brand, S. Lipocalin-2 Is a Disease Activity Marker in Inflammatory Bowel Disease Regulated by IL-17A, IL-22, and TNF-α and Modulated by IL23R Genotype Status. Inflamm. Bowel Dis. 2015, 21, 2327–2340. [Google Scholar] [CrossRef] [PubMed]
- Fagan, E.A.; Dyck, R.F.; Maton, P.N.; Hodgson, H.J.; Chadwick, V.S.; Petrie, A.; Pepys, M.B. Serum levels of C-reactive protein in Crohn’s disease and ulcerative colitis. Eur. J. Clin. Investig. 1982, 12, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Bermejo, F.; Pérez-Calle, J.-L.; Taxonera, C.; Vera, I.; McNicholl, A.G.; Algaba, A.; López, P.; López-Palacios, N.; Calvo, M.; et al. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm. Bowel Dis. 2009, 15, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Landers, C.J.; Tremaine, W.J.; Targan, S.R. Association of Antineutrophil Cytoplasmic Antibodies With Resistance to Treatment of Left-Sided Ulcerative Colitis: Results of a Pilot Study. Mayo Clin. Proc. 1996, 71, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Andalucía, C.; Martínez-Prat, L.; Bentow, C.; Aure, M.A.; Horn, M.P.; Mahler, M. Clinical Validity of Anti-Proteinase 3 Antibodies in Patients with Inflammatory Bowel Disease: A Short Meta-Analysis. Diagnostics 2023, 13, 3682. [Google Scholar] [CrossRef] [PubMed]
- Mavropoulou, E.; Mechie, N.C.; Knoop, R.; Petzold, G.; Ellenrieder, V.; Kunsch, S.; Pilavakis, Y.; Amanzada, A. Asso-ciation of serum interleukin-6 and soluble interleukin-2-receptor levels with disease activity status in patients with inflammatory bowel disease: A prospective observational study. PLoS ONE 2020, 15, e0233811. [Google Scholar] [CrossRef] [PubMed]
- Roseth, A.G.; Schmidt, P.N.; Fagerhol, M.K. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand. J. Gastroenterol. 1999, 34, 50–54. [Google Scholar]
- Liu, R.; Li, D.; Haritunians, T.; Ruan, Y.; Daly, M.J.; Huang, H.; McGovern, D.P.B. Profiling the inflammatory bowel diseases using genetics, serum biomarkers, and smoking information. iScience 2023, 26, 108053. [Google Scholar] [CrossRef]
- Hamilton, A.L.; Kamm, M.A.; De Cruz, P.; Wright, E.K.; Selvaraj, F.; Princen, F.; Gorelik, A.; Liew, D.; Lawrance, I.C.; An-drews, J.M.; et al. Serologic antibodies in relation to outcome in postoperative Crohn’s disease. J. Gastroenterol. Hepatol. 2016, 32, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Lodes, M.J.; Cong, Y.; Elson, C.O.; Mohamath, R.; Landers, C.J.; Targan, S.R.; Fort, M.; Hershberg, R.M. Bacterial flagellin is a dominant antigen in Crohn disease. J. Clin. Investig. 2004, 113, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Andreu-Sanchez, S.; Vogl, T.; Hu, S.; Vich Vila, A.; Gacesa, R.; Leviatan, S.; Kurilshikov, A.; Klompus, S.; Kalka, I.N.; et al. Phage-display immunoprecipitation sequencing of the antibody epitope repertoire in inflammatory bowel disease reveals distinct antibody signatures. Immunity 2023, 56, 1393–1409.e6. [Google Scholar] [CrossRef]
- Galipeau, H.J.; Caminero, A.; Turpin, W.; Bermudez-Brito, M.; Santiago, A.; Libertucci, J.; Constante, M.; Raygoza Garay, J.A.; Rueda, G.; Armstrong, S.; et al. Novel Fecal Biomarkers That Precede Clinical Diagnosis of Ulcerative Colitis. Gastroenterology 2021, 160, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Dai, Y.; Zhang, L.; Wang, D.; Hu, W.; Yu, Q.; Wang, X.; Yu, P.; Liu, W.; Ping, Y.; et al. Combined Use of Fecal Biomarkers in Inflammatory Bowel Diseases: Oncostatin M and Calprotectin. J. Inflamm. Res. 2021, 14, 6409–6419. [Google Scholar] [CrossRef] [PubMed]
- Røseth, A.G.; Fagerhol, M.K.; Aadland, E.; Schjønsby, H. Assessment of the Neutrophil Dominating Protein Calprotectin in Feces: A Methodologic Study. Scand. J. Gastroenterol. 1992, 27, 793–798. [Google Scholar] [CrossRef]
- Pathirana, W.G.W.; Chubb, S.P.; Gillett, M.J.; Vasikaran, S.D. Faecal Calprotectin. Clin. Biochem. Rev. 2018, 39, 77–90. [Google Scholar]
- Azramezani Kopi, T.; Shahrokh, S.; Mirzaei, S.; Asadzadeh Aghdaei, H.; Amini Kadijani, A. The role of serum cal-protectin as a novel biomarker in inflammatory bowel diseases: A review study. Gastroenterol. Hepatol. Bed. Bench. 2019, 12, 183–189. [Google Scholar] [PubMed]
- Hart, L.; Chavannes, M.; Kherad, O.; Maedler, C.; Mourad, N.; Marcus, V.; Afif, W.; Bitton, A.; Lakatos, P.L.; Brassard, P.; et al. Faecal Calprotectin Predicts Endoscopic and Histological Activity in Clinically Quiescent Ulcerative Colitis. J. Crohn’s Colitis 2020, 14, 46–52. [Google Scholar] [CrossRef]
- Tham, Y.S.; Yung, D.E.; Fay, S.; Yamamoto, T.; Ben-Horin, S.; Eliakim, R.; Koulaouzidis, A.; Kopylov, U. Fecal cal-protectin for detection of postoperative endoscopic recurrence in Crohn’s disease: Systematic review and meta-analysis. Therap. Adv. Gastroenterol. 2018, 11, 1756284818785571. [Google Scholar] [CrossRef]
- De Sloovere, M.M.W.; De Smet, D.; Baert, F.J.; Debrabandere, J.; Vanpoucke, H.J.M. Analytical and diagnostic performance of two automated fecal calprotectin immunoassays for detection of inflammatory bowel disease. Clin. Chem. Lab. Med. 2017, 55, 1435–1446. [Google Scholar] [CrossRef] [PubMed]
- Garnett, E.; Pagaduan, J.; Rajapakshe, D.; Tam, E.; Kellermayer, R.; Devaraj, S. Validation of the newly FDA-approved Buhlmann fCal Turbo assay for measurement of fecal calprotectin in a pediatric population. Pract. Lab. Med. 2020, 22, e00178. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, T.; Sunde, K.; Hansson, L.O.; Havelka, A.M.; Larsson, A. A novel turbidimetric immunoassay for fecal cal-protectin optimized for routine chemistry analyzers. J. Clin. Lab. Anal. 2017, 31, e22061. [Google Scholar] [CrossRef] [PubMed]
- Mandic-Havelka, A.; Nilsen, T.; Sunde, K.; Norell, M.; Hansson, L.O.; Larsson, A. Turbidimetric Determination of Fecal Calprotectin Using Two Table Top Chemistry Analyzers: Mindray BS-200E and Cobas(R) c111. Clin. Lab. 2017, 63, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Noebauer, B.; Ramic, L.; Konstantin, A.; Zachbauer, C.; Einwallner, E. Analytical evaluation of a fully automated immunoassay for faecal calprotectin in a paediatric setting. Biochem. Medica 2017, 27, 030710. [Google Scholar] [CrossRef] [PubMed]
- Handley, S.A.; Dote, N.P.; Wanandy, T.; Prentice, L. Verification of the Buhlmann fCAL turbo faecal calprotectin assay on the Binding Site Optilite benchtop analyzer. Pract. Lab. Med. 2023, 36, e00318. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, V.; Berodes, M.; Lukas, P.; Louis, E.; Cavalier, E.; Lutteri, L. New Faecal Calprotectin Assay by IDS: Validation and Comparison to DiaSorin Method. Diagnostics 2022, 12, 2338. [Google Scholar] [CrossRef]
- Labaere, D.; Smismans, A.; Van Olmen, A.; Christiaens, P.; D’haens, G.; Moons, V.; Cuyle, P.-J.; Frans, J.; Bossuyt, P. Comparison of six different calprotectin assays for the assessment of inflammatory bowel disease. United Eur. Gastroenterol. J. 2014, 2, 30–37. [Google Scholar] [CrossRef]
- Blad, N.; Palmqvist, R.; Karling, P. Pre-diagnostic faecal calprotectin levels in patients with colorectal cancer: A retro-spective study. BMC Cancer 2022, 22, 315. [Google Scholar] [CrossRef] [PubMed]
- Oka, A.; Kawashima, K.; Kishimoto, K.; Kotani, S.; Fukunaga, M.; Fukuba, N.; Mishima, Y.; Oshima, N.; Ishimura, N.; Awoniyi, M.; et al. Validation of rapid fecal calprotectin assay using particle enhanced turbidimetric immunoassay for inflammatory bowel disease. Sci. Rep. 2024, 14, 1653. [Google Scholar] [CrossRef]



| Within-Day (n = 40) | Between-Day (n = 40) | |||
|---|---|---|---|---|
| QC | Mean (µg/g) | %CV | Mean (µg/g) | %CV |
| QC Level 1 | 85 | 5.3 | 84 | 2.5 |
| QC Level 2 | 291 | 1.7 | 290 | 1.9 |
| N | Est | Mean | Residual | % Recovery | |
|---|---|---|---|---|---|
| 39.7 | 3 | 29.76 | 31.00 | 1.24 | 78.1 |
| 119.2 | 3 | 110.35 | 105.43 | −4.91 | 88.5 |
| 238.4 | 3 | 231.18 | 229.83 | −1.35 | 96.4 |
| 476.9 | 3 | 472.95 | 472.37 | −0.58 | 99.0 |
| 953.5 | 3 | 956.08 | 954.13 | −1.94 | 100.1 |
| 1430.6 | 3 | 1439.71 | 1463.60 | 23.89 | 102.3 |
| 1907.5 | 3 | 1923.15 | 1906.80 | −16.35 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sataranatarajan, K.; Adhikari, S.; Nguyen, N.; Narasimhan, M.; Balani, J.; Muthukumar, A. Performance Evaluation of Open Channel Buhlmann Fecal Calprotectin Turbo Assay on Abbott Alinity C Analyzer. Diagnostics 2024, 14, 1744. https://doi.org/10.3390/diagnostics14161744
Sataranatarajan K, Adhikari S, Nguyen N, Narasimhan M, Balani J, Muthukumar A. Performance Evaluation of Open Channel Buhlmann Fecal Calprotectin Turbo Assay on Abbott Alinity C Analyzer. Diagnostics. 2024; 14(16):1744. https://doi.org/10.3390/diagnostics14161744
Chicago/Turabian StyleSataranatarajan, Kavithalakshmi, Shishir Adhikari, Ngoc Nguyen, Madhusudhanan Narasimhan, Jyoti Balani, and Alagarraju Muthukumar. 2024. "Performance Evaluation of Open Channel Buhlmann Fecal Calprotectin Turbo Assay on Abbott Alinity C Analyzer" Diagnostics 14, no. 16: 1744. https://doi.org/10.3390/diagnostics14161744
APA StyleSataranatarajan, K., Adhikari, S., Nguyen, N., Narasimhan, M., Balani, J., & Muthukumar, A. (2024). Performance Evaluation of Open Channel Buhlmann Fecal Calprotectin Turbo Assay on Abbott Alinity C Analyzer. Diagnostics, 14(16), 1744. https://doi.org/10.3390/diagnostics14161744






