Benefit of Optical Coherence Tomography–Angiography in Patients Undergoing Transsphenoidal Pituitary Adenoma Surgery: A Prospective Controlled Study
Abstract
1. Introduction
2. Materials and Methods
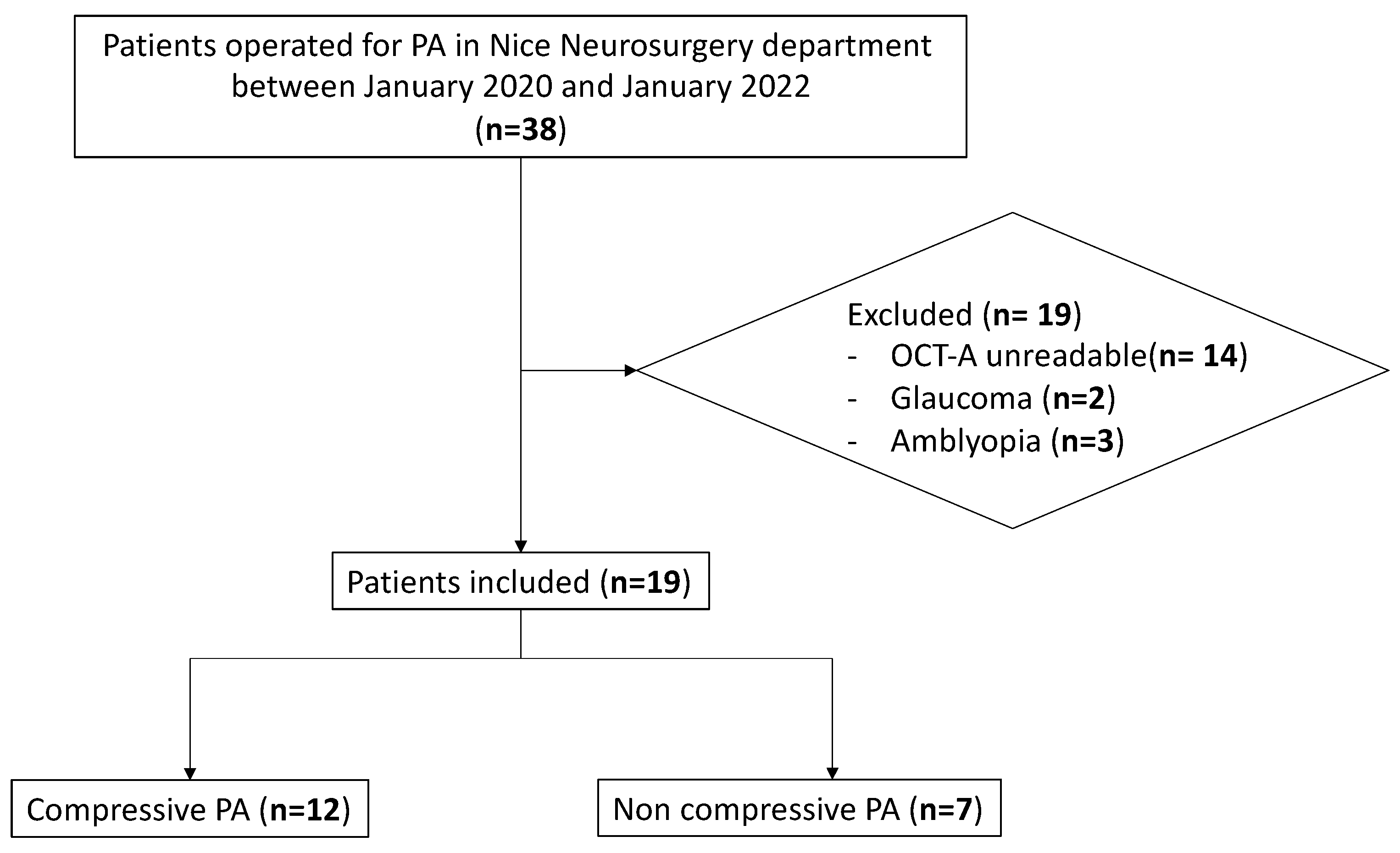
2.1. Study Design and Participants
2.2. Data Recorded
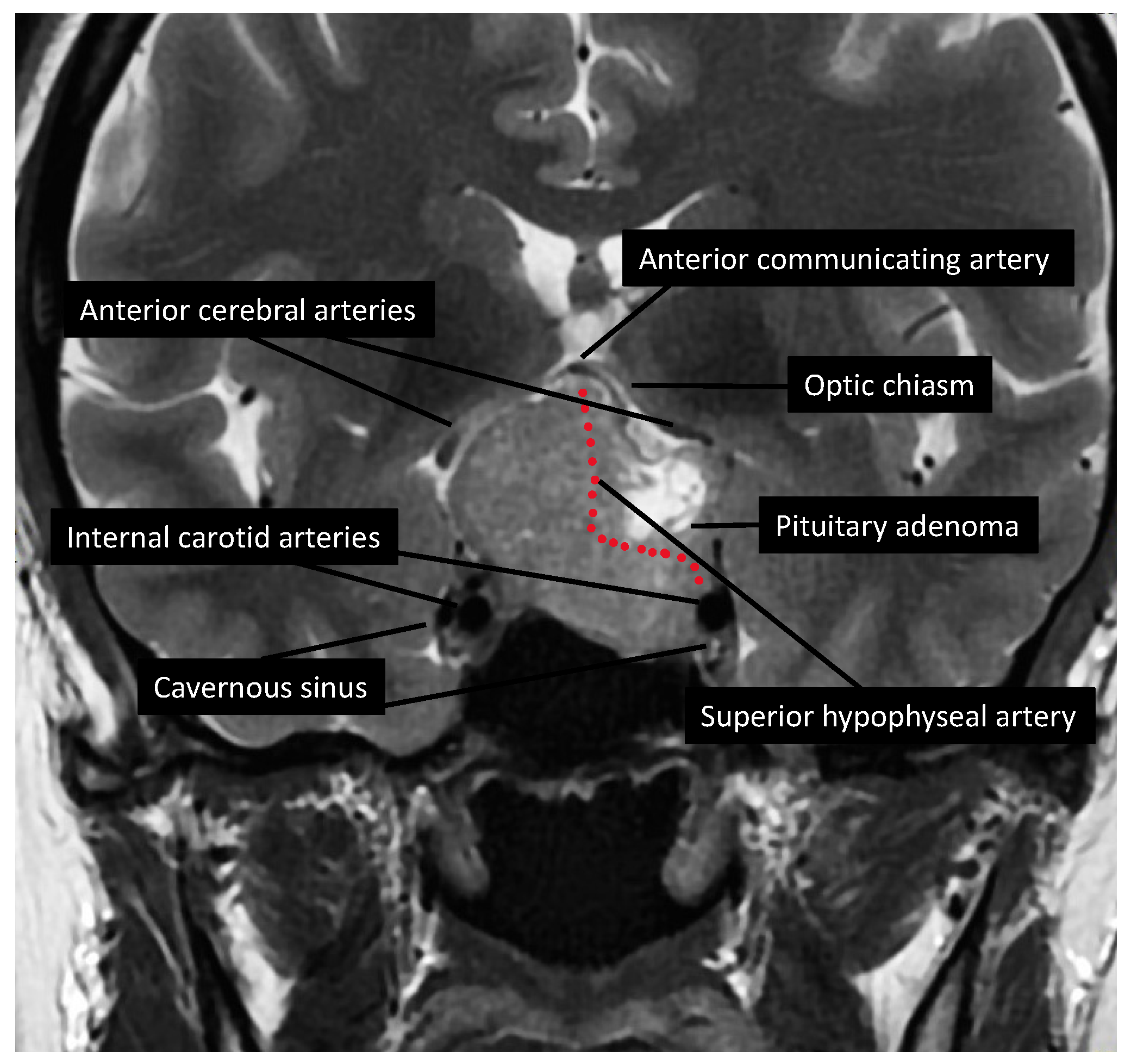
2.3. Brain Imaging
2.4. Static Automated Perimetry
2.5. Spectral-Domain Optical Coherence Tomography
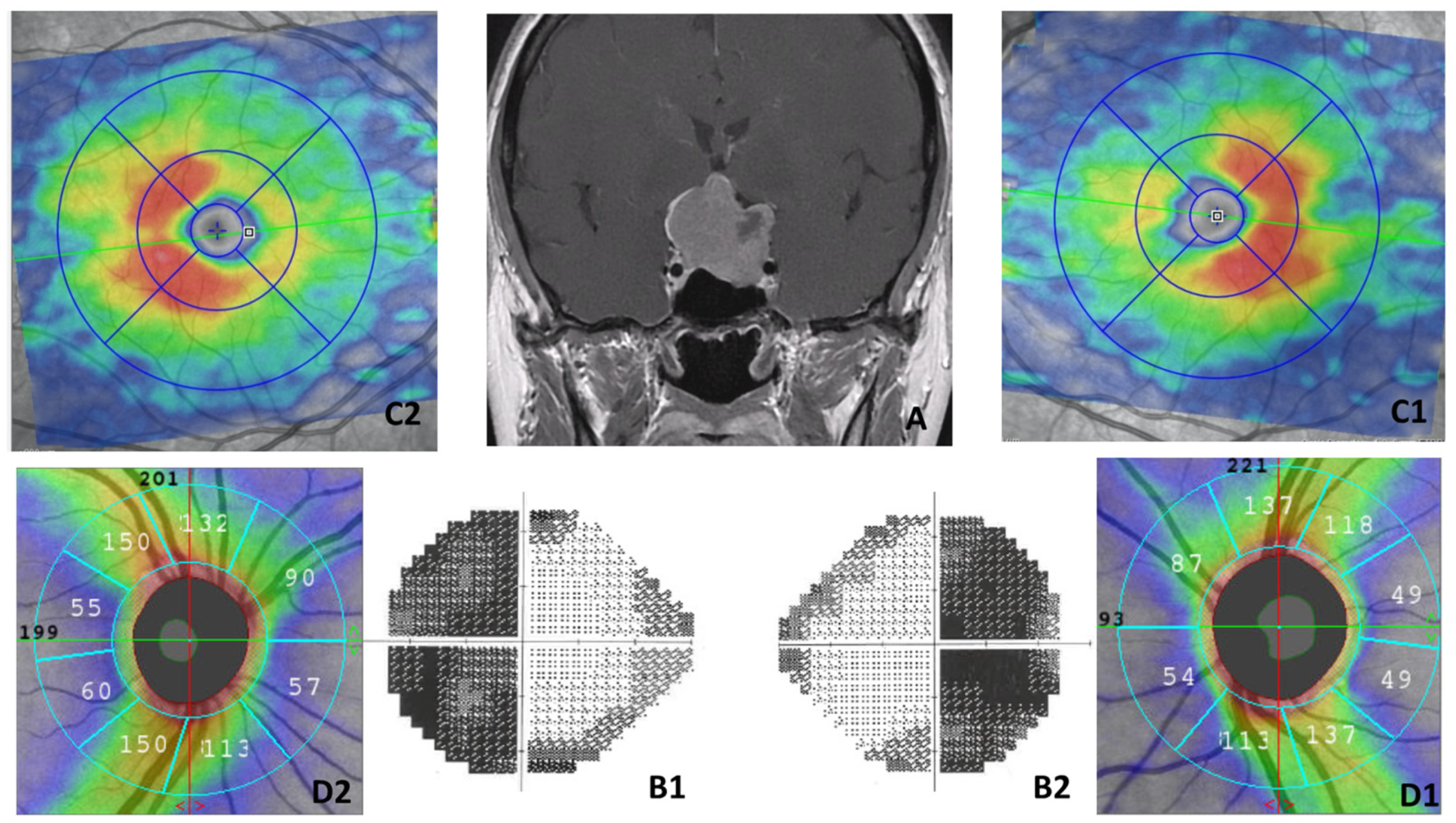
2.6. Optical Coherence Tomography–Angiography
2.7. Neurosurgery
2.8. Primary Outcomes
2.9. Statistics
3. Results
3.1. Patients’ Baseline Characteristics
3.2. Surgical Outcomes
3.3. VF and SD-OCT Parameter Outcomes
3.4. OCT-A Parameter Outcomes
3.5. Preoperative Prognostic Factors for Postoperative VF Improvement
4. Discussion
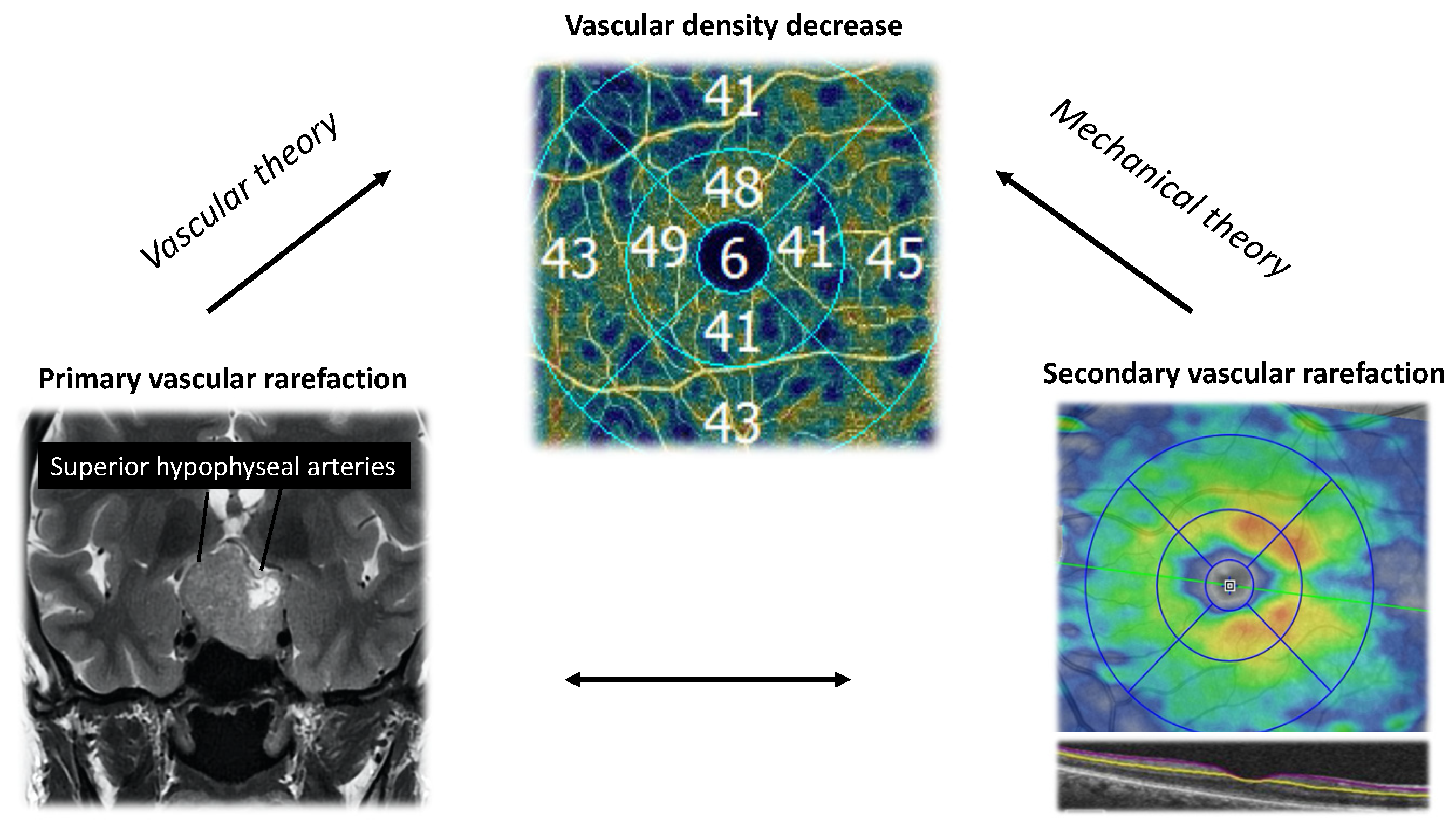
4.1. The Mechanical and Vascular Theories
4.2. Main Results
4.3. Study Strengths
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


| Author Year | Country | Number of Eyes (Case/Control) | Mean Age (Years) | Design | Mean Follow-Up (Months) | Mean Preoperative VF Mean Deviation (dB): Cases/Controls | Mean Preoperative Macular sVD (%): Cases/Controls | Mean Preoperative Disc ppVD (%): Cases/Controls | Main Findings |
|---|---|---|---|---|---|---|---|---|---|
| Our study | France | 38 (24/14) | 55 | Prospective comparative cohort | 6 | −6.09/−0.52 | 49.01/49.61 | 52.15/58.83 | 1. At baseline, the VF mean deviation, the RNFL and GCC thicknesses, and the papillary VD were significantly reduced in the CPA group. 2. Macular and papillary VD were not increased following surgical decompression, suggesting that the vascular theory is unlikely to explain the visual impairment in CPA. 3. None of the baseline OCT-A parameters were predictive of postoperative visual field improvement. |
| Ga-In Lee et al., 2021 [15] | Korea | 106 (44/62) | 50.2 | Retrospective cohort | 4–6 | −8.03/0.09 | 47.34/48.62 | 56.15/58.32 | 1. At baseline, the RNFL and GCC thicknesses, the macular sVD and the papillary VD were reduced in patients compared to control subjects. 2. Decrease in macular sVD, dVD and papillary VD after surgery compared to baseline values. 3. The postoperative visual field correlated with the postoperative macular sVD. |
| Lee et al., 2020 [16] | Korea | 99 (42/57) | 49 | Retrospective cohort | 10 | −7.27/0.07 | 47.46/48.67 | 56.91/58.41 | 1. The preoperative perimetric mean deviation and macular sVD correlated with the postoperative perimetric mean deviation. |
| Ben Ghezala et al., 2021 [14] | France | 56 (24/32) | 49.4 | Prospective cohort | 6 | −8.45/NA | NA | 57.53/58.53 | 1. The baseline VD was lower in patients with chiasmal compression compared to control subjects. 2. The postoperative VD was decreased compared to baseline values. |
| Dallorto et al., 2020 [13]. | France | 33 (16/17) | 48.8 | Retrospective cross-sectional | NA | NA | 44.87/48.96 | 47.81/50.52 | 1. The peripapillary VD and macular sVD were decreased in CPA patients compared to control subjects. 2. The peripapillary VD correlated with the RNFL thickness and VF mean deviation before surgery. |
References
- Cironi, K.A.; Decater, T.; Iwanaga, J.; Dumont, A.S.; Tubbs, R.S. Arterial supply to the pituitary gland: A comprehensive review. World Neurosurg. 2020, 142, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.C.; Kriss, F.C.; Falls, H.F. Prechiasmal infarction associated with intrachiasmal and suprasellar tumors. J. Neurosurg. 1970, 32, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Danesh-Meyer, H.V.; Carroll, S.C.; Foroozan, R.; Savino, P.J.; Fan, J.; Jiang, Y.; Hoorn, S.V. Relationship between retinal nerve fiber layer and visual field sensitivity as measured by optical coherence tomography in chiasmal compression. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4827–4835. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.H.; Hwang, S.C.; Kim, B.-T.; Ohn, Y.-H.; Park, T.K. Visual prognostic value of optical coherence tomography and photopic negative response in chiasmal compression. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8527–8533. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.; Raverot, G.; Jouanneau, E.; Borson-Chazot, F.; Perrin, G.; Rabilloud, M.; Tilikete, C.; Bernard, M.; Vighetto, A. Predicting visual outcome after treatment of pituitary adenomas with optical coherence tomography. Am. J. Ophthalmol. 2009, 147, 64–70.e2. [Google Scholar] [CrossRef]
- Moon, C.H.; Hwang, S.C.; Ohn, Y.-H.; Park, T.K. The time course of visual field recovery and changes of retinal ganglion cells after optic chiasmal decompression. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7966–7973. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, S.; Higashide, T.; Takeda, H.; Murotani, E.; Hayashi, Y.; Sugiyama, K. Relationship between macular ganglion cell complex parameters and visual field parameters after tumor resection in chiasmal compression. Jpn. J. Ophthalmol. 2012, 56, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kim, J.-A.; Kim, T.-W.; Kim, H.; Yang, H.K.; Hwang, J.-M. Glaucoma-like parapapillary choroidal microvasculature dropout in patients with compressive optic neuropathy. Ophthalmology 2020, 127, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- Ghahari, E.; Bowd, C.; Zangwill, L.M.; Proudfoot, J.; Hasenstab, K.A.; Hou, H.; Penteado, R.C.; Manalastas, P.I.C.; Moghimi, S.; Shoji, T.; et al. Association of macular and circumpapillary microvasculature with visual field sensitivity in advanced glaucoma. Am. J. Ophthalmol. 2019, 204, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kim, T.-W.; Kim, J.-A.; Kim, J.-A. Central visual field damage and parapapillary choroidal microvasculature dropout in primary open-angle glaucoma. Ophthalmology 2018, 125, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Gaier, E.D.; Wang, M.; Gilbert, A.L.; Rizzo, I.I.I.J.F.; Cestari, D.M.; Miller, J.B. Quantitative analysis of optical coherence tomographic angiography (OCT-A) in patients with non-arteritic anterior ischemic optic neuropathy (NAION) corresponds to visual function. PLoS ONE 2018, 13, e0199793. [Google Scholar] [CrossRef] [PubMed]
- Higashiyama, T.; Nishida, Y.; Ohji, M. Optical coherence tomography angiography in eyes with good visual acuity recovery after treatment for optic neuritis. PLoS ONE 2017, 12, e0172168. [Google Scholar] [CrossRef] [PubMed]
- Dallorto, L.; Lavia, C.; Jeannerot, A.L.; Shor, N.; Jublanc, C.; Boch, A.L.; Bodaghi, B.; Tadayoni, R.; Touitou, V.; Bonnin, S. Retinal microvasculature in pituitary adenoma patients: Is optical coherence tomography angiography useful? Acta Ophthalmol. 2020, 98, e585–e592. [Google Scholar] [CrossRef]
- Ben Ghezala, I.; Haddad, D.; Blanc, J.; Meillon, C.; Madkouri, R.; Borsotti, F.; Bron, A.M.; Creuzot-Garcher, C. Peripapillary microvascularization analysis using swept-source optical coherence tomography angiography in optic chiasmal compression. J. Ophthalmol. 2021, 2021, 5531959. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-I.; Park, K.-A.; Oh, S.Y.; Kong, D.-S. Changes in parafoveal and peripapillary perfusion after decompression surgery in chiasmal compression due to pituitary tumors. Sci. Rep. 2021, 11, 3464. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-I.; Park, K.-A.; Oh, S.Y.; Kong, D.-S. Parafoveal and peripapillary perfusion predict visual field recovery in chiasmal compression due to pituitary tumors. J. Clin. Med. 2020, 9, 697. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-I.; Park, K.-A.; Oh, S.Y.; Kong, D.-S. Analysis of optic chiasmal compression caused by brain tumors using optical coherence tomography angiography. Sci. Rep. 2020, 10, 2088. [Google Scholar] [CrossRef] [PubMed]
- Parrozzani, R.; Leonardi, F.; Frizziero, L.; Trevisson, E.; Clementi, M.; Pilotto, E.; Fusetti, S.; Miglionico, G.; Midena, E. Retinal vascular and neural remodeling secondary to optic nerve axonal degeneration: A study using OCT angiography. Ophthalmol. Retin. 2018, 2, 827–835. [Google Scholar] [CrossRef]
- Suzuki, A.C.F.; Zacharias, L.C.; Preti, R.C.; Cunha, L.P.; Monteiro, M.L. Circumpapillary and macular vessel density assessment by optical coherence tomography angiography in eyes with temporal hemianopia from chiasmal compression. Correlation with retinal neural and visual field loss. Eye 2020, 34, 695–703. [Google Scholar] [CrossRef]
- Aghi, M.K.; Chen, C.C.; Fleseriu, M.; Newman, S.A.; Lucas, J.W.; Kuo, J.S.; Barkhoudarian, G.; Farrell, C.J.; Sheehan, J.; Ziu, M.; et al. Congress of neurological surgeons systematic review and evidence-based guidelines on the management of patients with nonfunctioning pituitary adenomas: Executive summary. Neurosurgery 2016, 79, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Iida, Y.; Nakanishi, H.; Terada, N.; Morooka, S.; Yamada, H.; Hasegawa, T.; Yokota, S.; Yoshikawa, M.; Yoshimura, N. Microvascular density in glaucomatous eyes with hemifield visual field defects: An optical coherence tomography angiography study. Am. J. Ophthalmol. 2016, 168, 237–249. [Google Scholar] [CrossRef]
- Suh, M.H.; Zangwill, L.M.; Manalastas, P.I.C.; Belghith, A.; Yarmohammadi, A.; Medeiros, F.A.; Diniz-Filho, A.; Saunders, L.J.; Weinreb, R.N. Deep retinal layer microvasculature dropout detected by the optical coherence tomography angiography in glaucoma. Ophthalmology 2016, 123, 2509–2518. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Lee, S.H.; Kim, J.-A.; Kim, T.-W. Parapapillary deep-layer microvasculature dropout in glaucoma: Topographic association with glaucomatous damage. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3004–3010. [Google Scholar] [CrossRef] [PubMed]
- Feucht, N.; Maier, M.; Lepennetier, G.; Pettenkofer, M.; Wetzlmair, C.; Daltrozzo, T.; Scherm, P.; Zimmer, C.; Hoshi, M.-M.; Hemmer, B.; et al. Optical coherence tomography angiography indicates associations of the retinal vascular network and disease activity in multiple sclerosis. Mult. Scler. J. 2019, 25, 224–234. [Google Scholar] [CrossRef] [PubMed]
| Patients’ Characteristics | All Groups (n = 19) | CPA (n = 12) | NCPA (n = 7) | p * | |
|---|---|---|---|---|---|
| Number of eyes | 38 | 24 | 14 | 0.39 | |
| Age (years), mean (range) [SD] | 55 (33–78) [14] | 59 (33–78) [13] | 50 (36–76) [15] | 0.17 | |
| Gender, n (%) | Male | 6 (31.6) | 6 (50) | 0 | 0.04 |
| Female | 13 (68.4) | 6 (50) | 7 (100) | ||
| Secreting PA, n (%) | 8 (42.1) | 2 (16.7) | 6 (66.7) | <0.01 | |
| Duration of optic chiasm compression, n (%) | Acute | NA | 1 (8.3) | NA | |
| Subacute | NA | 6 (50) | NA | ||
| Chronic | NA | 5 (41.7) | NA | ||
| Hormone replacement therapy, n (%) | 8 (42.1) | 2 (16.7) | 6 (66.7) | <0.01 | |
| Diabetes, n (%) | 1 (5.3) | 0 | 1 (14.29) | 0.37 | |
| High blood pressure, n (%) | 4 (21.1) | 2 (16.67) | 2 (28.57) | 0.60 | |
| Smoking, n (%) | 0 | 0 | 0 | 1.00 | |
| Cataract, n (%) | 4 (21.1) | 3 (25.00) | 1 (14.29) | 1.00 | |
| IOP (mmHg), mean (range) [SD] | 15.60 (13–21) [2.34] | 15.30 (13–20) [1.85] | 16.10 (13–21) [3.02] | 0.64 | |
| Disc pallor, n (%) | 5 (19) | 5 (41.67) | 0 | 0.11 | |
| BCVA (LogMAR), mean (range) [SD] | 0.13 (0.00–0.07) [0.23] | 0.20 (0.00–0.07) [0.28] | 0.02 (0.00–0.02) [0.04] | 0.06 | |
| VF mean deviation (dB), mean (range) [SD] | −6.09 (−20.18–1.1) [6.40] | −9.34 (−20.18–0.28) [5.96] | −0.52 (−1.79–1.10) [0.86] | <0.01 | |
| RNFL thickness (μm), mean (range) [SD] | 97.69 (59.50–115.25) [15.22] | 91.13 (59.50–115.25) [15.61] | 108.93 (95.84–114.75) [3.50] | <0.01 | |
| GCC thickness (μm), mean (range) [SD] | 43.96 (20.50–57.25) [8.76] | 40.97 (20.50–57.25) [9.63] | 49.09 (42.76–53.75) [3.01] | 0.02 | |
| Whole disc VD (%), mean (range) [SD] | 53.18 (43.70–59.30) [3.74] | 51.50 (43.70–59.30) [3.49] | 55.90 (50.08–59.30) [2.23] | <0.01 | |
| Inside disc VD (%), mean (range) [SD] | 59.23 (47.50–65.60) [3.81] | 59.07 (47.50–64.00) [3.59] | 59.61 (51.70–65.60) [4.16] | 0.72 | |
| Disc peripapillary VD (%), mean (range) [SD] | 54.61 (41.30–64.70) [5.05] | 52.15 (41.30–56.80) [4.47] | 58.83 (55.10–64.70) [2.59] | <0.01 | |
| Macular parafoveal sVD (%), mean (range) [SD] | 49.01 (39.00–82.25) [6.77] | 48.66 (39.00–82.25) [8.29] | 49.61 (45.00–53.50) [2.91] | 0.33 | |
| Macular parafoveal dVD (%), mean (range) [SD] | 51.39 (26.25–64.25) [8.25] | 51.73 (36.50–61.50) [7.48] | 50.83 (26.25–64.25) [9.73] | 0.79 | |
| Foveal avascular zone (%), mean (range) [SD] | 22.32 (6.00–82.00) [12.24] | 22.54 (6.00–82.00) [14.90] | 19.36 (12.00–26.00) [4.33] | 0.33 | |
| Pituitary adenoma volume (cm3), mean (range) [SD] | 8.11 (0.16–13.20) [1.51] | 10.65 (5.30–13.20) [1.91] | 3.77 (0.16–8.50) [1.48] | <0.01 | |
| Surgery duration (min), mean (range) [SD] | 122.2 (74.00–390.00) [68.46] | 137.4 (74.00–390.00) [82.61] | 98.8 (79.00–125.00) [16.84] | 0.06 | |
| Intraoperative complication, n (%) | 1 (5.27) | 1 (8.33) | 0 (0) | 1.00 | |
| Ki67 (%), mean (range) [SD] | 1.68 (0–10.00) [2.56] | 1.17 (0–5.00) [1.85] | 2.57 (0–10.00) [3.46] | 0.27 | |
| Cell mitosis (% per 10 field), mean (range) [SD] | 0.68 (0.00–6.00) [1.45] | 0.33 (0.00–6.00) [0.65] | 1.29 (0.00–2.00) [2.21] | 0.36 | |
| NCPA | CPA | |||||||
|---|---|---|---|---|---|---|---|---|
| M0 | M1 | M6 | p * | M0 | M1 | M6 | p * | |
| Mean BCVA (LogMAR) | 0.02 | 0.01 | 0.02 | 1.00 | 0.2 | 0.03 | 0.04 | 0.02 |
| VF Mean Deviation (dB), mean | −0.52 | −0.24 | −0.60 | 0.85 | −9.34 | −3.35 | −2.01 | <0.01 |
| VF Improvement, n (%) | NA | 0 | 0 | NA | NA | 4 (33) | 6 (50) | NA |
| Mean RNFL Thickness (μm) | 108.93 | 110.48 | 107.92 | 0.56 | 91.13 | 91.23 | 89.26 | 0.30 |
| Mean GCC Thickness (μm) | 49.09 | 48.27 | 49.23 | 1.00 | 40.97 | 39.28 | 38.01 | 0.03 |
| Mean Tumor Size (cm3) | 3.17 | NA | 0.13 | 0.02 | 8.84 | NA | 3.50 | <0.01 |
| Mean Macular sVD (%) | Mean Whole Disc VD (%) | Mean Inside Disc VD (%) | Mean Disc ppVD (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M0 | M6 | i ≥ 10%, n | VD Change (%) | M0 | M6 | i ≥ 10%, n | VD Change (%) | M0 | M6 | i ≥ 10%, n | VD Change (%) | M0 | M6 | i ≥ 10%, n | VD Change (%) | ||
| NCPA | 49.61 | 48.95 | 0 | −1.33 | 55.9 | 56.44 | 0 | 0.97 | 59.61 | 59.51 | 0 | −0.17 | 58.83 | 58.86 | 0 | 0.05 | |
| CPA | total | 48.66 | 46.18 * | 1 | −5.10 | 51.5 | 51.16 | 0 | −0.66 | 59.07 | 60.03 | 0 | 1.63 | 52.15 | 51.48 | 0 | −1.28 |
| with VF improvement | 47.63 | 47.02 | 1 | −1.28 | 52.81 | 52.60 | 0 | −0.40 | 58.02 | 59.25 | 0 | 2.12 | 53.86 | 53.46 | 0 | −0.74 | |
| without VF improvement | 49.71 | 45.33 | 0 | −9.66 | 50.19 | 49.73 | 0 | −0.92 | 60.12 | 60.82 | 0 | 1.16 | 50.43 | 49.48 | 0 | −1.88 | |
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR, 95% CI | p-Value | OR, 95% CI | p-Value | |
| Age | 0.49 | |||
| Gender | 11 (1.14–106.43) | 0.04 | 0.76 (0.56–1.01) | 0.06 |
| Secreting PA | 0.15 | |||
| Diabetes | 1.00 | |||
| High blood pressure | 0.75 | |||
| Smoking | 1.00 | |||
| IOP | 0.55 | |||
| Disc pallor | 0.13 | |||
| BCVA | 0.90 | |||
| VF mean deviation | 0.79 (0.63–0.98) | 0.04 | 15.81 (0.76–327.08) | 0.07 |
| Duration of optic chiasm compression | 0.30 | |||
| RNFL thickness | 0.81 | |||
| GCC thickness | 0.61 | |||
| Whole disc VD | 0.78 | |||
| Inside disc VD | 0.23 | |||
| Disc peripapillary VD | 0.63 | |||
| Macular parafoveal sVD | 0.43 | |||
| Macular parafoveal dVD | 0.82 | |||
| Foveal avascular zone | 0.90 | |||
| Surgery duration | 0.51 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toumi, E.; Almairac, F.; Mondot, L.; Themelin, A.; Decoux-Poullot, A.-G.; Paquis, P.; Chevalier, N.; Baillif, S.; Nahon-Esteve, S.; Martel, A. Benefit of Optical Coherence Tomography–Angiography in Patients Undergoing Transsphenoidal Pituitary Adenoma Surgery: A Prospective Controlled Study. Diagnostics 2024, 14, 1747. https://doi.org/10.3390/diagnostics14161747
Toumi E, Almairac F, Mondot L, Themelin A, Decoux-Poullot A-G, Paquis P, Chevalier N, Baillif S, Nahon-Esteve S, Martel A. Benefit of Optical Coherence Tomography–Angiography in Patients Undergoing Transsphenoidal Pituitary Adenoma Surgery: A Prospective Controlled Study. Diagnostics. 2024; 14(16):1747. https://doi.org/10.3390/diagnostics14161747
Chicago/Turabian StyleToumi, Elsa, Fabien Almairac, Lydiane Mondot, Albert Themelin, Anne-Gaëlle Decoux-Poullot, Philippe Paquis, Nicolas Chevalier, Stéphanie Baillif, Sacha Nahon-Esteve, and Arnaud Martel. 2024. "Benefit of Optical Coherence Tomography–Angiography in Patients Undergoing Transsphenoidal Pituitary Adenoma Surgery: A Prospective Controlled Study" Diagnostics 14, no. 16: 1747. https://doi.org/10.3390/diagnostics14161747
APA StyleToumi, E., Almairac, F., Mondot, L., Themelin, A., Decoux-Poullot, A.-G., Paquis, P., Chevalier, N., Baillif, S., Nahon-Esteve, S., & Martel, A. (2024). Benefit of Optical Coherence Tomography–Angiography in Patients Undergoing Transsphenoidal Pituitary Adenoma Surgery: A Prospective Controlled Study. Diagnostics, 14(16), 1747. https://doi.org/10.3390/diagnostics14161747







