Temporal Pattern Analysis of Ultrasound Surveillance Data in Vascular Connective Tissue Disorders
Abstract
1. Introduction
2. Methods
2.1. IRB Approval
2.2. Design
2.3. Data
2.4. Analysis
3. Results
3.1. Sample Characteristics
3.2. Combining Imaging Modalities
3.3. Consecutive Ultrasound Studies
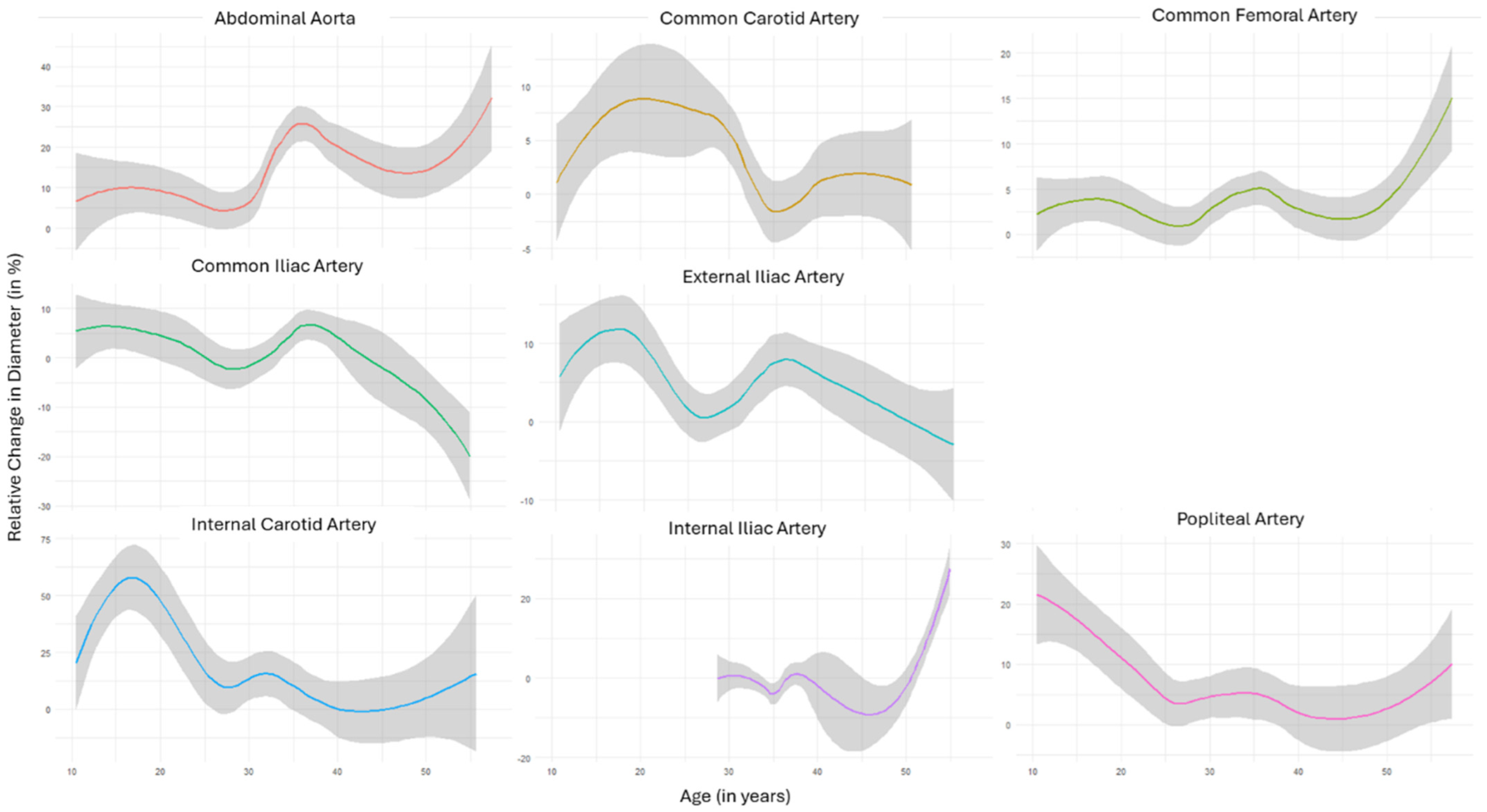
3.4. Temporal Patterns
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loeys, B.L.; Chen, J.; Neptune, E.R.; Judge, D.P.; Podowski, M.; Holm, T.; Meyers, J.; Leitch, C.C.; Katsanis, N.; Sharifi, N.; et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat. Genet. 2005, 37, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Bertoli-Avella, A.M.; Gillis, E.; Morisaki, H.; Verhagen, J.M.A.; De Graaf, B.M.; Van De Beek, G.; Gallo, E.; Kruithof, B.P.T.; Venselaar, H.; Myers, L.A.; et al. Mutations in a TGF-β Ligand, TGFB3, Cause Syndromic Aortic Aneurysms and Dissections. J. Am. Coll. Cardiol. 2015, 65, 1324–1336. [Google Scholar] [CrossRef] [PubMed]
- Van Laer, L.; Dietz, H.; Loeys, B. Loeys-Dietz syndrome. Adv. Exp. Med. Biol. 2014, 802, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Judge, D.P.; Dietz, H.C. Marfan’s syndrome. Lancet 2005, 366, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Zhang, D.; Zhuang, Y.; Xia, Q.; Wen, T.; Jia, H. The Molecular Genetics of Marfan Syndrome. Int. J. Med. Sci. 2021, 18, 2752–2766. [Google Scholar] [CrossRef] [PubMed]
- Salik, I.; Rawla, P. Marfan Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, Finland, 2023. [Google Scholar]
- Parapia, L.A.; Jackson, C. Ehlers-Danlos syndrome—A historical review. Br. J. Haematol. 2008, 141, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Malfait, F.; Francomano, C.; Byers, P.; Belmont, J.; Berglund, B.; Black, J.; Bloom, L.; Bowen, J.M.; Brady, A.F.; Burrows, N.P.; et al. The 2017 international classification of the Ehlers–Danlos syndromes. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Byers, P.H. Vascular Ehlers-Danlos Syndrome. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Meester, J.A.N.; Verstraeten, A.; Schepers, D.; Alaerts, M.; Van Laer, L.; Loeys, B.L. Differences in manifestations of Marfan syndrome, Ehlers-Danlos syndrome, and Loeys-Dietz syndrome. Ann. Cardiothorac. Surg. 2017, 6, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Shalhub, S.; Byers, P.H.; Hicks, K.L.; Coleman, D.M.; Davis, F.M.; De Caridi, G.; Weaver, K.N.; Miller, E.M.; Schermerhorn, M.L.; Shean, K.; et al. A multi-institutional experience in vascular Ehlers-Danlos syndrome diagnosis. J. Vasc. Surg. 2020, 71, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Byers, P.H.; Belmont, J.; Black, J.; De Backer, J.; Frank, M.; Jeunemaitre, X.; Johnson, D.; Pepin, M.; Robert, L.; Sanders, L.; et al. Diagnosis, natural history, and management in vascular Ehlers-Danlos syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.C.S. Management of Marfan syndrome. Heart Br. Card. Soc. 2002, 88, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Isselbacher, E.M.; Preventza, O.; Hamilton Black, J.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 80, e223–e393. [Google Scholar] [CrossRef] [PubMed]
- Loeys, B.L.; Dietz, H.C. Loeys-Dietz Syndrome. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Velchev, J.D.; Van Laer, L.; Luyckx, I.; Dietz, H.; Loeys, B. Loeys-Dietz Syndrome. Adv. Exp. Med. Biol. 2021, 1348, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Van De Laar, I.M.B.H.; Baas, A.F.; De Backer, J.; Blankenstein, J.D.; Dulfer, E.; Helderman-van Den Enden, A.T.J.M.; Houweling, A.C.; Kempers, M.J.; Loeys, B.; Malfait, F.; et al. Surveillance and monitoring in vascular Ehlers-Danlos syndrome in European Reference Network For Rare Vascular Diseases (VASCERN). Eur. J. Med. Genet. 2022, 65, 104557. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.; Adham, S.; Seigle, S.; Legrand, A.; Mirault, T.; Henneton, P.; Albuisson, J.; Denarié, N.; Mazzella, J.-M.; Mousseaux, E.; et al. Vascular Ehlers-Danlos Syndrome. J. Am. Coll. Cardiol. 2019, 73, 1948–1957. [Google Scholar] [CrossRef]
- Groenink, M.; Lohuis, T.A.; Tijssen, J.G.; Naeff, M.S.; Hennekam, R.C.; van der Wall, E.E.; Mulder, B.J. Survival and complication free survival in Marfan’s syndrome: Implications of current guidelines. Heart Br. Card. Soc. 1999, 82, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Sulli, A.; Talarico, R.; Scirè, C.A.; Avcin, T.; Castori, M.; Ferraris, A.; Frank, C.; Grunert, J.; Paolino, S.; Bombardieri, S.; et al. Ehlers-Danlos syndromes: State of the art on clinical practice guidelines. RMD Open 2018, 4, e000790. [Google Scholar] [CrossRef] [PubMed]
- Pomposelli, F. Arterial imaging in patients with lower extremity ischemia and diabetes mellitus. J. Vasc. Surg. 2010, 100, 412–423. [Google Scholar] [CrossRef]
- Takehara, Y.; Yamashita, S.; Sakahara, H.; Masui, T.; Isoda, H. Magnetic resonance angiography of the aorta. Ann. Vasc. Dis. 2011, 4, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Baliyan, V.; Shaqdan, K.; Hedgire, S.; Ghoshhajra, B. Vascular computed tomography angiography technique and indications. Cardiovasc. Diagn. Ther. 2019, 9, S14–S27. [Google Scholar] [CrossRef] [PubMed]
- Brower, C.; Rehani, M.M. Radiation risk issues in recurrent imaging. Br. J. Radiol. 2021, 94, 20210389. [Google Scholar] [CrossRef] [PubMed]
- Schäberle, W.; Leyerer, L.; Schierling, W.; Pfister, K. Ultrasound diagnostics of the abdominal aorta: English version. Gefässchirurgie 2015, 20, 22–27. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gupta, P.; Lyons, S.; Hedgire, S. Ultrasound imaging of the arterial system. Cardiovasc. Diagn. Ther. 2019, 9, S2–S13. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hossack, J.A.; Klibanov, A.L. From Anatomy to Functional and Molecular Biomarker Imaging and Therapy: Ultrasound Is Safe, Ultrafast, Portable, and Inexpensive. Investig. Radiol. 2020, 55, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Ghouri, M.A.; Gupta, N.; Bhat, A.P.; Thimmappa, N.D.; Saboo, S.S.; Khandelwal, A.; Nagpal, P. CT and MR imaging of the upper extremity vasculature: Pearls, pitfalls, and challenges. Cardiovasc. Diagn. Ther. 2019, 9, S152–S173. [Google Scholar] [CrossRef] [PubMed]
- Pepin, M.G.; Schwarze, U.; Rice, K.M.; Liu, M.; Leistritz, D.; Byers, P.H. Survival is affected by mutation type and molecular mechanism in vascular Ehlers–Danlos syndrome (EDS type IV). Genet. Med. 2014, 16, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Mila, L.; Teixido Tura, G.; Granato, C.; Limeres, J.; Servato, L.; Lopez-Sainz, A.; Gutierrez, L.; Galian, L.; Gonzalez-Alujas, T.; Rodriguez-Palomares, J.; et al. 6126Peripheral aneurysms in Marfan patients are common and are related to age and advanced aortic disease. Eur. Heart J. 2019, 40, ehz746.0152. [Google Scholar] [CrossRef]
- Sprouse, L.R.; Meier, G.H.; Lesar, C.J.; Demasi, R.J.; Sood, J.; Parent, F.N.; Marcinzyck, M.J.; Gayle, R.G. Comparison of abdominal aortic aneurysm diameter measurements obtained with ultrasound and computed tomography: Is there a difference? J. Vasc. Surg. 2003, 38, 466–471; discussion 471–472. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.J.; Kristmundsson, T.; Sonesson, B.; Resch, T. Abdominal aortic aneurysm diameter: A comparison of ultrasound measurements with those from standard and three-dimensional computed tomography reconstruction. J. Vasc. Surg. 2009, 50, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Itzchak, Y.; Dorfman, G.; Glickman, M.; Pingoud, E. Relationship of pressure and flow to arterial diameter. Investig. Radiol. 1982, 17, 265–270. [Google Scholar]
- Yuan, X.; Mitsis, A.; Nienaber, C.A. Current Understanding of Aortic Dissection. Life 2022, 12, 1606. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.I.; Burton, K.J.; Gray, J.; Bosner, M.S.; Kouchoukos, N.T.; Roman, M.J.; Boxer, M.; Devereux, R.B.; Tsipouras, P. Life expectancy in the Marfan syndrome. Am. J. Cardiol. 1995, 75, 157–160. [Google Scholar] [CrossRef] [PubMed]


| Ehlers–Danlos | Loeys–Dietz | Marfan | Total | |
|---|---|---|---|---|
| Number | 13 | 3 | 3 | 19 |
| Age | 31.4 (10.9) | 29.6/31.7/38.7 | 24.0/33.5/52.2 | 32.5 (9.6) |
| Arterial Hypertension | 4 (30.8%) | 0 (0%) | 2 (66.7%) | 6 (31.6%) |
| History of cardiovascular surgery | 5 (38.5%) | 1 (33.3%) | 2 (66.7%) | 8 (42.1%) |
| History of aneurysm rupture | 3 (23.1%) | 0 (0%) | 0 (0%) | 3 (15.8%) |
| History of arterial dissection | 3 (23.1%) | 0 (0%) | 2 (66.7%) | 5 (26.3%) |
| Territory | Increase | Decrease | ||
|---|---|---|---|---|
| Patients | Controls | Patients | Controls | |
| Common Carotid | 8 (36.4%) | 0 (0%) | 1 (5.6%) | 4 (66.7%) |
| Internal Carotid | 6 (30.0%) | 0 (0%) | 3 (18.8%) | 2 (50.0%) |
| External Carotid | 2 (33.3%) | 0 (0%) | 0 (0%) | 1 (50.0%) |
| Abdominal Aorta | 6 (40.0%) | 1 (20.0%) | 0 (0%) | 1 (20.0%) |
| Common Iliac | 5 (16.7%) | 3 (37.5%) | 5 (16.7%) | 1 (12.5%) |
| Internal Iliac | 7 (35.0%) | 1 (16.7%) | 0 (0%) | 1 (16.7%) |
| External Iliac | 11 (36.7%) | 1 (12.5%) | 4 (15.4%) | 1 (12.5%) |
| Common Femoral | 12 (50.0%) | 4 (66.7%) | 3 (12.5%) | 0 (0%) |
| Popliteal | 7 (31.8%) | 1 (50.0%) | 2 (9.1%) | (0%) |
| Territory | Increase | Decrease |
|---|---|---|
| Common Carotid | 3 (15.0%) | 0 (0%) |
| Internal Carotid | 6 (30.0%) | 1 (5.0%) |
| External Carotid | 0 (0%) | 0 (0%) |
| Abdominal Aorta | 7 (58.3%) | 0 (0%) |
| Common Iliac | 4 (20.0%) | 3 (15.0%) |
| Internal Iliac | 1 (12.5%) | 0 (0%) |
| External Iliac | 4 (20.0%) | 1 (5.0%) |
| Common Femoral | 8 (42.1%) | 0 (0%) |
| Popliteal | 8 (36.4%) | 2 (9.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walter, C.; Leinweber, M.E.; Mlekusch, I.; Assadian, A.; Hofmann, A.G. Temporal Pattern Analysis of Ultrasound Surveillance Data in Vascular Connective Tissue Disorders. Diagnostics 2024, 14, 1749. https://doi.org/10.3390/diagnostics14161749
Walter C, Leinweber ME, Mlekusch I, Assadian A, Hofmann AG. Temporal Pattern Analysis of Ultrasound Surveillance Data in Vascular Connective Tissue Disorders. Diagnostics. 2024; 14(16):1749. https://doi.org/10.3390/diagnostics14161749
Chicago/Turabian StyleWalter, Corinna, Maria Elisabeth Leinweber, Irene Mlekusch, Afshin Assadian, and Amun Georg Hofmann. 2024. "Temporal Pattern Analysis of Ultrasound Surveillance Data in Vascular Connective Tissue Disorders" Diagnostics 14, no. 16: 1749. https://doi.org/10.3390/diagnostics14161749
APA StyleWalter, C., Leinweber, M. E., Mlekusch, I., Assadian, A., & Hofmann, A. G. (2024). Temporal Pattern Analysis of Ultrasound Surveillance Data in Vascular Connective Tissue Disorders. Diagnostics, 14(16), 1749. https://doi.org/10.3390/diagnostics14161749







