Prognostic Value of Optic Nerve Sheath Diameters after Acute Ischemic Stroke According to Slice Thickness on Computed Tomography
Abstract
:1. Introduction
2. Materials and Methods
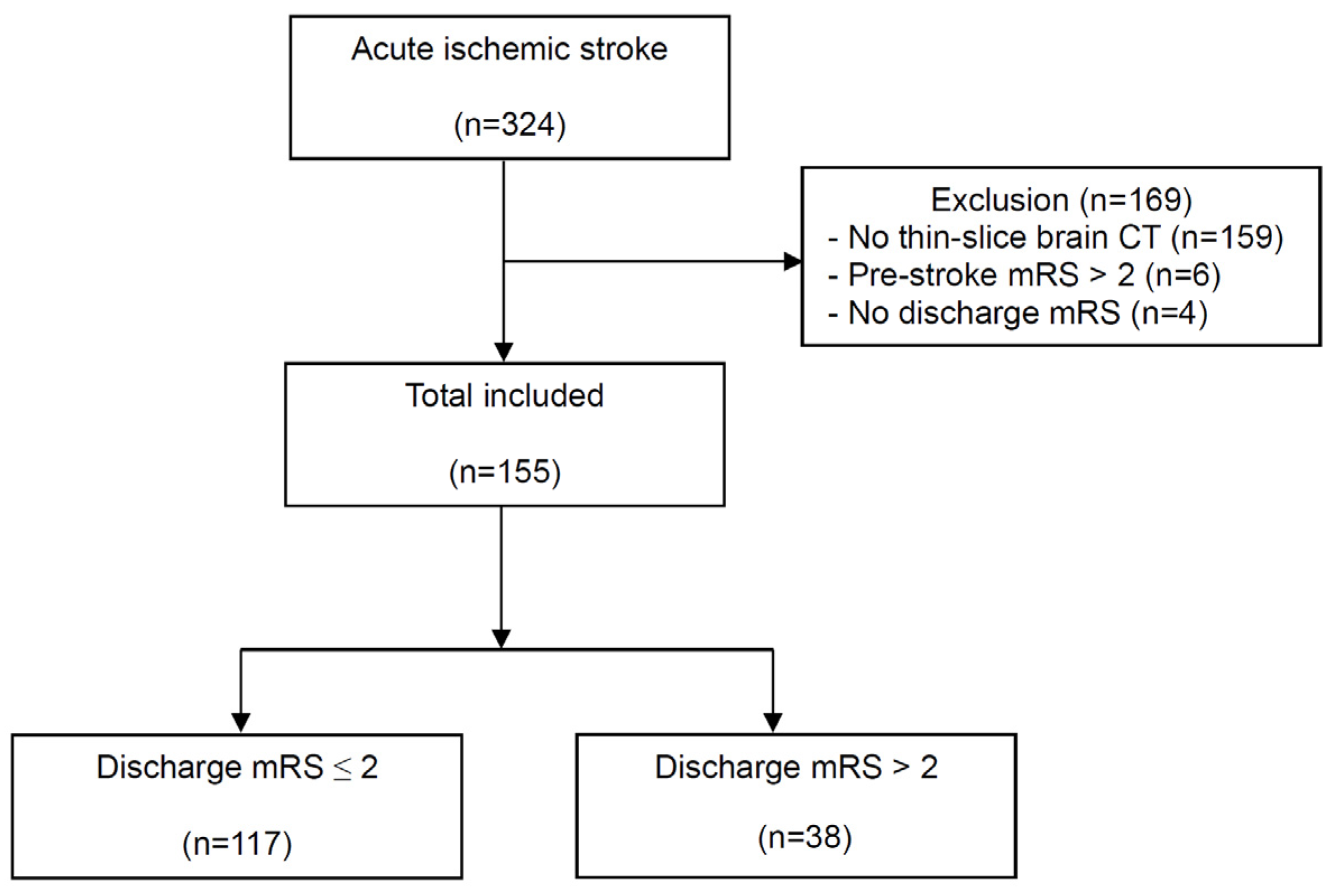
2.1. Study Design and Patients
2.2. Demographic and Clinical Data Information
2.3. Radiological Data
2.4. Outcome Measurement
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Participants
3.2. Comparison of ONSD-Related Variables between the Outcome Groups
3.3. Prognostic Values of Outcome Predictors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopez, A.D.; Mathers, C.D.; Ezzati, M.; Jamison, D.T.; Murray, C.J. Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet 2006, 367, 1747–1757. [Google Scholar] [CrossRef] [PubMed]
- Warlow, C.P. Epidemiology of stroke. Lancet 1998, 352 (Suppl. S3), Siii1–Siii4. [Google Scholar] [CrossRef] [PubMed]
- Donkor, E.S. Stroke in the 21(st) Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res. Treat. 2018, 2018, 3238165. [Google Scholar] [PubMed]
- Moran, A.; Forouzanfar, M.; Sampson, U.; Chugh, S.; Feigin, V.; Mensah, G. The epidemiology of cardiovascular diseases in sub-Saharan Africa: The Global Burden of Diseases, Injuries and Risk Factors 2010 Study. Prog. Cardiovasc. Dis. 2013, 56, 234–239. [Google Scholar] [CrossRef]
- Wouters, A.; Nysten, C.; Thijs, V.; Lemmens, R. Prediction of Outcome in Patients With Acute Ischemic Stroke Based on Initial Severity and Improvement in the First 24 h. Front. Neurol. 2018, 9, 308. [Google Scholar] [CrossRef] [PubMed]
- Vogt, G.; Laage, R.; Shuaib, A.; Schneider, A. Initial lesion volume is an independent predictor of clinical stroke outcome at day 90: An analysis of the Virtual International Stroke Trials Archive (VISTA) database. Stroke 2012, 43, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [PubMed]
- Roque, P.J.; Wu, T.S.; Barth, L.; Drachman, D.; Khor, K.N.; Lovecchio, F.; Stapczynski, S. Optic nerve ultrasound for the detection of elevated intracranial pressure in the hypertensive patient. Am. J. Emerg. Med. 2012, 30, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.; Kariman, H.; Arhami Dolatabadi, A.; Hatamabadi, H.R.; Derakhshanfar, H.; Mansouri, B.; Safari, S.; Eqtesadi, R. Use of the sonographic diameter of optic nerve sheath to estimate intracranial pressure. Am. J. Emerg. Med. 2013, 31, 236–239. [Google Scholar] [CrossRef]
- Amini, A.; Eghtesadi, R.; Feizi, A.M.; Mansouri, B.; Kariman, H.; Dolatabadi, A.A.; Hatamabadi, H.; Kabir, A. Sonographic Optic Nerve Sheath Diameter as a Screening Tool for Detection of Elevated Intracranial Pressure. Emergency 2013, 1, 15–19. [Google Scholar]
- Golshani, K.; Ebrahim Zadeh, M.; Farajzadegan, Z.; Khorvash, F. Diagnostic Accuracy of Optic Nerve Ultrasonography and Ophthalmoscopy in Prediction of Elevated Intracranial Pressure. Emergency 2015, 3, 54–58. [Google Scholar]
- Lee, S.J.; Choi, M.H.; Lee, S.E.; Park, J.H.; Park, B.; Lee, J.S.; Hong, J.M. Optic nerve sheath diameter change in prediction of malignant cerebral edema in ischemic stroke: An observational study. BMC Neurol. 2020, 20, 354. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lee, S.H.; Oh, J.H.; Cho, I.S.; Lee, Y.H.; Han, C.; Choi, W.J.; Sohn, Y.D. Optic nerve sheath diameter measured using early unenhanced brain computed tomography shows no correlation with neurological outcomes in patients undergoing targeted temperature management after cardiac arrest. Resuscitation 2018, 128, 144–150. [Google Scholar] [CrossRef]
- Monnin, P.; Sfameni, N.; Gianoli, A.; Ding, S. Optimal slice thickness for object detection with longitudinal partial volume effects in computed tomography. J. Appl. Clin. Med. Phys. 2017, 18, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Gökcen, E.; Caltekin, İ.; Savrun, A.; Korkmaz, H.; Savrun, Ş.T.; Yıldırım, G. Alterations in optic nerve sheath diameter according to cerebrovascular disease sub-groups. Am. J. Emerg. Med. 2017, 35, 1607–1611. [Google Scholar] [CrossRef]
- Kozaci, N.; Avci, M.; Caliskan, G.; Yuksel, S. Variability of optic nerve sheath diameter in acute ischemic stroke. Hong Kong J. Emerg. Med. 2019, 27, 223–228. [Google Scholar] [CrossRef]
- Maetani, K.; Namiki, J.; Matsumoto, S.; Matsunami, K.; Narumi, A.; Tsuneyoshi, T.; Kishikawa, M. Routine Head Computed Tomography for Patients in the Emergency Room with Trauma Requires Both Thick- and Thin-Slice Images. Emerg. Med. Int. 2016, 2016, 5781790. [Google Scholar] [CrossRef]
- Bello, H.R.; Graves, J.A.; Rohatgi, S.; Vakil, M.; McCarty, J.; Van Hemert, R.L.; Geppert, S.; Peterson, R.B. Skull Base-related Lesions at Routine Head CT from the Emergency Department: Pearls, Pitfalls, and Lessons Learned. Radiographics 2019, 39, 1161–1182. [Google Scholar] [CrossRef]
- Zhu, S.; Cheng, C.; Wang, L.L.; Zhao, D.J.; Zhao, Y.L.; Liu, X.Z. Prognostic values of optic nerve sheath diameter for comatose patients with acute stroke: An observational study. World J. Clin. Cases 2022, 10, 12175–12183. [Google Scholar] [CrossRef]
- Kwon, S.H.; Oh, S.H.; Jang, J.; Kim, S.H.; Park, K.N.; Youn, C.S.; Kim, H.J.; Lim, J.Y.; Kim, H.J.; Bang, H.J. Can Optic Nerve Sheath Images on a Thin-Slice Brain Computed Tomography Reconstruction Predict the Neurological Outcomes in Cardiac Arrest Survivors? J. Clin. Med. 2022, 11, 3677. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Seyedhosseini, J.; Aghili, M.; Vahidi, E.; Shirani, F. Association of optic nerve sheath diameter in ocular ultrasound with prognosis in patients presenting with acute stroke symptoms. Turk. J. Emerg. Med. 2019, 19, 132–135. [Google Scholar] [CrossRef]
- Yüzbaşıoğlu, Y.; Yüzbaşıoğlu, S.; Coşkun, S.; İçme, F.; Öz, T.; Kunt, R.; Becel, S.; Akçay, E.; Şahin Kavaklı, H. Bedside measurement of the optic nerve sheath diameter with ultrasound in cerebrovascular disorders. Turk. J. Med. Sci. 2018, 48, 93–99. [Google Scholar] [CrossRef]
- Wang, L.J.; Chen, L.M.; Chen, Y.; Bao, L.Y.; Zheng, N.N.; Wang, Y.Z.; Xing, Y.Q. Ultrasonography Assessments of Optic Nerve Sheath Diameter as a Noninvasive and Dynamic Method of Detecting Changes in Intracranial Pressure. JAMA Ophthalmol. 2018, 136, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Aspide, R.; Bertolini, G.; Belotti, L.M.B.; Albini Riccioli, L.; Toni, F.; Mazzatenta, D.; Palandri, G.; Vetrugno, L.; Biasucci, D.G. The CLOSED protocol to assess optic nerve sheath diameter using color-Doppler: A comparison study in a cohort of idiopathic normal pressure hydrocephalus patients. Ultrasound J. 2022, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, Y.O.; Baek, J.S.; Ryu, J.A. The prognostic value of optic nerve sheath diameter in patients with subarachnoid hemorrhage. Crit. Care 2019, 23, 65. [Google Scholar] [CrossRef] [PubMed]
- Albert, A.F.; Kirkman, M.A. Clinical and Radiological Predictors of Malignant Middle Cerebral Artery Infarction Development and Outcomes. J. Stroke Cerebrovasc. Dis. 2017, 26, 2671–2679. [Google Scholar] [CrossRef] [PubMed]
- Yavaşi, Ö.; Metin, N.O.; Metin, Y.; Çelik, A.; Tüfekçi, A.; Çeliker, F.B. The role of optic nerve sheath diameter measurement on CT in differentiating transient ischemic attack and acute ischemic stroke. Clin. Neurol. Neurosurg. 2022, 212, 107094. [Google Scholar] [CrossRef] [PubMed]
- Vaiman, M.; Gottlieb, P.; Bekerman, I. Quantitative relations between the eyeball, the optic nerve, and the optic canal important for intracranial pressure monitoring. Head Face Med. 2014, 10, 32. [Google Scholar] [CrossRef]
- Ropper, A.H.; Shafran, B. Brain edema after stroke. Clinical syndrome and intracranial pressure. Arch. Neurol. 1984, 41, 26–29. [Google Scholar] [CrossRef]
- Hacke, W.; Schwab, S.; Horn, M.; Spranger, M.; De Georgia, M.; von Kummer, R. ‘Malignant’ middle cerebral artery territory infarction: Clinical course and prognostic signs. Arch. Neurol. 1996, 53, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Heinsius, T.; Bogousslavsky, J.; Van Melle, G. Large infarcts in the middle cerebral artery territory. Etiology and outcome patterns. Neurology 1998, 50, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Rost, N.S.; Bottle, A.; Lee, J.M.; Randall, M.; Middleton, S.; Shaw, L.; Thijs, V.; Rinkel, G.J.; Hemmen, T.M. Stroke Severity Is a Crucial Predictor of Outcome: An International Prospective Validation Study. J. Am. Heart Assoc. 2016, 5, e002433. [Google Scholar] [CrossRef] [PubMed]
- Hwan Kim, Y.; Ho Lee, J.; Kun Hong, C.; Won Cho, K.; Hoon Yeo, J.; Ju Kang, M.; Weon Kim, Y.; Yul Lee, K.; Joo Kim, J.; Youn Hwang, S. Feasibility of optic nerve sheath diameter measured on initial brain computed tomography as an early neurologic outcome predictor after cardiac arrest. Acad. Emerg. Med. 2014, 21, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, H.; Jaiswal, R.; Bruck, I.; Matari, H.; Ghobadi, F.; Weedon, J.; Hassen, G.W. Correlation of optic nerve sheath diameter measurements by computed tomography and magnetic resonance imaging. Am. J. Emerg. Med. 2013, 31, 1595–1597. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, I.O.; Aksay, E.; Bayram, B.; Oray, N.C.; Karabay, N. Interrater reliability of emergency medicine residents in measurement of optic nerve sheath diameter with computed tomography. Turk. J. Emerg. Med. 2021, 21, 117–121. [Google Scholar] [PubMed]
- Aspide, R.; Bertolini, G.; Belotti, L.M.B.; Albini Riccioli, L.; Toni, F.; Mazzatenta, D.; Palandri, G.; Vetrugno, L.; Biasucci, D.G. Magnetic Resonance-Based Assessment of Optic Nerve Sheath Diameter: A Prospective Observational Cohort Study on Inter- and Intra-Rater Agreement. J. Clin. Med. 2023, 12, 2713. [Google Scholar] [CrossRef]
- Mikhail, P.; Le, M.G.D.; Mair, G. Computational Image Analysis of Nonenhanced Computed Tomography for Acute Ischaemic Stroke: A Systematic Review. J. Stroke Cerebrovasc. Dis. 2020, 29, 104715. [Google Scholar] [CrossRef]
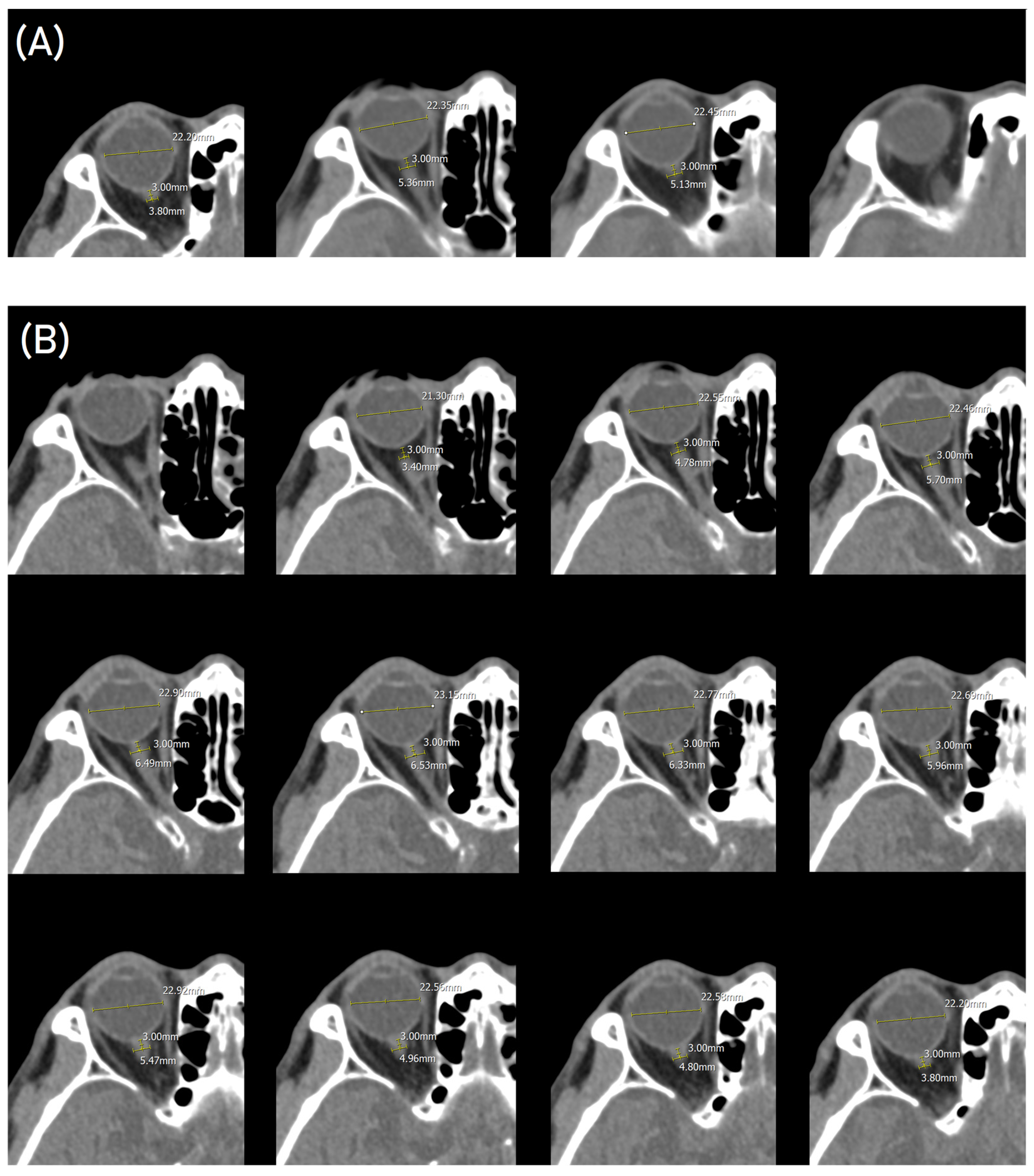



| mRS Score ≤ 2 (n = 117) | mRS Score > 2 (n = 38) | p-Value | |
|---|---|---|---|
| Male | 70 (59.8) | 19 (50.0) | 0.287 |
| Age, years, mean ± SD | 68.9 ± 12.8 | 75.1 ± 12.1 | 0.009 |
| Comorbidity | |||
| Hypertension | 67 (57.3) | 25 (65.8) | 0.353 |
| Diabetes mellitus | 35 (29.9) | 11 (28.9) | 0.910 |
| Cerebrovascular disease | 23 (19.7) | 9 (23.7) | 0.594 |
| Coronary artery disease | 10 (8.5) | 3 (7.9) | 0.900 |
| Chronic kidney disease | 6 (5.1) | 1 (2.6) | 0.520 |
| Malignancy | 9 (7.7) | 4 (10.5) | 0.584 |
| NIHSS score on initial presentation | 2.0 (1.0–4.0) | 5.0 (2.8–9.3) | <0.001 |
| mRS score on initial presentation | 2.0 (1.0–3.0) | 4.0 (3.0–4.0) | <0.001 |
| Glasgow Coma Scale | 15.0 (15.0–15.0) | 15.0 (13.0–15.0) | <0.001 |
| Symptom onset-to-CT interval, h (IQR) | 24.5 (13.4–53.8) | 21.6 (5.2–70.6) | 0.548 |
| ONSD-related variables | |||
| Routine ONSD, mm | 4.97 ± 0.53 | 5.21 ± 0.78 | 0.085 |
| Thin ONSD, mm | 5.59 ± 0.52 | 5.85 ± 0.73 | 0.047 |
| Difference between both ONSDs, mm | 0.63 ± 0.30 | 0.65 ± 0.35 | 0.739 |
| Routine ETD | 22.37 ± 1.08 | 22.33 ± 0.91 | 0.827 |
| Thin ETD | 22.79 ± 1.08 | 22.68 ± 0.89 | 0.570 |
| Routine ONSD/ETD ratio | 0.22 ± 0.02 | 0.23 ± 0.03 | 0.034 |
| Thin ONSD/ETD ratio | 0.25 ± 0.02 | 0.26 ± 0.03 | 0.010 |
| Reperfusion therapy | 1 (0.9) | 1 (2.6) | 0.399 |
| NIHSS score at hospital discharge a | 1.0 (0.0–2.5) | 4.0 (2.0–7.0) | <0.001 |
| mRS score at hospital discharge | 1.0 (1.0–2.0) | 4.0 (3.0–4.0) | <0.001 |
| Length of hospital stay, day | 6.0 (5.0–8.0) | 12.0 (7.8–24.8) | <0.001 |
| Univariable | Multivariable | Multivariable | Multivariable | Multivariable | |
|---|---|---|---|---|---|
| OR (95% CI), p | OR (95% CI), p | OR (95% CI), p | OR (95% CI), p | OR (95% CI), p | |
| Age, per year | 1.04 (1.01–1.08), 0.011 | 1.05 (1.01–1.09), 0.009 | 1.05 (1.01–1.09), 0.009 | 1.05 (1.01–1.09), 0.010 | 1.05 (1.01–1.09), 0.010 |
| Initial NIHSS score | 1.40 (1.20–1.64), <0.001 | 1.44 (1.22–1.69), <0.001 | 1.44 (1.22–1.70), <0.001 | 1.43 (1.22–1.69), <0.001 | 1.44 (1.22–1.69), <0.001 |
| Routine ONSD, mm | 1.90 (1.03–3.48), 0.039 | 1.62 (0.76–3.45), 0.214 | – | – | – |
| Thin ONSD, mm | 2.12 (1.12–4.01), 0.021 | – | 1.94 (0.88–4.28), 0.102 | – | – |
| Routine ONSD/ETD × 10 | 4.26 (1.08–16.75), 0.038 | – | – | 2.61 (0.47–14.51), 0.273 | – |
| Thin ONSD/ETD × 10 | 6.46 (1.46–28.66), 0.014 | – | – | – | 4.75 (0.77–29.16), 0.093 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-B.; Oh, S.H.; Jang, J.; Koo, J.; Bang, H.J.; Lee, M.H. Prognostic Value of Optic Nerve Sheath Diameters after Acute Ischemic Stroke According to Slice Thickness on Computed Tomography. Diagnostics 2024, 14, 1754. https://doi.org/10.3390/diagnostics14161754
Lee H-B, Oh SH, Jang J, Koo J, Bang HJ, Lee MH. Prognostic Value of Optic Nerve Sheath Diameters after Acute Ischemic Stroke According to Slice Thickness on Computed Tomography. Diagnostics. 2024; 14(16):1754. https://doi.org/10.3390/diagnostics14161754
Chicago/Turabian StyleLee, Han-Bin, Sang Hoon Oh, Jinhee Jang, Jaseong Koo, Hyo Jin Bang, and Min Hwan Lee. 2024. "Prognostic Value of Optic Nerve Sheath Diameters after Acute Ischemic Stroke According to Slice Thickness on Computed Tomography" Diagnostics 14, no. 16: 1754. https://doi.org/10.3390/diagnostics14161754





