Common Non-Rheumatic Medical Conditions Mimicking Fibromyalgia: A Simple Framework for Differential Diagnosis
Abstract
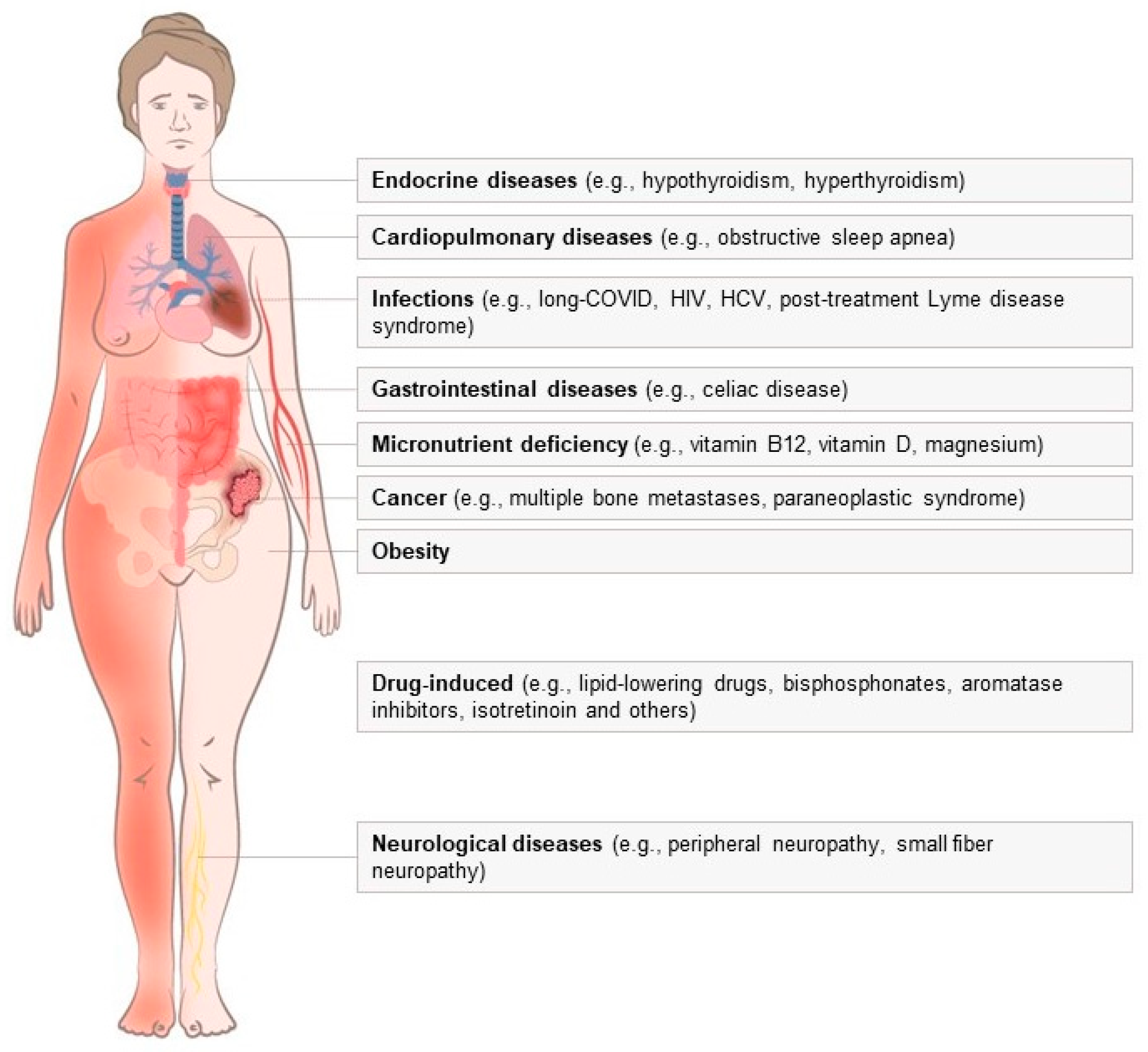
:1. Introduction
2. Micronutrient Deficiency
2.1. Vitamin B12 Deficiency
- Summary: Suspicion of vitamin B12 deficiency should arise in patients presenting with FM symptoms such as pain, fatigue, sleepiness, paresthesia, generalized pain, or sensory disturbances, especially in the presence of macrocytosis, glossitis, a vegan diet, gastrointestinal illness, or medications that could interfere with absorption. Diagnosis can be confirmed by low plasma cobalamin and high homocysteine levels.
2.2. Vitamin D Deficiency
- Summary: In FM patients experiencing widespread musculoskeletal/bone pain and muscle weakness, consideration should be given to the possibility of vitamin D deficiency. Evaluation of serum 25-hydroxyvitamin D level is recommended.
2.3. Iron Deficiency
- Summary: Iron status, including ferritin and TSAT, should be evaluated in patients with suspected FM and typical symptoms of iron deficiency (fatigue, weakness, exercise intolerance, dyspnea, restless leg syndrome, frail hair, koilonychia, atrophic glossitis, angular cheilosis, or pica) and/or microcytosis.
2.4. Magnesium Deficiency
- Summary: In FM patients presenting with predominant muscular and cognitive symptoms, consideration should be given to the possibility of magnesium deficiency.
2.5. Vitamin C Deficiency
- Summary: Vitamin C deficiency should be suspected in FM patients with risk factors such as alcoholism, advanced age, low socioeconomic status, extreme dietary habits, or severe gastrointestinal or renal conditions.
3. Endocrine Diseases
3.1. Thyroid Diseases
- Summary: Thyroid dysfunction may manifest with nonspecific symptoms resembling those of FM, such as fatigue, weight loss/increase, tremor, anxiety, heat/cold intolerance, palpitations, or insomnia. Evaluation of TSH levels is recommended, and if abnormal, further assessment of FT3 and FT4 is suggested.
3.2. Obesity
- Summary: There is a well-known association between obesity and FM; subjects with obesity could present weakness, sleep disturbances, musculoskeletal pain, and mood disturbances. In those patients, weight loss should be prioritized, not only to improve global health and reduce the incidence of obesity-associated chronic diseases but also to alleviate FM symptoms.
4. Cardiopulmonary Diseases
Obstructive Sleep Apnea
- Summary: Individuals with obstructive sleep apnea (OSA) may exhibit typical FM symptoms such as fatigue, sleep disturbances, and decreased concentration and memory. High suspicion for OSA arises when daytime sleepiness is reported or in the presence of predisposing factors such as obesity or anatomical abnormalities causing narrowing of the upper airways.
5. Neurological Diseases
- Summary: Patients experiencing widespread pain should be evaluated for peripheral neuropathy, with small fiber neuropathy (SFN) being of particular concern. SFN should be considered in FM patients, especially when neuropathic symptoms such as paresthesia, autonomic disturbances, sweating abnormalities, and hypoesthesia to warm or cold stimuli, along with skin changes, are present.
6. Infections
- Summary: Chronic infections can manifest with musculoskeletal symptoms resembling FM. Testing for HIV and HCV should be considered in sexually active individuals on intravenous drug users, while a recent history of COVID-19 may indicate long COVID-19. Additionally, testing for Lyme disease may be warranted in patients potentially exposed to tick bites, especially in endemic areas.
7. Cancer
- Summary: In patients presenting with multifocal musculoskeletal pain along with worsening fatigue, constitutional symptoms, or organ-related manifestations, particularly in the presence of risk factors, the possibility of cancer should always be considered.
8. Celiac Disease
- Summary: CD should be considered in patients exhibiting musculoskeletal symptoms suggestive of FM alongside concomitant gastrointestinal symptoms.
9. Drug-Induced Musculoskeletal Pain
9.1. Lipid-Lowering Drugs
9.2. Bisphosphonates
9.3. Aromatase Inhibitors
9.4. Isotretinoin
9.5. Others
- Summary: Several drugs commonly used to treat chronic diseases have the potential to induce musculoskeletal symptoms resembling FM. A detailed drug history should be obtained in all patients, and a trial of treatment discontinuation, if feasible, may be warranted to establish a causative role of the medication.
10. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Häuser, W.; Fluß, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; et al. EULAR revised recommendations for the management of fibromyalgia. Ann. Rheum. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Chinn, S.; Caldwell, W.; Gritsenko, K. Fibromyalgia Pathogenesis and Treatment Options Update. Curr. Pain Headache Rep. 2016, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Hunt, A.; Harrington, D.; Robinson, S. Vitamin B12 deficiency. BMJ 2014, 349, g5226. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.-L.; Brito, A.; Guéant, J.-L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.-H.; et al. Vitamin B12 deficiency. Nat. Rev. Dis. Primer 2017, 3, 17040. [Google Scholar] [CrossRef] [PubMed]
- Andrès, E.; Zulfiqar, A.-A.; Vogel, T. State of the art review: Oral and nasal vitamin B12 therapy in the elderly. QJM Mon. J. Assoc. Phys. 2020, 113, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, R.; Lester, S.E.; Babatunde, T. The prevalence of cobalamin deficiency among vegetarians assessed by serum vitamin B12: A review of literature. Eur. J. Clin. Nutr. 2014, 68, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Salinas, M.; Flores, E.; López-Garrigós, M.; Leiva-Salinas, C. Vitamin B12 deficiency and clinical laboratory: Lessons revisited and clarified in seven questions. Int. J. Lab. Hematol. 2018, 40 (Suppl. S1), 83–88. [Google Scholar] [CrossRef] [PubMed]
- Lindenbaum, J.; Healton, E.B.; Savage, D.G.; Brust, J.C.; Garrett, T.J.; Podell, E.R.; Marcell, P.D.; Stabler, S.P.; Allen, R.H. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N. Engl. J. Med. 1988, 318, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N. Neurologic aspects of cobalamin (B12) deficiency. Handb. Clin. Neurol. 2014, 120, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Devalia, V.; Hamilton, M.S.; Molloy, A.M. British Committee for Standards in Haematology Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br. J. Haematol. 2014, 166, 496–513. [Google Scholar] [CrossRef]
- Wolffenbuttel, B.H.R.; Wouters, H.J.C.M.; Heiner-Fokkema, M.R.; van der Klauw, M.M. The Many Faces of Cobalamin (Vitamin B12) Deficiency. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 200–214. [Google Scholar] [CrossRef]
- Gharibpoor, F.; Ghavidel-Parsa, B.; Sattari, N.; Bidari, A.; Nejatifar, F.; Montazeri, A. Effect of vitamin B12 on the symptom severity and psychological profile of fibromyalgia patients; a prospective pre-post study. BMC Rheumatol. 2022, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- British Columbia Guidelines on Cobalamin (Vitamin B12) and Folate Deficiency. Available online: https://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/bc-guidelines/vitamin-b12 (accessed on 19 April 2024).
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Looker, A.C.; Johnson, C.L.; Lacher, D.A.; Pfeiffer, C.M.; Schleicher, R.L.; Sempos, C.T. Vitamin D Status: United States, 2001–2006; NCHS data brief, no 59; National Center for Health Statistics: Hyattsville, MD, USA, 2011. Available online: https://www.cdc.gov/nchs/data/databriefs/db59.pdf (accessed on 27 March 2024).
- Sprague, S.; Petrisor, B.; Scott, T.; Devji, T.; Phillips, M.; Spurr, H.; Bhandari, M.; Slobogean, G.P. What Is the Role of Vitamin D Supplementation in Acute Fracture Patients? A Systematic Review and Meta-Analysis of the Prevalence of Hypovitaminosis D and Supplementation Efficacy. J. Orthop. Trauma 2016, 30, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Książek, A.; Zagrodna, A.; Słowińska-Lisowska, M. Vitamin D, Skeletal Muscle Function and Athletic Performance in Athletes-A Narrative Review. Nutrients 2019, 11, 1800. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-T.; Tavera-Mendoza, L.E.; Laperriere, D.; Libby, E.; MacLeod, N.B.; Nagai, Y.; Bourdeau, V.; Konstorum, A.; Lallemant, B.; Zhang, R.; et al. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol. Endocrinol. 2005, 19, 2685–2695. [Google Scholar] [CrossRef]
- Neal, S.; Sykes, J.; Rigby, M.; Hess, B. A review and clinical summary of vitamin D in regard to bone health and athletic performance. Phys. Sportsmed. 2015, 43, 161–168. [Google Scholar] [CrossRef]
- Owens, D.J.; Allison, R.; Close, G.L. Vitamin D and the Athlete: Current Perspectives and New Challenges. Sports Med. Auckl. NZ 2018, 48, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Shipton, E.E.; Shipton, E.A. Vitamin D Deficiency and Pain: Clinical Evidence of Low Levels of Vitamin D and Supplementation in Chronic Pain States. Pain Ther. 2015, 4, 67–87. [Google Scholar] [CrossRef]
- Powell, H.S.; Greenberg, D. Tackling vitamin D deficiency. Postgrad. Med. 2006, 119, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.D.; Kelly, S.T.; Shurlock, J.H.; Hepburn, A.L.N. The role of vitamin D testing and replacement in fibromyalgia: A systematic literature review. BMC Rheumatol. 2018, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Martins, Y.A.; Cardinali, C.A.E.F.; Ravanelli, M.I.; Brunaldi, K. Is hypovitaminosis D associated with fibromyalgia? A systematic review. Nutr. Rev. 2020, 78, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet Lond. Engl. 2016, 387, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- Stugiewicz, M.; Tkaczyszyn, M.; Kasztura, M.; Banasiak, W.; Ponikowski, P.; Jankowska, E.A. The influence of iron deficiency on the functioning of skeletal muscles: Experimental evidence and clinical implications. Eur. J. Heart Fail. 2016, 18, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Pamuk, G.E.; Pamuk, O.N.; Set, T.; Harmandar, O.; Yeşil, N. An increased prevalence of fibromyalgia in iron deficiency anemia and thalassemia minor and associated factors. Clin. Rheumatol. 2008, 27, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Hamarat, H.; Gürcü, S.; Kıvanç, B.K.; Aydemir, A.E. Ferric carboxymaltose therapy reduces pain and improves the quality of life in female patients with fibromyalgia. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 10375–10380. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.-C.; Chen, H.-J.; Leong, K.-H.; Chang, K.-L.; Wang, Y.-T.T.; Wu, L.-C.; Tung, P.-Y.; Kuo, C.-F.; Lin, C.-C.; Tsai, S.-Y. The risk of fibromyalgia in patients with iron deficiency anemia: A nationwide population-based cohort study. Sci. Rep. 2021, 11, 10496. [Google Scholar] [CrossRef]
- Beard, J.L.; Connor, J.R.; Jones, B.C. Iron in the Brain. Nutr. Rev. 2009, 51, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Snook, J.; Bhala, N.; Beales, I.L.P.; Cannings, D.; Kightley, C.; Logan, R.P.; Pritchard, D.M.; Sidhu, R.; Surgenor, S.; Thomas, W.; et al. British Society of Gastroenterology guidelines for the management of iron deficiency anaemia in adults. Gut 2021, 70, 2030–2051. [Google Scholar] [CrossRef] [PubMed]
- Pavord, S.; Daru, J.; Prasannan, N.; Robinson, S.; Stanworth, S.; Girling, J. BSH Committee UK guidelines on the management of iron deficiency in pregnancy. Br. J. Haematol. 2020, 188, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.I.; Cittadini, A. Chemistry and biochemistry of magnesium. Mol. Aspects Med. 2003, 24, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, K.; Kocot, J.; Horecka, A. Biochemistry of magnesium. J. Elem. 2012, 15, 601–616. [Google Scholar] [CrossRef]
- Castiglioni, S.; Maier, J.A.M. Magnesium and cancer: A dangerous liason. Magnes. Res. 2011, 24, S92–S100. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Bergkvist, L.; Wolk, A. Magnesium intake in relation to risk of colorectal cancer in women. JAMA 2005, 293, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, S.; Chakraborti, T.; Mandal, M.; Mandal, A.; Das, S.; Ghosh, S. Protective role of magnesium in cardiovascular diseases: A review. Mol. Cell. Biochem. 2002, 238, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-J.; Na, H.-S.; Do, S.-H. Magnesium and Pain. Nutrients 2020, 12, 2184. [Google Scholar] [CrossRef]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [PubMed]
- Saris, N.E.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium. An update on physiological, clinical and analytical aspects. Clin. Chim. Acta Int. J. Clin. Chem. 2000, 294, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Grzebisz, W. Magnesium—Food and human health. J. Elemntology 2011, 16, 299–323. [Google Scholar] [CrossRef]
- Boulis, M.; Boulis, M.; Clauw, D. Magnesium and Fibromyalgia: A Literature Review. J. Prim. Care Community Health 2021, 12, 21501327211038433. [Google Scholar] [CrossRef] [PubMed]
- Blaszczyk, U.; Duda-Chodak, A. Magnesium: Its role in nutrition and carcinogenesis. Rocz. Panstw. Zakl. Hig. 2013, 64, 165–171. [Google Scholar] [PubMed]
- Andretta, A.; Dias Batista, E.; Madalozzo Schieferdecker, M.E.; Rasmussen Petterle, R.; Boguszewski, C.L.; Dos Santos Paiva, E. Relation between magnesium and calcium and parameters of pain, quality of life and depression in women with fibromyalgia. Adv. Rheumatol. Lond. Engl. 2019, 59, 55. [Google Scholar] [CrossRef] [PubMed]
- Macian, N.; Dualé, C.; Voute, M.; Leray, V.; Courrent, M.; Bodé, P.; Giron, F.; Sonneville, S.; Bernard, L.; Joanny, F.; et al. Short-Term Magnesium Therapy Alleviates Moderate Stress in Patients with Fibromyalgia: A Randomized Double-Blind Clinical Trial. Nutrients 2022, 14, 2088. [Google Scholar] [CrossRef]
- Kisters, K. What is the correct magnesium supplement? Magnes. Res. 2013, 26, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Léger, D. Scurvy: Reemergence of nutritional deficiencies. Can. Fam. Phys. Med. Fam. Can. 2008, 54, 1403–1406. [Google Scholar]
- Weinstein, M.; Babyn, P.; Zlotkin, S. An orange a day keeps the doctor away: Scurvy in the year 2000. Pediatrics 2001, 108, E55. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; McCall, C. The role of vitamin C in the treatment of pain: New insights. J. Transl. Med. 2017, 15, 77. [Google Scholar] [CrossRef] [PubMed]
- Fain, O. Musculoskeletal manifestations of scurvy. Jt. Bone Spine 2005, 72, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Aïm, F.; Klouche, S.; Frison, A.; Bauer, T.; Hardy, P. Efficacy of vitamin C in preventing complex regional pain syndrome after wrist fracture: A systematic review and meta-analysis. Orthop. Traumatol. Surg. Res. 2017, 103, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Chaker, L.; Bianco, A.C.; Jonklaas, J.; Peeters, R.P. Hypothyroidism. Lancet Lond. Engl. 2017, 390, 1550–1562. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Albrecht, D.; Scholz, A.; Gutierrez-Buey, G.; Lazarus, J.H.; Dayan, C.M.; Okosieme, O.E. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 2018, 14, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Persani, L.; Brabant, G.; Dattani, M.; Bonomi, M.; Feldt-Rasmussen, U.; Fliers, E.; Gruters, A.; Maiter, D.; Schoenmakers, N.; van Trotsenburg, A.S.P. 2018 European Thyroid Association (ETA) Guidelines on the Diagnosis and Management of Central Hypothyroidism. Eur. Thyroid J. 2018, 7, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Garber, J.R.; Cobin, R.H.; Gharib, H.; Hennessey, J.V.; Klein, I.; Mechanick, J.I.; Pessah-Pollack, R.; Singer, P.A.; Woeber, K.A. American Association Of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid Off. J. Am. Thyroid Assoc. 2012, 22, 1200–1235. [Google Scholar] [CrossRef]
- Ross, D.S.; Burch, H.B.; Cooper, D.S.; Greenlee, M.C.; Laurberg, P.; Maia, A.L.; Rivkees, S.A.; Samuels, M.; Sosa, J.A.; Stan, M.N.; et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid Off. J. Am. Thyroid Assoc. 2016, 26, 1343–1421. [Google Scholar] [CrossRef] [PubMed]
- Franklyn, J.A.; Boelaert, K. Thyrotoxicosis. Lancet Lond. Engl. 2012, 379, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Hegedüs, L. Graves’ Disease. N. Engl. J. Med. 2016, 375, 1552–1565. [Google Scholar] [CrossRef] [PubMed]
- Bahn, R.S. Graves’ ophthalmopathy. N. Engl. J. Med. 2010, 362, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Janssen, J.A.M.J.L. Insulin-like Growth Factor-I Receptor and Thyroid-Associated Ophthalmopathy. Endocr. Rev. 2019, 40, 236–267. [Google Scholar] [CrossRef]
- De Leo, S.; Lee, S.Y.; Braverman, L.E. Hyperthyroidism. Lancet Lond. Engl. 2016, 388, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kwon, J.-S.; Park, Y.-B.; Park, J.W. Is thyroid autoimmunity a predisposing factor for fibromyalgia? A systematic review and meta-analysis. Clin. Exp. Rheumatol. 2022, 40, 1210–1220. [Google Scholar] [CrossRef]
- Haliloglu, S.; Ekinci, B.; Uzkeser, H.; Sevimli, H.; Carlioglu, A.; Macit, P.M. Fibromyalgia in patients with thyroid autoimmunity: Prevalence and relationship with disease activity. Clin. Rheumatol. 2017, 36, 1617–1621. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R. Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2016, 22 (Suppl. S3), 1–203. [Google Scholar] [CrossRef]
- Bray, G.A.; Frühbeck, G.; Ryan, D.H.; Wilding, J.P.H. Management of obesity. Lancet 2016, 387, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC) Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet Lond. Engl. 2016, 387, 1377–1396. [CrossRef] [PubMed]
- Hales, C.M.; Fryar, C.D.; Carroll, M.D.; Freedman, D.S.; Aoki, Y.; Ogden, C.L. Differences in Obesity Prevalence by Demographic Characteristics and Urbanization Level Among Adults in the United States, 2013-2016. JAMA 2018, 319, 2419–2429. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.; Puhl, R.M.; Cummings, D.E.; Eckel, R.H.; Ryan, D.H.; Mechanick, J.I.; Nadglowski, J.; Ramos Salas, X.; Schauer, P.R.; Twenefour, D.; et al. Joint international consensus statement for ending stigma of obesity. Nat. Med. 2020, 26, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Ley, S.H.; Manson, J.E.; Willett, W.; Satija, A.; Hu, F.B.; Stokes, A. Weight History and All-Cause and Cause-Specific Mortality in Three Prospective Cohort Studies. Ann. Intern. Med. 2017, 166, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Yancy, W.S.; Olsen, M.K.; Westman, E.C.; Bosworth, H.B.; Edelman, D. Relationship between obesity and health-related quality of life in men. Obes. Res. 2002, 10, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Kucharz, E.J.; Kopeć-Mędrek, M.; Kramza, J.; Chrzanowska, M.; Kotyla, P. Dercum’s disease (adiposis dolorosa): A review of clinical presentation and management. Reumatologia 2019, 57, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Naty, S.; Grembiale, R.D. Fibromyalgia and obesity: The hidden link. Rheumatol. Int. 2011, 31, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- D’Onghia, M.; Ciaffi, J.; Lisi, L.; Mancarella, L.; Ricci, S.; Stefanelli, N.; Meliconi, R.; Ursini, F. Fibromyalgia and obesity: A comprehensive systematic review and meta-analysis. Semin. Arthritis Rheum. 2021, 51, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Ciaffi, J.; Lisi, L.; Mari, A.; Mancarella, L.; Brusi, V.; Pignatti, F.; Ricci, S.; Vitali, G.; Stefanelli, N.; Assirelli, E.; et al. Efficacy, safety and tolerability of very low-calorie ketogenic diet in obese women with fibromyalgia: A pilot interventional study. Front. Nutr. 2023, 10, 1219321. [Google Scholar] [CrossRef] [PubMed]
- Epstein, L.J.; Kristo, D.; Strollo, P.J.; Friedman, N.; Malhotra, A.; Patil, S.P.; Ramar, K.; Rogers, R.; Schwab, R.J.; Weaver, E.M.; et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2009, 5, 263–276. [Google Scholar]
- Jordan, A.S.; McSharry, D.G.; Malhotra, A. Adult obstructive sleep apnoea. Lancet Lond. Engl. 2014, 383, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Lévy, P.; Kohler, M.; McNicholas, W.T.; Barbé, F.; McEvoy, R.D.; Somers, V.K.; Lavie, L.; Pépin, J.-L. Obstructive sleep apnoea syndrome. Nat. Rev. Dis. Primer 2015, 1, 15015. [Google Scholar] [CrossRef] [PubMed]
- Younes, M. Pathogenesis of Obstructive Sleep Apnea. Clin. Chest Med. 2019, 40, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Greenstone, M.; Hack, M. Obstructive sleep apnoea. BMJ 2014, 348, g3745. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, V.W.; Rutledge, D.N.; Stern, J.M. Polysomnography with quantitative EEG in patients with and without fibromyalgia. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 2015, 32, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Meresh, E.S.; Artin, H.; Joyce, C.; Birch, S.; Daniels, D.; Owens, J.H.; La Rosa, A.J.; Rao, M.S.; Halaris, A. Obstructive sleep apnea co-morbidity in patients with fibromyalgia: A single-center retrospective analysis and literature review. Open Access Rheumatol. Res. Rev. 2019, 11, 103–109. [Google Scholar] [CrossRef]
- Sateia, M.J. International classification of sleep disorders-third edition: Highlights and modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Tanna, N.; Smith, B.D.; Zapanta, P.E.; Karanetz, I.; Andrews, B.T.; Urata, M.M.; Bradley, J.P. Surgical Management of Obstructive Sleep Apnea. Plast. Reconstr. Surg. 2016, 137, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Feltner, C.; Wallace, I.F.; Aymes, S.; Cook Middleton, J.; Hicks, K.L.; Schwimmer, M.; Baker, C.; Balio, C.P.; Moore, D.; Voisin, C.E.; et al. Screening for Obstructive Sleep Apnea in Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2022, 328, 1951–1971. [Google Scholar] [CrossRef] [PubMed]
- Tomfohr, L.M.; Ancoli-Israel, S.; Loredo, J.S.; Dimsdale, J.E. Effects of continuous positive airway pressure on fatigue and sleepiness in patients with obstructive sleep apnea: Data from a randomized controlled trial. Sleep 2011, 34, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Borsook, D. Neurological diseases and pain. Brain J. Neurol. 2012, 135, 320–344. [Google Scholar] [CrossRef] [PubMed]
- Azhary, H.; Farooq, M.U.; Bhanushali, M.; Majid, A.; Kassab, M.Y. Peripheral neuropathy: Differential diagnosis and management. Am. Fam. Phys. 2010, 81, 887–892. [Google Scholar]
- Hanewinckel, R.; Drenthen, J.; van Oijen, M.; Hofman, A.; van Doorn, P.A.; Ikram, M.A. Prevalence of polyneuropathy in the general middle-aged and elderly population. Neurology 2016, 87, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Barrell, K.; Smith, A.G. Peripheral Neuropathy. Med. Clin. N. Am. 2019, 103, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.C.; Price, R.S.; Feldman, E.L. Distal Symmetric Polyneuropathy: A Review. JAMA 2015, 314, 2172–2181. [Google Scholar] [CrossRef] [PubMed]
- Alport, A.R.; Sander, H.W. Clinical approach to peripheral neuropathy: Anatomic localization and diagnostic testing. Contin. Minneap. Minn 2012, 18, 13–38. [Google Scholar] [CrossRef] [PubMed]
- Raasing, L.R.M.; Vogels, O.J.M.; Veltkamp, M.; van Swol, C.F.P.; Grutters, J.C. Current View of Diagnosing Small Fiber Neuropathy. J. Neuromuscul. Dis. 2021, 8, 185–207. [Google Scholar] [CrossRef] [PubMed]
- Grayston, R.; Czanner, G.; Elhadd, K.; Goebel, A.; Frank, B.; Üçeyler, N.; Malik, R.A.; Alam, U. A systematic review and meta-analysis of the prevalence of small fiber pathology in fibromyalgia: Implications for a new paradigm in fibromyalgia etiopathogenesis. Semin. Arthritis Rheum. 2019, 48, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Bailly, F. The challenge of differentiating fibromyalgia from small-fiber neuropathy in clinical practice. Jt. Bone Spine 2021, 88, 105232. [Google Scholar] [CrossRef] [PubMed]
- Beynon, A.M.; Hebert, J.J.; Hodgetts, C.J.; Boulos, L.M.; Walker, B.F. Chronic physical illnesses, mental health disorders, and psychological features as potential risk factors for back pain from childhood to young adulthood: A systematic review with meta-analysis. Eur. Spine J. 2020, 29, 480–496. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.W.; Brown, V.; Jacobs, S.; Horne, L.; Langenberg, P.; Greenberg, P. Urinary Tract Infection and Inflammation at Onset of Interstitial Cystitis/Painful Bladder Syndrome. Urology 2008, 71, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Hickie, I.; Davenport, T.; Wakefield, D.; Vollmer-Conna, U.; Cameron, B.; Vernon, S.D.; Reeves, W.C.; Lloyd, A. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: Prospective cohort study. BMJ 2006, 333, 575. [Google Scholar] [CrossRef] [PubMed]
- Kallio-Laine, K.; Seppänen, M.; Lokki, M.-L.; Lappalainen, M.; Notkola, I.-L.; Seppälä, I.; Koskinen, M.; Valtonen, V.; Kalso, E. Widespread unilateral pain associated with herpes simplex virus infections. J. Pain 2008, 9, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Navis, A.; Jiao, J.; George, M.C.; Simpson, D.; Robinson-Papp, J. Comorbid Pain Syndromes in HIV-Associated Peripheral Neuropathy. Pain Med. 2018, 19, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Madden, V.J.; Parker, R.; Goodin, B.R. Chronic pain in people with HIV: A common comorbidity and threat to quality of life. Pain Manag. 2020, 10, 253–260. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. Electronic address: [email protected]; European Association for the Study of the Liver EASL Recommendations on Treatment of Hepatitis C 2018. J. Hepatol. 2018, 69, 461–511. [Google Scholar] [CrossRef] [PubMed]
- Moretti, R.; Caruso, P.; Dal Ben, M.; Gazzin, S.; Tiribelli, C. Hepatitis C-related cryoglobulinemic neuropathy: Potential role of oxcarbazepine for pain control. BMC Gastroenterol. 2018, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Cacoub, P.; Saadoun, D. Extrahepatic Manifestations of Chronic HCV Infection. N. Engl. J. Med. 2021, 384, 1038–1052. [Google Scholar] [CrossRef]
- Fernandez-de-Las-Peñas, C.; Notarte, K.I.; Macasaet, R.; Velasco, J.V.; Catahay, J.A.; Ver, A.T.; Chung, W.; Valera-Calero, J.A.; Navarro-Santana, M. Persistence of post-COVID symptoms in the general population two years after SARS-CoV-2 infection: A systematic review and meta-analysis. J. Infect. 2024, 88, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Ciaffi, J.; Mancarella, L.; Lisi, L.; Brusi, V.; Cavallari, C.; D’Onghia, M.; Mari, A.; Borlandelli, E.; Faranda Cordella, J.; et al. Fibromyalgia: A new facet of the post-COVID-19 syndrome spectrum? Results from a web-based survey. RMD Open 2021, 7, e001735. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Ruscitti, P.; D’Angelo, S.; Cacciapaglia, F.; De Angelis, R.; Campochiaro, C.; Caso, F.; De Santis, M.; Di Cola, I.; Parisi, S.; et al. Broad clinical spectrum of SARS-CoV-2-associated inflammatory joint disease in adults: A report of 35 cases from the COVID-19 & Autoimmune Systemic Disease Italian study group. Ann. Rheum. Dis. 2021, 80, 1498–1501. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Ruscitti, P.; Addimanda, O.; Foti, R.; Raimondo, V.; Murdaca, G.; Caira, V.; Pigatto, E.; Cuomo, G.; Lo Gullo, A.; et al. Inflammatory rheumatic diseases with onset after SARS-CoV-2 infection or COVID-19 vaccination: A report of 267 cases from the COVID-19 and ASD group. RMD Open 2023, 9, e003022. [Google Scholar] [CrossRef] [PubMed]
- Ciaffi, J.; Vanni, E.; Mancarella, L.; Brusi, V.; Lisi, L.; Pignatti, F.; Naldi, S.; Assirelli, E.; Neri, S.; Reta, M.; et al. Post-Acute COVID-19 Joint Pain and New Onset of Rheumatic Musculoskeletal Diseases: A Systematic Review. Diagnostics 2023, 13, 1850. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Ruscitti, P.; Raimondo, V.; De Angelis, R.; Cacciapaglia, F.; Pigatto, E.; Olivo, D.; Di Cola, I.; Galluccio, F.; Francioso, F.; et al. Systemic syndromes of rheumatological interest with onset after COVID-19 vaccine administration: A report of 30 cases. Clin. Rheumatol. 2022, 41, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Lantos, P.M.; Rumbaugh, J.; Bockenstedt, L.K.; Falck-Ytter, Y.T.; Aguero-Rosenfeld, M.E.; Auwaerter, P.G.; Baldwin, K.; Bannuru, R.R.; Belani, K.K.; Bowie, W.R.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 Guidelines for the Prevention, Diagnosis and Treatment of Lyme Disease. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 72, e1–e48. [Google Scholar] [CrossRef] [PubMed]
- Stanek, G.; Wormser, G.P.; Gray, J.; Strle, F. Lyme borreliosis. Lancet Lond. Engl. 2012, 379, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Dinerman, H.; Steere, A.C. Lyme disease associated with fibromyalgia. Ann. Intern. Med. 1992, 117, 281–285. [Google Scholar] [CrossRef] [PubMed]
- WHO Fact SHeets. Available online: https://www.who.int/health-topics/cancer#tab=tab_1 (accessed on 6 April 2024).
- Mercadante, S. Cancer Pain Treatment Strategies in Patients with Cancer. Drugs 2022, 82, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.P.; Kupelnick, B.; Miller, K.; Devine, D.; Lau, J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J. Natl. Cancer Inst. Monogr. 2004, 32, 40–50. [Google Scholar] [CrossRef] [PubMed]
- McBeth, J.; Symmons, D.P.; Silman, A.J.; Allison, T.; Webb, R.; Brammah, T.; Macfarlane, G.J. Musculoskeletal pain is associated with a long-term increased risk of cancer and cardiovascular-related mortality. Rheumatology 2008, 48, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Koo, M.M.; Swann, R.; McPhail, S.; Abel, G.A.; Elliss-Brookes, L.; Rubin, G.P.; Lyratzopoulos, G. Presenting symptoms of cancer and stage at diagnosis: Evidence from a cross-sectional, population-based study. Lancet Oncol. 2020, 21, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, A.; Smith, T.J. Total Pain #417. J. Palliat. Med. 2021, 24, 1100–1101. [Google Scholar] [CrossRef] [PubMed]
- Silvestris, F.; Lombardi, L.; De Matteo, M.; Bruno, A.; Dammacco, F. Myeloma bone disease: Pathogenetic mechanisms and clinical assessment. Leuk. Res. 2007, 31, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.; Clézardin, P. Bone-Targeted Therapies in Cancer-Induced Bone Disease. Calcif. Tissue Int. 2018, 102, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Han, I.; Kang, S.; Lee, S.A.; Kim, H.-S. Non-spine bone metastasis as an initial manifestation of cancer in Korea. J. Korean Med. Sci. 2014, 29, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Falk, S.; Bannister, K.; Dickenson, A.H. Cancer pain physiology. Br. J. Pain 2014, 8, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.; Vargas, G.; Pape, F.L.; Clézardin, P. Cancer Cell Colonisation in the Bone Microenvironment. Int. J. Mol. Sci. 2016, 17, 1674. [Google Scholar] [CrossRef] [PubMed]
- Panaroni, C.; Yee, A.J.; Raje, N.S. Myeloma and Bone Disease. Curr. Osteoporos. Rep. 2017, 15, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Minisola, S.; Pepe, J.; Piemonte, S.; Cipriani, C. The diagnosis and management of hypercalcaemia. BMJ 2015, 350, h2723. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Zhang, G.; Li, M.; Zhou, X. Whole-Body MRI vs. PET/CT for the Detection of Bone Metastases in Patients With Prostate Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 633833. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.F.; Jones, L.W.; Andersen, J.L.; Daugaard, G.; Rorth, M.; Hojman, P. Muscle dysfunction in cancer patients. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, A.G.S.; Allard, A.B.; Callen, J.P.; Chinoy, H.; Chung, L.; Fiorentino, D.; George, M.D.; Gordon, P.; Kolstad, K.; Kurtzman, D.J.B.; et al. A systematic review and meta-analysis to inform cancer screening guidelines in idiopathic inflammatory myopathies. Rheumatol. Oxf. Engl. 2021, 60, 2615–2628. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, N.; Atalay, N.S.; Selcuk, S.T.; Alkan, H.; Catalbas, N.; Sahin, F. Frequency of fibromyalgia syndrome in breast cancer patients. Int. J. Clin. Oncol. 2013, 18, 285–292. [Google Scholar] [CrossRef]
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac disease: A comprehensive current review. BMC Med. 2019, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Therrien, A.; Kelly, C.P.; Silvester, J.A. Celiac Disease: Extraintestinal Manifestations and Associated Conditions. J. Clin. Gastroenterol. 2020, 54, 8–21. [Google Scholar] [CrossRef]
- Rodrigo, L.; Blanco, I.; Bobes, J.; de Serres, F.J. Remarkable prevalence of coeliac disease in patients with irritable bowel syndrome plus fibromyalgia in comparison with those with isolated irritable bowel syndrome: A case-finding study. Arthritis Res. Ther. 2013, 15, R201. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Sanders, D.S.; Green, P.H.R. Coeliac disease. Lancet Lond. Engl. 2018, 391, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Laurikka, P.; Salmi, T.; Collin, P.; Huhtala, H.; Mäki, M.; Kaukinen, K.; Kurppa, K. Gastrointestinal Symptoms in Celiac Disease Patients on a Long-Term Gluten-Free Diet. Nutrients 2016, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Sansotta, N.; Amirikian, K.; Guandalini, S.; Jericho, H. Celiac Disease Symptom Resolution: Effectiveness of the Gluten-free Diet. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Insani, W.N.; Whittlesea, C.; Alwafi, H.; Man, K.K.C.; Chapman, S.; Wei, L. Prevalence of adverse drug reactions in the primary care setting: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0252161. [Google Scholar] [CrossRef]
- Conforti, A.; Chiamulera, C.; Moretti, U.; Colcera, S.; Fumagalli, G.; Leone, R. Musculoskeletal adverse drug reactions: A review of literature and data from ADR spontaneous reporting databases. Curr. Drug Saf. 2007, 2, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Mammen, A.L. Statin-Associated Myalgias and Muscle Injury-Recognizing and Managing Both While Still Lowering the Low-Density Lipoprotein. Rheum. Dis. Clin. N. Am. 2022, 48, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Cholesterol Treatment Trialists’ Collaboration Effect of statin therapy on muscle symptoms: An individual participant data meta-analysis of large-scale, randomised, double-blind trials. Lancet Lond. Engl. 2022, 400, 832–845. [CrossRef] [PubMed]
- Akimoto, H.; Negishi, A.; Oshima, S.; Okita, M.; Numajiri, S.; Inoue, N.; Ohshima, S.; Kobayashi, D. Onset timing of statin-induced musculoskeletal adverse events and concomitant drug-associated shift in onset timing of MAEs. Pharmacol. Res. Perspect. 2018, 6, e00439. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, R.S.; Whipple, M.O.; Vincent, A. Statin Therapy and Symptom Burden in Patients With Fibromyalgia: A Prospective Questionnaire Study. Mayo Clin. Proc. Innov. Qual. Outcomes 2021, 5, 1036–1041. [Google Scholar] [CrossRef]
- Tomaszewski, M.; Stępień, K.M.; Tomaszewska, J.; Czuczwar, S.J. Statin-induced myopathies. Pharmacol. Rep. PR 2011, 63, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Simard, C.; Poirier, P. Ezetimibe-associated myopathy in monotherapy and in combination with a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Can. J. Cardiol. 2006, 22, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Molokhia, M.; McKeigue, P.; Curcin, V.; Majeed, A. Statin induced myopathy and myalgia: Time trend analysis and comparison of risk associated with statin class from 1991-2006. PLoS ONE 2008, 3, e2522. [Google Scholar] [CrossRef]
- Ding, L.; Chen, C.; Yang, Y.; Fang, J.; Cao, L.; Liu, Y. Musculoskeletal Adverse Events Associated with PCSK9 Inhibitors: Disproportionality Analysis of the FDA Adverse Event Reporting System. Cardiovasc. Ther. 2022, 2022, 9866486. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, T.A.; Zimmerman, F.H. Fibrates in combination with statins in the management of dyslipidemia. J. Clin. Hypertens. Greenwich Conn. 2006, 8, 35–41; quiz 42–43. [Google Scholar] [CrossRef] [PubMed]
- Sieber, P.; Lardelli, P.; Kraenzlin, C.A.; Kraenzlin, M.E.; Meier, C. Intravenous bisphosphonates for postmenopausal osteoporosis: Safety profiles of zoledronic acid and ibandronate in clinical practice. Clin. Drug Investig. 2013, 33, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Bock, O.; Boerst, H.; Thomasius, F.E.; Degner, C.; Stephan-Oelkers, M.; Valentine, S.M.; Felsenberg, D. Common musculoskeletal adverse effects of oral treatment with once weekly alendronate and risedronate in patients with osteoporosis and ways for their prevention. J. Musculoskelet. Neuronal Interact. 2007, 7, 144–148. [Google Scholar] [PubMed]
- Wysowski, D.K.; Chang, J.T. Alendronate and risedronate: Reports of severe bone, joint, and muscle pain. Arch. Intern. Med. 2005, 165, 346–347. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.R.D.; Dowsett, M. Aromatase inhibitors for breast cancer: Lessons from the laboratory. Nat. Rev. Cancer 2003, 3, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Henry, N.L.; Giles, J.T.; Ang, D.; Mohan, M.; Dadabhoy, D.; Robarge, J.; Hayden, J.; Lemler, S.; Shahverdi, K.; Powers, P.; et al. Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res. Treat. 2008, 111, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Beckwée, D.; Leysen, L.; Meuwis, K.; Adriaenssens, N. Prevalence of aromatase inhibitor-induced arthralgia in breast cancer: A systematic review and meta-analysis. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2017, 25, 1673–1686. [Google Scholar] [CrossRef] [PubMed]
- Vallerand, I.A.; Lewinson, R.T.; Farris, M.S.; Sibley, C.D.; Ramien, M.L.; Bulloch, A.G.M.; Patten, S.B. Efficacy and adverse events of oral isotretinoin for acne: A systematic review. Br. J. Dermatol. 2018, 178, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.; Haettich, B. Rheumatological symptoms due to retinoids. Baillieres Clin. Rheumatol. 1991, 5, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Kapała, J.; Lewandowska, J.; Placek, W.; Owczarczyk-Saczonek, A. Adverse Events in Isotretinoin Therapy: A Single-Arm Meta-Analysis. Int. J. Environ. Res. Public. Health 2022, 19, 6463. [Google Scholar] [CrossRef] [PubMed]
- Bannwarth, B. Drug-induced musculoskeletal disorders. Drug Saf. 2007, 30, 27–46. [Google Scholar] [CrossRef] [PubMed]
| Medical Condition | Predisposing Factors | Typical Features | Clues to Diagnosis in FM Patients | First-Level Diagnostic Test |
|---|---|---|---|---|
| Micronutrient deficiency | ||||
| Vitamin B12 deficiency | Vegetarian or vegan diet, atrophic gastritis, drugs (PPIs, metformin, colchicine), inflammatory bowel disease, history of gastrointestinal surgery | Megaloblastic anemia, extravascular hemolysis, pancytopenia, neurological manifestations, glossitis | Chronic widespread pain and/or fatigue, with macrocytosis, or anemia/pancytopenia, glossitis, and prominent neurological manifestations (sleepiness, muscle weakness, paresthesia, ataxia) | ↓ Vitamin B12 ↑ Homocysteine |
| Vitamin D deficiency | Low sunlight exposure, dark skin pigmentation, obesity, inflammatory bowel disease, chronic kidney disease, chronic liver disease, history of gastrointestinal surgery | Bone fragility | Chronic widespread pain/bone pain and/or fatigue with proximal muscle weakness | ↓ 25-hydroxyvitamin D |
| Iron deficiency | Vegetarian or vegan diet, celiac disease, inflammatory bowel disease, chronic bleeding, history of gastrointestinal surgery, drugs (PPIs, antiacids) | Microcytic anemia, dry/frail hair, koilonychia, atrophic glossitis, angular cheilosis, restless leg syndrome, pica | Chronic fatigue/exercise intolerance with microcytosis or restless leg syndrome, frail hair, koilonychia, atrophic glossitis, and angular cheilosis or pica | ↓ Ferritin ↓ TSAT |
| Magnesium deficiency | Alcoholism, diabetes mellitus, inflammatory bowel disease, chronic kidney disease, thyroid/parathyroid disorders), drugs (PPIs) | Neuromuscular hyperexcitability, cardiac arrhythmias, hypokalemia, hypocalcemia | Chronic widespread pain and/or fatigue with prominent muscular (e.g., cramps) and cognitive (agitation, restlessness) symptoms or palpitations/arrhythmia | ↓ Magnesium |
| Vitamin C | Alcoholism, insufficient dietary intake (raw vegetables and citrus fruits), chronic kidney disease, inflammatory bowel disease | Scurvy | Chronic widespread pain and/or fatigue with easy bruising and bleeding or teeth loosening | ↓ Vitamin C |
| Endocrine disorders | ||||
| Thyroid diseases | Female sex, family history of thyroid or autoimmune diseases, iodine deficiency, radioiodine therapy, neck radiation, drugs (amiodarone, lithium, metoclopramide, tyrosine kinase inhibitors, interferon-alfa, steroids) | Hypothyroidism: fatigue, mood disturbances, slowing of thought and speech, lethargy, bradycardia, cold sensitivity, weight gain, dry skin, hair loss, and menstrual irregularities Hyperthyroidism: fatigue, nervousness, anxiety, insomnia, tachycardia, tremor, heat intolerance, increased sweating, tachycardia, involuntary weight loss and (in case of Graves’ disease), eye symptoms (irritation, pain, discomfort in eye motion, diplopia) | Prominent fatigue with or without chronic widespread pain, mood and cognitive disturbances, insomnia, temperature sensitivity, tremor, weight change, and palpitation | ↑ TSH (hypothyroidism) ↓ TSH (hyperthyroidism) |
| Obesity | Family inheritance, unhealthy diet, inactivity, drugs (antidepressants, antipsychotics, beta-blockers, steroids, insulin) | BMI ≥ 30 kg/m2, weakness, shortness of breath “adiposis dolorosa” | Chronic widespread pain, fatigue, exercise intolerance, sleep disturbances, “adiposis dolorosa” in patients with increased BMI | BMI calculation |
| Infections | ||||
| HIV | High-risk sexual behavior, intravenous drug use, needle-stick accidents, unsterile cutting or piercings, blood transfusions and organ transplants | Acquired immunodeficiency syndrome (AIDS) | Chronic widespread pain and/or fatigue in patients with high-risk sexual behavior, history of other sexually transmitted infections (e.g., syphilis, herpes, chlamydia, gonorrhea), recurrent infections, or lymphopenia | Positive HIV antibody test |
| HCV | High-risk sexual behavior, intravenous drug use, needle-stick accidents, unsterile cutting or piercings, blood transfusions and organ transplants | Acute or chronic hepatitis | Chronic widespread pain and/or fatigue in patients with high-risk sexual behavior, a history of other sexually transmitted infections (e.g., syphilis, herpes, chlamydia, gonorrhea) | Positive HCV antibody test |
| SARS-CoV-2 | Close personal contact with infected individuals | COVID-19; long-COVID-19 | Chronic widespread pain and/or fatigue with onset after SARS-CoV-2 infection | No specific tests available for post-COVID-19 conditions |
| Borrelia burgdorferi | Risk factors for tick bite exposure in an endemic area (e.g., gardening, hunting, or walking in high grasses) | Erythema migrans, neuroborreliosis, carditis, arthritis | Chronic widespread pain and/or fatigue in patients with a past history of tick bite or erythema migrans following potential exposure to tick bite | Positive Borrelia antibody test |
| Others | ||||
| Peripheral Neuropathy | Diabetes, alcohol abuse, malnutrition, monoclonal gammopathy, drugs (chemotherapy, amiodarone, colchicine, gold salts) heavy metals intoxication | Sensory (pins and needles, burning or sharp pain, numbness, decreased sensitivity to pain or temperature), motor (muscle weakness/paralysis; twitching or muscle cramps) or autonomic (sicca syndrome, accommodation problems, orthostatic hypotension, hypohidrosis or hyperhidrosis, hot flashes, intestinal problems, gastroparesis, palpitations) disturbances | Chronic widespread pain with prominent neuropathic features (burning, shooting, pricking, pins and needles, squeezing, or freezing pain) and/or accompanied by allodynia, hyperalgesia, paresthesia, weakness, imbalance, and/or autonomic dysfunction | Nerve conduction studies; skin biopsy (small fiber neuropathy) |
| Obstructive sleep apnea | Obesity, heart failure, acromegaly, hypothyroidism, smoking, craniofacial abnormalities | Daytime sleepiness, fatigue, non-refreshing sleep, snoring, paroxysmal nocturnal dyspnea, decreased concentration and memory, headache, nocturia | Chronic fatigue and/or widespread pain with snoring or prominent sleep disturbances (e.g., non-refreshing sleep) and daytime cognitive symptoms | Polysomnography |
| Celiac disease | Family history, type 1 diabetes, Hashimoto thyroiditis, microscopic colitis, Addison’s disease, Down syndrome, William syndrome, Turner syndrome | Abdominal pain, diarrhea or constipation, weight loss, bloating, nausea/vomiting | Chronic widespread pain and/or fatigue with prominent gastrointestinal symptoms, unexplained iron deficiency or low bone mass | Positive tTG-IgA |
| Cancer | Previous history of cancer, aging, smoking, obesity, alcohol abuse, infectious agents (e.g., HPV), radiation and/or chemical exposure, chronic inflammation, immunosuppression | Cancer-specific manifestations; bone metastasis; paraneoplastic syndromes (e.g., cancer-associated myositis) | Progressive, treatment-resistant fatigue/muscle weakness and/or multifocal bone pain in patients with known risk factors associated with organ-specific or constitutional symptoms or unexplained weight loss | No universal screening test is available; consider the possibility of further investigation based on demographic/epidemiological factors and specific symptoms |
| Drugs | Chronic treatment | - | Chronic widespread pain and/or fatigue in patients exposed to medications notoriously associated with musculoskeletal adverse events | Lipid-lowering drugs, bisphosphonates, aromatase inhibitors, isotretinoin, and others |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amuri, A.; Greco, S.; Pagani, M.; Presciuttini, B.; Ciaffi, J.; Ursini, F. Common Non-Rheumatic Medical Conditions Mimicking Fibromyalgia: A Simple Framework for Differential Diagnosis. Diagnostics 2024, 14, 1758. https://doi.org/10.3390/diagnostics14161758
D’Amuri A, Greco S, Pagani M, Presciuttini B, Ciaffi J, Ursini F. Common Non-Rheumatic Medical Conditions Mimicking Fibromyalgia: A Simple Framework for Differential Diagnosis. Diagnostics. 2024; 14(16):1758. https://doi.org/10.3390/diagnostics14161758
Chicago/Turabian StyleD’Amuri, Andrea, Salvatore Greco, Mauro Pagani, Barbara Presciuttini, Jacopo Ciaffi, and Francesco Ursini. 2024. "Common Non-Rheumatic Medical Conditions Mimicking Fibromyalgia: A Simple Framework for Differential Diagnosis" Diagnostics 14, no. 16: 1758. https://doi.org/10.3390/diagnostics14161758





