Abstract
Structured follow-up visits should be accessible for children at risk for developmental delay. Follow-up visits should include a serial neuromotor assessment in the first two years of life (e.g., 3–6, 12, 24 months corrected age), which are repeated during the transition to school. The diagnosis of neuromotor development may be prognostic for important skills later in life. The early diagnosis of a child’s general movements can be helpful in planning appropriately for proper treatment and intervention. These diagnostic assessments should be conducted by qualified healthcare professionals. The evaluation of neuromotor developmental health is specified in the national guidelines and funded by either a national government or public or private healthcare insurance and based on standardized assessment scales. The aim of this study is to show what elements of follow-up visits are recommended. Objectives: The group of patients for whom the structured follow-up systems are intended were children born very preterm (<32 weeks gestation) or full-term born children with severe neonatal complications. Material and methods: The methods for monitoring neurodevelopment include the following: The General Movements Assessment (GMA), the Ages and Stages Questionnaire (ASQ-3), the Bayley Scales of Infant and Toddler Development (BSID-4), and the Parent Report of Children’s Abilities-Revised (PARCA-R). Results: The results of follow-up visits should be registered. Conclusions: The benefits of follow-up neuromotor development assessments can be observed at school age and even in adulthood.
1. Introduction
Human development is shaped both by nature and nurture. Human development in the first few years of life is exceptionally rapid across almost all domains, such as social, emotional, cognitive, language, and motor development [1]. The National Resource Center For Health and Safety In Child Care and Early Education advises professionals to perform screening assessments on infants to improve their development [2]. According to the Swiss National Cohort, thanks to follow-up care, 63% percent of very preterm infants do not have any cognitive or psychomotor impairments by the time they reach school age [3].
Several countries have decided to support the psychomotor development of children systemically, not only due to ethical reasons but also due to socioeconomic status. The program for Transmural Developmental Support for Very Preterm Infants and Their Parents in the Netherlands has demonstrated positive outcomes on a child’s development until 5 years of age. This is why Transmural Developmental Support for Very Preterm Infants and Their Parents has established a foundation of legitimacy for implementation [4]. Additionally, a comparative analysis of data from this program, involving 90 infants in the intervention group and 85 infants in the control group, revealed saved costs due to fewer referrals to paramedical professionals, reduced hospital readmissions, and a lower incidence of child abuse during the first year of life in the intervention group compared to the control group (10% vs. 22%, 29% vs. 40%, and 1.2% vs. 6.4%, respectively). In a Business Case, savings in healthcare provision were compared to the estimated costs of the Transmural Developmental Support for Very Preterm Infants and Their Parents program. However, the Business Case’s political will and positive results were fundamental to establishing structural financial support for implementing this program, showing that the savings outweigh the cost. The cost of healthcare provision was calculated in EUR [5].
The European Foundation for the Care of Newborn Infants (EFCNIs), a collaborative organization comprising over 220 healthcare professionals, parents, and industry specialists from more than 30 countries, has created the European Standards of Care for Newborn Health (ESCNH)—a set of standards for newborn care from hospital to school age [6]. These guidelines aim to ensure the best possible care for newborns across Europe. Each country that participates in implementing these standards has its own national guidelines, protocols, and laws (depending on the local situation) that refer to ESCNH. The most vulnerable group included is very preterm infants (<32 weeks’ gestation) or children with severe developmental complications [5]. Therefore, these standards have been developed for the following:
- Full-term infants who require intensive care.
- Infants born before 32 + 0 weeks’ gestation.
- Infants born after 32 + 0 gestation who have or had one or more significant risk factors, such as
- ○
- A brain lesion in neuroimaging that is likely associated with developmental problems or disorders (for example, grade 3 or 4 intraventricular hemorrhage or cystic periventricular leukomalacia);
- ○
- Grade 2 or 3 hypoxic ischaemic encephalopathy in the neonatal period;
- ○
- Severe fetal growth restriction (microcephalic—symmetric growth);
- ○
- Severe social or family problems with safety issues for the child;
- ○
- Neonatal bacterial or viral meningitis/encephalitis [7].
Another group of patients who benefit from follow-up care are children who have hypothermia [8], congenital heart diseases [9] and chromosomal abnormalities [10].
It is important to address several factors of infant development, including growth, feeding, general health, hearing, visual abilities, speech, cognition, behaviors, and motor skills. If necessary, interventions such as family-centered developmental support, physiotherapy, speech therapy, dietetics, occupational therapy, and psychological support should be provided. According to the National Guideline Alliance, screening assessments should be conducted at 3, 6, 9, 12, 24 (corrected age), and 48 months of age [11,12,13] (Table 1).

Table 1.
Typical developmental milestones at 4, 6, 9, and 12 months of age related to motor development, social-emotional skills, and infant communication abilities [14,15,16,17,18].
2. Infants at Risk of Acquiring Developmental Disabilities
In cases where a child presents with symptoms such as excessive head lag, persistent fisting of the hands beyond four months of age, stiffness or tightness in the legs, posturing, persistent primitive reflexes, an asymmetry of movement, motor delay, or inharmonic gross motor milestones that appear to be acquired excessively early on or in an unusual sequence (such as rolling by arching their back at the age of 1 month and the lack of fidgety movement in the third month of age) cerebral palsy (CP) may be suspected [19,20]. These clinical signs may indicate potential motor dysfunction and should be evaluated by a pediatric neurologist or other healthcare provider with experience in diagnosing and managing CP. Recognizing and diagnosing CP as early as possible in patients is essential to initiating appropriate interventions and optimizing health outcomes. This is possible, especially in the USA, because early intervention programs are not only available for preterm infants but for all infants and are available in every state and territory of the USA [21]. Early intervention is critical in supporting the healthy development of infants and toddlers with developmental delays and disabilities [22]. Moreover, the Centers for Disease Control and Prevention recommend that “If you, your child’s doctor, or other care provider is concerned about your child’s development, ask to be connected with your state or territory’s early intervention program to find out if your child can get services to help” [23]. Similarly, in Switzerland, due to continuous medical care, if clinicians see a symptom of CP, a child is seen earlier than the third month of life [24].
Infants with motor delays have restricted opportunities to interact with their environment and possess limited abilities to learn through action [1]. Current evidence shows that a child’s brain has more potential for neuroplasticity early in life, which may encourage reorganization after perinatal injury. Early therapeutic interventions can help reduce neuromotor conditions and enhance cognitive and motor abilities for infants at risk of developing a neuromotor delay [25]. One particular program, called Coping with and Caring for Infants with Special Needs (COPCAs), was developed in the Netherlands in the early 2000s, specifically for infants with a high physiological risk for developing neurological disabilities [26]. This intervention focuses on teaching parents how to stimulate infant development during daily routines. Physiotherapists treat family members as active and responsible partners in the intervention process. The objective is to expand the child’s range of physical activities and improve their ability to adapt their movements under different circumstances [26,27]. Randomized controlled trials have shown that COPCA’s coaching strategies are effective in infants with a high risk of developing neurodevelopmental disorders [28,29,30,31]. It was found that encouraging infants with CP at 18 months of age to independently engage in motor behaviors through the repetition of trial-and-error experiences and caregiver coaching as elements of the COPCA intervention was beneficial for improving their motor developmental outcomes compared to standard physiotherapy care [28,29,30,31]. A study conducted by Van Balen et al. (2019) revealed that infants participating in the COPCA program exhibited higher abilities to imitate typical development, which was linked to a higher level of overall anticipatory activation at 18 months of age [32]. This was consistent with another randomized controlled trial from 2021 that found that the COPCA program was associated with greater motor outcomes in infants born before 32 weeks than standard infant physiotherapy [26].
The aim of this study is to present recommendations from follow-up visits and the group of individuals who will benefit most from the monitoring of their neuromotor development. The other purpose of this study is to show the role of follow-up visits across almost all continents.
3. Materials and Methods
A literature review was performed from January to March 2024. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMAs) guidelines were applied to evaluate the methodological quality of the manuscripts [33]. Computer-based searches of the following medical and public databases were performed: PubMed, Medline Complete, Science Direct, Scopus, and Web of Science. Guidelines were published by the European Foundation for the Care of Newborn Infants, the National Institute for Health and Care Excellence (NICE), the American Academy of Pediatrics (AAP), the National Guideline Alliance, the Australian Children’s Education and Care Quality Authority, and the Japan Environment and Children’s Study [34,35,36,37]. These recommendations were divided into the context of standards described in medical articles [38]. The following combinations of keywords were used to search for the intervention of interest: “preterm infant + follow-up consultations”, and “preterm infant + follow up”.
The following inclusion criteria were included in this review: (1) Identification: studies conducted on preterm infants; (2) Screening: systematic and periodic early intervention until school age with no restrictions on the pathogenesis and etiology of prematurity; (3) Eligibility: reviews of the full text excluding any inappropriate studies; and (4) Inclusion: studies must investigate the effects of follow-up screening and early intervention.
Exclusion criteria were as follows: (1) Identification: studies not conducted on preterm infants, including animal models; (2) Screening: a lack of systematic, periodic follow-up visits until the school age (3) Eligibility: no full text and language other than English used, and (4) Indication: the lack of identification of the effects of follow-up visits.
The Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) system was used [39]. These recommendations were divided into high- and moderate-quality categories. Weak-quality recommendations were not considered.
The term “high quality” means that a specific recommendation is unlikely to be changed due to the number of articles and audit reports showing supporting evidence of it [40]. Papers that show the application of these recommendations are often randomized trials and the quality of the results and evidence presented are high. Moreover, these articles are deemed high quality since they refer to reliability, compliance with best practices, potential benefits for patients, and efficacy in achieving the intended goals. In medicine, the term “high quality” indicates that a particular service or practice meets the highest professional standards and is safe, effective, and beneficial for the population. “High quality” also means that further studies with a high confidence level do not change the conclusions drawn from the presented research.
The term “Moderate quality” indicates a moderate level of certainty that newer studies will not alter the conclusions of regarding the direction of the presented effects; however, new research may change the conclusions regarding the magnitude of this effect. On the other hand, it combines the certainty of evidence with the effect size of evidence and shows interventions [41]. This recommendation is mostly based on prognostic and prospective studies, randomized controlled trials, and systematic reviews where weaker diagnostic criteria and reference standards were presented [40]. To the best of our knowledge, we avoided using any studies that had been used as evidence for this type of recommendation and showed that methodological flaws were inconsistent or provided indirect evidence [41] (Table 2).

Table 2.
From the Lee SW, Koo MJ. PRISMA 2020 statement and guidelines for systematic review and meta-analysis articles, and their underlying mathematic Life Cycle Committee Recommendations. Life Cycle 2022, 2, e9. https://doi.org/10.54724/lc.2022.e9 [42].
4. Results
We reviewed and analyzed 313 papers. After including the GRADE and PRISMA guidelines, we came up with 23 results (Figure 1).
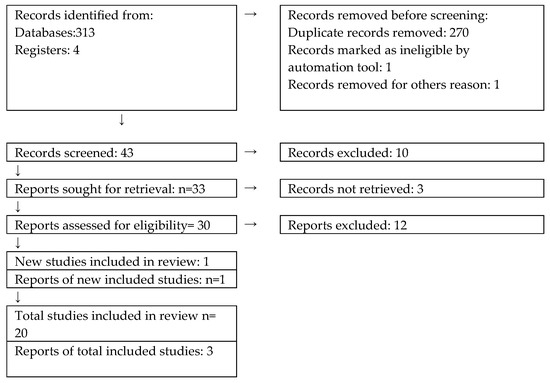
Figure 1.
The selection process referring to PRISMA from [33].
Next, we rejected “very low”, “low”, and “moderate” recommendations and focused our findings on those that were based on The National Guideline Alliance, The European Foundation for the Care of Newborn Infants, and the American Academy of Pediatrics (AAP). From our search, 20 papers included the following high-quality findings, as presented in Table 3.

Table 3.
The contents of the results.
5. Discussion
Follow-up visits are essential for monitoring the psychomotor development of these preterm infants. These visits should include preterm children born before 32 weeks of gestation and full-term born children who are at risk of developmental disorders, such as brain lesions; stage II or stage III hypoxic-ischemic encephalopathy (HIE) in the neonatal period; severe fetal growth restrictions; severe social or family problems with safety issues for the child; and neonatal bacterial or viral meningitis/encephalitis. Therefore, knowing, in general, that preterm children are at risk for developing motor delays, intellectual disability, visual and hearing impairments, attention-deficit concerns, impulsivity, and hyperactivity, the NICE has developed relevant recommendations, which have been made mandatory in many countries such as Switzerland, the Netherlands, and the US [13,26,55]. These recommendations state that infants who are born before 32 weeks of gestation or those who are born between 32 and 36 weeks of life with specific and identifiable risk factors should receive supplementary support and monitoring until they turn two years old. Infants born at or before 28 weeks of gestation are provided with support and monitoring until they are four years old without any age adjustments. This is due to their heightened risk of having special educational requirements [13].
Assessments should be administered periodically since, nowadays, specialists can detect children at risk of CP from a very early age, for instance, before 3–4 months of life and before the transition from preschool to primary school [56]. This is very important because parents often have to make the difficult decision about which school to choose for their child to attend. Some families who have chosen mainstream schools have observed that their children achieve many practical skills, such as literacy, in contrast to those who attend special schools. The greatest benefit for families is a higher level of independence for their children. Even when a brain lesion has been identified in perinatal life, later problems may not appear due to the neuroprotective role of IFCDC, but mostly only if a more independent-oriented therapeutic approach is utilized [57,58,59]. Applying the principles of IFCDC in the long term may reduce the adverse effects of brain lesions. Brain lesions in perinatal life may lead to CP in five to nine percent of preterm children [26]. Studies from the current multicenter study CP-EDIT (Early Diagnosis and Intervention Trial) showed that improving the care of patients with CP is possible even before they have an established diagnosis [60]. The standardized motor assessment scales are an essential component for the early diagnosis of CP.
In 2018, a comprehensive systematic review and meta-analysis was conducted to provide an overview of the prevalence of CP and motor and cognitive delays in very preterm (VPT) and very low-birthweight (VLBW) infants born within the past decade. This has enabled practitioners to show congruent abnormal findings indicative of CP. Their role is to approach diagnostic-specific early interventions to optimize infant motor and cognitive plasticity, prevent secondary complications, and enhance caregiver well-being [12,58]. Most of the studies included in the analysis evaluated motor and cognitive development at approximately 24 months of age. The most commonly used outcome measure was the BSID scale. Interestingly, the third edition of this scale showed a lower combined prevalence of motor and cognitive delays than the second edition. The findings from this paper revealed that taken as a whole, nearly one in six very preterm infants and one in five VLBW infants had cognitive or motor delays according to developmental scales at around 24 months of age, with approximately 1 in 15 developing CP [59].
In another big cohort study conducted by Pascal et al. (2020), trained physiotherapists and educational psychologists assessed preterm infants’ (<31 weeks’ gestation and or/<1500 g birthweight) motor, cognitive, and language performance using the BSID-III scale. The study found that at 2 years of corrected age, 25.2% of VPT and VLBW infants had mild NDI, while 10.9% had moderate-to-severe impairment. CP was diagnosed in 4.3% of the patients. However, in the subgroup of infants born at less than 26 weeks of gestational age, over 60% were found to have developed nephrogenic diabetes insipidus by 2 years of age, highlighting the significance of consistent follow-up care for a correct diagnosis, especially in the most vulnerable of infants. This clinical study concluded that lower gestational age and birthweight are linked to increased rates of adverse neurodevelopmental outcomes [60].
The studies that presented these results used scales such as the Bayley Scales of Infant and Toddler Development (BSID), or ASQ, which means that many aspects of development were considered (i.e., motor, cognitive, social) and focused on the family, not just on the child [55,56]. The WHO and The European Foundation for the Care of Newborn Infants have also recommended using PARKA since the COVID-19 pandemic. Using standardized tools of assessment enables a practitioner to register each patient, periodically compare their results, analyze the results, and also consult on their state with an appropriate specialist, even if the specialist is far away from the center where the follow-up visit takes place [57]. The children who attend follow-up visits present fewer cognitive and psychomotor problems, even at school age [3].
Follow-up visits should be conducted in a structured way that is standardized and family-friendly. Therefore, there is no need to create new assessment tools because those that already exist and are available are effective, as shown in many studies. Countries that would like to provide follow-up consultations should base their plans on those countries that have already conducted follow-up consultations for many years, such as Norway, Belgium, Switzerland, and the USA.
Unfortunately, little is known about early diagnosis in 50% of all CP cases, which are only discernible later in infancy. This is why follow-up visits should also be performed in late infancy, the toddler stage, and before the transition to school. Regular monitoring during this period is essential for detecting developmental coordination disorder (DCD), attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), and other specific learning disorders [61,62,63,64]. An early proper diagnosis enables a child and their family to obtain relevant support and teaches the child practical skills that they can use in their future life.
Follow-up visits should be accessible, routinely revised, consistent, funded, and supported. It seems unethical not to conduct these assessments because it is generally acknowledged that preterm children are at risk of many problems that can be detected early. Proper interventions should be conducted, which can then positively impact these children in school and later in adulthood [65].
Limitations of this study include the lack of a Polish version for the follow-up visit. The reason why it was not described in the article is the need to show only those follow-up visits that are based on structured, standardized, and validated scales presented by such organizations as The European Foundation for the Care of Newborn Infants, The National Institute for Health and Care Excellence (NICE) and the American Academy of Pediatrics (AAP). The aim of this article is to show what follow-up visits look like in most countries, where they bring many benefits to a population, including social, economic, and certainly health advantages.
6. Conclusions
This study summarizes how important it is to conduct follow-up consultations for children at risk of developmental delay in order to further widen the application of this intervention across several countries. Follow-up visits for preterm infants are critically important for their development. A follow-up visit helps a practitioner and family plan for the appropriate therapy for children at risk of motor developmental delay as well as children with a diagnosis of cerebral palsy. Many benefits of the regular assessment of preterm children have been proven clinically, and therefore, an early assessment allowing for early intervention is obligatory in many countries. The way regular, systematic, periodic patronage visits are supposed to proceed is already well described and tested. There are standardized tools readily available to practitioners to evaluate psychomotor development and help in conducting appropriate therapy, for instance, The General Movement Assessment (GMA), the Ages and Stages Questionnaire (ASQ-3), The Wechsler Preschool and Primary Scales of Intelligence (WPPSI-IV), and the Bayley Scales of Infant and Toddler Development (BSID-4). Follow-up visits should be widespread and mandatory in many countries to make opportunities more equal for all children at risk of developmental delay.
Author Contributions
Conceptualization, R.M. and E.M.; methodology, R.M.; software, W.S.; validation, R.M.; formal analysis, R.M.; investigation, R.M., J.K., O.K. and E.M.; resources, R.M.; data curation, R.M. and E.M.; writing—original draft preparation, R.M. and B.F.; writing—review and editing, B.F. and E.M.; visualization, A.K., M.T. (Maria Tuczyńska) and M.T. (Magdalena Tuczyńska); supervision, E.M. and W.S.; project administration, R.M.; funding acquisition, R.M., W.S. and E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
This project was undertaken thanks to conduct internships at (1) the Division of Child Development & Growth, Dept. of Woman, Child and Adolescent University Hospital, Geneva, Switzerland, with thanks to the invitation of Petra Huppi, who is a professor of pediatrics and Vice-Dean of the Faculty of Medicine at the University of Geneva; (2) University Hospitals Leuven, Belgium, with thanks to the invitation of Gunnar Naulaers, Bieke Bollen, and all nurses and neonatologists from the clinic of neonatology, especially Kelly Janssens and physiotherapist Sofie Vuylsteke who prepared each neonate for the follow-up assessment. (3) We also thank Michal Nowicki, Vice-President for Science and International Cooperation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Agazzi, H.; Shaffer-Hudkins, E.J.; Armstrong, K.H.; Hayford, H. Promoting Positive Behavioral Outcomes for Infants and Toddlers: An Evidence-Based Guide to Early Intervention; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Hutchins, H.; Abercrombie, J.; Lipton, C. Promotion of early childhood development and mental health in quality rating and improvement systems for early care and education: A review of state quality indicators. Early Child. Res. Q. 2023, 64, 229–241. [Google Scholar] [CrossRef]
- Pittet-Metrailler, M.P.; Mürner-Lavanchy, I.M.; Adams, M.; Bickle-Graz, M.; Pfister, R.E.; Natalucci, G.; Grunt, S.; Borradori Tolsa, C. Neurodevelopmental outcome at early school age in a Swiss national cohort of very preterm children. Swiss Med. Wkly. 2019, 149, w20084. [Google Scholar] [CrossRef]
- Halbmeijer, N.M.; Jeukens-Visser, M.; Onland, W.; Flierman, M.; Van Kaam, A.H.; Leemhuis, A. Neurodevelopmental Outcomes at Two Years’ Corrected Age of Very Preterm Infants after Implementation of a Post-Discharge Responsive Parenting Intervention Program (TOP Program). J. Pediatr. 2023, 257, 113381. [Google Scholar] [CrossRef]
- Jeukens-Visser, M.; Koldewijn, K.; Van Wassenaer-Leemhuis, A.G.; Flierman, M.; Nollet, F.; Wolf, M. Development and nationwide implementation of a postdischarge responsive parenting intervention program for very preterm born children: The TOP program. Infant. Ment. Health J. 2021, 42, 423–437. [Google Scholar] [CrossRef]
- Lindacher, V.; Altebaeumer, P.; Marlow, N.; Matthaeus, V.; Straszewski, I.N.; Thiele, N.; Pfeil, J.M.; Zimmermann, L.J.; Mader, S. European Standards of Care for Newborn Health—A project protocol. Acta Paediatr. 2021, 110, 1433–1438. [Google Scholar] [CrossRef]
- de Ceano-Vivas, M.; García, M.L.; Velázquez, A.; del Valle, F.M.; Menasalvas, A.; Cilla, A.; Epalza, C.; Romero, M.P.; Cabrerizo, M.; Calvo, C. Neurodevelopmental Outcomes of Infants Younger Than 90 Days Old Following Enterovirus and Parechovirus Infections of the Central Nervous System. Front. Pediatr. 2021, 9, 719119. [Google Scholar] [CrossRef]
- Zubcevic, S.; Heljic, S.; Catibusic, F.; Uzicanin, S.; Sadikovic, M.; Krdzalic, B. Neurodevelopmental Follow Up After Therapeutic Hypothermia for Perinatal Asphyxia. Med. Arch. 2015, 69, 362. [Google Scholar] [CrossRef]
- Abell, B.R.; Eagleson, K.; Auld, B.; Bora, S.; Justo, R.; Parsonage, W.; Sharma, P.; Kularatna, S.; McPhail, S.M. Implementing neurodevelopmental follow-up care for children with congenital heart disease: A scoping review with evidence mapping. Dev. Med. Child. Neurol. 2024, 66, 161–175. [Google Scholar] [CrossRef]
- Wojcik, M.H.; Stewart, J.E.; Waisbren, S.E.; Litt, J.S. Developmental Support for Infants with Genetic Disorders. Pediatrics 2020, 145, e20190629. [Google Scholar] [CrossRef]
- Dusing, S.C.; Burnsed, J.C.; E Brown, S.; Harper, A.D.; Hendricks-Munoz, K.D.; Stevenson, R.D.; Thacker, L.R.; Molinini, R.M. Efficacy of Supporting Play Exploration and Early Development Intervention in the First Months of Life for Infants Born Very Preterm: 3-Arm Randomized Clinical Trial Protocol. Phys. Ther. 2020, 100, 1343–1352. [Google Scholar] [CrossRef]
- Morgan, C.; Fetters, L.; Adde, L.; Badawi, N.; Bancale, A.; Boyd, R.N.; Chorna, O.; Cioni, G.; Damiano, D.L.; Darrah, J.; et al. Early Intervention for Children Aged 0 to 2 Years with or at High Risk of Cerebral Palsy: International Clinical Practice Guideline Based on Systematic Reviews. JAMA Pediatr. 2021, 175, 846. [Google Scholar] [CrossRef] [PubMed]
- National Guideline Alliance (UK). Developmental Follow-Up of Children and Young People Born Preterm; National Institute for Health and Care Excellence (NICE): London, UK, 2017. Available online: http://www.ncbi.nlm.nih.gov/books/NBK447731/ (accessed on 13 March 2024).
- World Health Organization. Improving Early Childhood Development: WHO Guideline; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Bundy, D.A.P.; De Silva, N.; Horton, S.; Jamison, D.T.; Patton, G.C. Child and Adolescent Health and Development, 3rd ed.; World Bank Group: Washington, DC, USA, 2017. [Google Scholar]
- World Health Organization. WHO Guidelines on Parenting Interventions to Prevent Maltreatment and Enhance Parent-Child Relationships with Children Aged 0–17 Years; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Fauls, J.R.; Thompson, B.L.; Johnston, L.M. Validity of the Ages and Stages Questionnaire to identify young children with gross motor difficulties who require physiotherapy assessment. Dev. Med. Child. Neurol. 2020, 62, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Zubler, J.; Whitaker, T. CDC’s Revised Developmental Milestone Checklists. Am. Fam. Physician 2022, 106, 370–371. [Google Scholar] [PubMed]
- Paul, S.; Nahar, A.; Bhagawati, M.; Kunwar, A.J. A Review on Recent Advances of Cerebral Palsy. Oxid. Med. Cell Longev. 2022, 2022, 2622310. [Google Scholar] [CrossRef]
- Malak, R.; Borek, J.; Sikorska, D.; Keczmer, P.; Samborski, W. Assessment of general movement among infants not at risk of developmental delay. J. Med. Sci. 2020, 89, e393. [Google Scholar] [CrossRef]
- CDC. What is “Early Intervention”? Available online: https://www.cdc.gov/ncbddd/actearly/parents/states.html#:~:text=Programs%20are%20available%20in%20every,any%20child%20who%20is%20eligible (accessed on 13 March 2024).
- Boyle, C.A.; Cordero, J.F.; Trevathan, E. The National Center on Birth Defects and Developmental Disabilities. Am. J. Prev. Med. 2012, 43, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Abercrombie, J.; Wiggins, L.; Green, K.K. CDC’s “Learn the Signs. Act Early.” Developmental Milestone Resources to Improve Early Identification of Children with Developmental Delays, Disorders, and Disabilities. Zero Three 2022, 43, 5–12. [Google Scholar] [PubMed]
- Davis, B.E.; Leppert, M.O.C.; German, K.; Lehmann, C.U.; Adams-Chapman, I. Primary Care Framework to Monitor Preterm Infants for Neurodevelopmental Outcomes in Early Childhood. Pediatrics 2023, 152, e2023062511. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Anastasopoulos, S.; Deregnier, R.-A.; Pouppirt, N.; Barlow, A.K.; Patrick, C.; O’brien, M.K.; Babula, S.; Sukal-Moulton, T.; Peyton, C.; et al. Protocol for a randomized controlled trial to evaluate a year-long (NICU-to-home) evidence-based, high dose physical therapy intervention in infants at risk of neuromotor delay. PLoS ONE 2023, 18, e0291408. [Google Scholar] [CrossRef]
- Akhbari Ziegler, S.; von Rhein, M.; Meichtry, A.; Wirz, M.; Hielkema, T.; Hadders-Algra, M.; Swiss Neonatal Network & Follow-Up Group. The Coping with and Caring for Infants with Special Needs intervention was associated with improved motor development in preterm infants. Acta Paediatr. 2021, 110, 1189–1200. [Google Scholar] [CrossRef]
- Akhbari Ziegler, S.; De Souza Morais, R.L.; Magalhães, L.; Hadders-Algra, M. The potential of COPCA’s coaching for families with infants with special needs in low- and middle-income countries. Front. Pediatr. 2023, 11, 983680. [Google Scholar] [CrossRef]
- Blauw-Hospers, C.H.; Dirks, T.; Hulshof, L.J.; Bos, A.F.; Hadders-Algra, M. Pediatric Physical Therapy in Infancy: From Nightmare to Dream? A Two-Arm Randomized Trial. Phys. Ther. 2011, 91, 1323–1338. [Google Scholar] [CrossRef]
- Hadders-Algra, M. Early human motor development: From variation to the ability to vary and adapt. Neurosci. Biobehav. Rev. 2018, 90, 411–427. [Google Scholar] [CrossRef]
- Hielkema, T.; Hamer, E.G.; A Reinders-Messelink, H.; Maathuis, C.G.; Bos, A.F.; Dirks, T.; van Doormaal, L.; Verheijden, J.; Vlaskamp, C.; Lindeman, E.; et al. LEARN 2 MOVE 0-2 years: Effects of a new intervention program in infants at very high risk for cerebral palsy; a randomized controlled trial. BMC Pediatr. 2010, 10, 76. [Google Scholar] [CrossRef]
- Hielkema, T.; Hamer, E.G.; Boxum, A.G.; La Bastide-Van Gemert, S.; Dirks, T.; Reinders-Messelink, H.A.; Maathuis, C.G.B.; Verheijden, J.; Geertzen, J.H.B.; Hadders-Algra, M.; et al. LEARN2MOVE 0–2 years, a randomized early intervention trial for infants at very high risk of cerebral palsy: Neuromotor, cognitive, and behavioral outcome. Disabil. Rehabil. 2020, 42, 3752–3761. [Google Scholar] [CrossRef]
- Van Balen, L.C.; Dijkstra, L.J.; Dirks, T.; Bos, A.F.; Hadders-Algra, M. Early Intervention and Postural Adjustments During Reaching in Infants at Risk of Cerebral Palsy. Pediatr. Phys. Ther. 2019, 31, 175–183. [Google Scholar] [CrossRef]
- Lee, S.W.; Koo, M.J. PRISMA 2020 statement and guidelines for systematic review and meta-analysis articles, and their underlying mathematics: Life Cycle Committee Recommendations. Life Cycle 2022, 2, e9. [Google Scholar] [CrossRef]
- Shonkoff, J.P.; Richter, L.; Van Der Gaag, J.; Bhutta, Z.A. An Integrated Scientific Framework for Child Survival and Early Childhood Development. Pediatrics 2012, 129, e460–e472. [Google Scholar] [CrossRef]
- Yasumitsu-Lovell, K.; Thompson, L.; Fernell, E.; Eitoku, M.; Suganuma, N.; Gillberg, C.; The Japan Environment and Children’s Study Group. Birth month and infant gross motor development: Results from the Japan Environment and Children’s Study (JECS). PLoS ONE 2021, 16, e0251581. [Google Scholar] [CrossRef] [PubMed]
- Faez, N.; Hmami, F.; Kojmane, W.; Atmani, S. Developmentally supportive care in neonatology: Correlational study of the knowledge and declared practices of professionals. Ann. Med. Surg. 2022, 84, 104928. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, N.; Spence, K.; Galea, C.; Psaila, K.; Foureur, M.; Sinclair, L. The effects of education levels of developmental care in Australia: Perceptions and challenges. Aust. Crit. Care 2021, 34, 370–377. [Google Scholar] [CrossRef]
- Kaplan, S.L.; Coulter, C.; Sargent, B. Physical Therapy Management of Congenital Muscular Torticollis: A 2018 Evidence-Based Clinical Practice Guideline From the APTA Academy of Pediatric Physical Therapy. Pediatr. Phys. Ther. 2018, 30, 240–290. [Google Scholar] [CrossRef]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef]
- Velde, A.T.; Morgan, C.; Finch-Edmondson, M.; McNamara, L.; McNamara, M.; Paton, M.C.B.; Stanton, E.; Webb, A.; Badawi, N.; Novak, I. Neurodevelopmental Therapy for Cerebral Palsy: A Meta-analysis. Pediatrics 2022, 149, e2021055061. [Google Scholar] [CrossRef]
- van Trotsenburg, P.; Stoupa, A.; Léger, J.; Rohrer, T.; Peters, C.; Fugazzola, L.; Cassio, A.; Heinrichs, C.; Beauloye, V.; Pohlenz, J.; et al. Congenital Hypothyroidism: A 2020–2021 Consensus Guidelines Update—An ENDO-European Reference Network Initiative Endorsed by the European Society for Pediatric Endocrinology and the European Society for Endocrinology. Thyroid 2021, 31, 387–419. [Google Scholar] [CrossRef]
- Janssen, A.J.; Akkermans, R.P.; Steiner, K.; de Haes, O.A.; Oostendorp, R.A.; Kollée, L.A.; Nijhuis-van der Sanden, M.W. Unstable longitudinal motor performance in preterm infants from 6 to 24 months on the Bayley Scales of Infant Development—Second edition. Res. Dev. Disabil. 2011, 32, 1902–1909. [Google Scholar] [CrossRef]
- Romeo, D.M.; Apicella, M.; Velli, C.; Brogna, C.; Ricci, D.; Pede, E.; Sini, F.; Coratti, G.; Gallini, F.; Cota, F.; et al. Hammersmith Infant Neurological Examination in low-risk infants born very preterm: A longitudinal prospective study. Dev. Med. Child Neurol. 2022, 64, 863–870. [Google Scholar] [CrossRef]
- Romeo, D.M.; Ricci, D.; Brogna, C.; Mercuri, E. Use of the Hammersmith Infant Neurological Examination in infants with cerebral palsy: A critical review of the literature. Dev. Med. Child Neurol. 2016, 58, 240–245. [Google Scholar] [CrossRef]
- Heineman, K.R.; Hadders-Algra, M. Evaluation of Neuromotor Function in Infancy–A Systematic Review of Available Methods. J. Dev. Behav. Pediatr. 2008, 29, 315–323. [Google Scholar] [CrossRef]
- Barnett, A.L. Can the Griffiths scales predict neuromotor and perceptual-motor impairment in term infants with neonatal encephalopathy? Arch. Dis. Child 2004, 89, 637–643. [Google Scholar] [CrossRef]
- Hadders-Algra, M. The neuromotor examination of the preschool child and its prognostic significance. Ment. Retard. Dev. Disabil. Res. Rev. 2005, 11, 180–188. [Google Scholar] [CrossRef]
- Surveillance of Cerebral Palsy in Europe (SCPE). Surveillance of cerebral palsy in Europe: A collaboration of cerebral palsy surveys and registers. Dev. Med. Child Neurol. 2001, 42, 816. [Google Scholar] [CrossRef]
- Rosenbaum, P.L.; Walter, S.D.; Hanna, S.E.; Palisano, R.J.; Russell, D.J.; Raina, P.; Wood, E.; Bartlett, D.J.; Galuppi, B.E. Prognosis for Gross Motor Function in Cerebral Palsy: Creation of Motor Development Curves. JAMA 2002, 288, 1357. [Google Scholar] [CrossRef]
- Gorter, J.W.; Ketelaar, M.; Rosenbaum, P.; Helders, P.J.; Palisano, R. Use of the GMFCS in infants with CP: The need for reclassification at age 2 years or older. Dev. Med. Child Neurol. 2009, 51, 46–52. [Google Scholar] [CrossRef]
- Virella, D.; Pennington, L.; Andersen, G.L.; Andrada, M.d.G.; Greitane, A.; Himmelmann, K.; Prasauskiene, A.; Rackauskaite, G.; De La Cruz, J.; Colver, A.; et al. Classification systems of communication for use in epidemiological surveillance of children with cerebral palsy. Dev. Med. Child Neurol. 2016, 58, 285–291. [Google Scholar] [CrossRef]
- mithers-Sheedy, H.; Badawi, N.; Blair, E.; Cans, C.; Himmelmann, K.; Krägeloh-Mann, I.; McIntyre, S.; Slee, J.; Uldall, P.; Watson, L.; et al. What constitutes cerebral palsy in the twenty-first century? Dev. Med. Child Neurol. 2014, 56, 323–328. [Google Scholar] [CrossRef]
- Bosanquet, M.; Copeland, L.; Ware, R.; Boyd, R. A systematic review of tests to predict cerebral palsy in young children. Dev. Med. Child Neurol. 2013, 55, 418–426. [Google Scholar] [CrossRef]
- Long, S.H.; Galea, M.P.; Eldridge, B.J.; Harris, S.R. Performance of 2-year-old children after early surgery for congenital heart disease on the Bayley Scales of Infant and Toddler Development, Third Edition. Early Hum. Dev. 2012, 88, 603–607. [Google Scholar] [CrossRef]
- Ehrhardt, H.; Aubert, A.M.; Ådén, U.; Draper, E.S.; Gudmundsdottir, A.; Varendi, H.; Weber, T.; Zemlin, M.; Maier, R.F.; Zeitlin, J.; et al. Apgar Score and Neurodevelopmental Outcomes at Age 5 Years in Infants Born Extremely Preterm. JAMA Netw. Open 2023, 6, e2332413. [Google Scholar] [CrossRef]
- Schonhaut, L.; Pérez, M.; Armijo, I.; Maturana, A. Comparison between Ages & Stages Questionnaire and Bayley Scales, to predict cognitive delay in school age. Early Hum. Dev. 2020, 141, 104933. [Google Scholar] [CrossRef]
- Hoei-Hansen, C.E.; Weber, L.; Johansen, M.; Fabricius, R.; Hansen, J.K.; Viuff, A.-C.F.; Rønde, G.; Hahn, G.H.; Østergaard, E.; Duno, M.; et al. Cerebral Palsy—Early Diagnosis and Intervention Trial: Protocol for the prospective multicentre CP-EDIT study with focus on diagnosis, prognostic factors, and intervention. BMC Pediatr. 2023, 23, 544. [Google Scholar] [CrossRef]
- Pascal, A.; Govaert, P.; Oostra, A.; Naulaers, G.; Ortibus, E.; Broeck, C.V.D. Neurodevelopmental outcome in very preterm and very-low-birthweight infants born over the past decade: A meta-analytic review. Dev. Med. Child Neurol. 2018, 60, 342–355. [Google Scholar] [CrossRef]
- Pascal, A.; Naulaers, G.; Ortibus, E.; Oostra, A.; De Coen, K.; Michel, S.; Cloet, E.; Casaer, A.; D’Haese, J.; Laroche, S.; et al. Neurodevelopmental outcomes of very preterm and very-low-birthweight infants in a population-based clinical cohort with a definite perinatal treatment policy. Eur. J. Paediatr. Neurol. 2020, 28, 133–141. [Google Scholar] [CrossRef]
- Kallioinen, M.; Eadon, H.; Murphy, M.S.; Baird, G. Developmental follow-up of children and young people born preterm: Summary of NICE guidance. BMJ 2017, 358, j351. [Google Scholar] [CrossRef]
- Ketelaar, M.; Vermeer, A.; Hart, H.; Van Petegem-van Beek, E.; Helders, P.J. Effects of a Functional Therapy Program on Motor Abilities of Children With Cerebral Palsy. Phys. Ther. 2001, 81, 1534–1545. [Google Scholar] [CrossRef]
- Bottos, M.; Feliciangeli, A.; Sciuto, L.; Gericke, C.; Vianello, A. Functional status of adults with cerebral palsy and implications for treatment of children. Dev. Med. Child Neurol. 2001, 43, 516. [Google Scholar] [CrossRef]
- Novak, I.; Morgan, C.; Adde, L.; Blackman, J.; Boyd, R.N.; Brunstrom-Hernandez, J.; Cioni, G.; Damiano, D.; Darrah, J.; Eliasson, A.-C.; et al. Early, Accurate Diagnosis and Early Intervention in Cerebral Palsy: Advances in Diagnosis and Treatment. JAMA Pediatr. 2017, 171, 897. [Google Scholar] [CrossRef]
- Hadders-Algra, M. The developing brain: Challenges and opportunities to promote school readiness in young children at risk of neurodevelopmental disorders in low- and middle-income countries. Front. Pediatr. 2022, 10, 989518. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).