Abstract
Meningiomas represent approximately 40% of all primary tumors of the central nervous system (CNS) and, based on the latest World Health Organization (WHO) guidelines, are classified into three grades and fifteen subtypes. The optimal treatment comprises gross total tumor resection. The WHO grade and the extent of tumor resection assessed by the Simpson grading system are the most important predictors of recurrence. Atypical meningiomas, a grade 2 meningioma, which represent almost a fifth of all meningiomas, have a recurrence rate of around 50%. Currently, different histopathologic, cytogenetic, and molecular genetic alterations have been associated with different meningioma phenotypes; however, the data are insufficient to enable the development of specific treatment plans. The optimal treatment, in terms of adjuvant radiotherapy and postoperative systemic therapy in atypical meningiomas, remains controversial, with inconclusive evidence in the literature and existing studies. We review the recent literature to identify studies investigating relevant atypical meningioma biomarkers and their clinical application and effects on treatment options.
1. Introduction
Meningiomas account for around 40% of all primary tumors of the central nervous system (CNS) in adults, with 4.2 cases per 100,000 surgically relevant cases per year [1,2,3,4]. Based on their cytological features, meningiomas were thought to be derived from arachnoid cap cells; however, the exact origin remains unknown, with a possible origin being the arachnoid barrier cells, since they share the expression of prostaglandin D synthase with meningiomas [5,6,7]. The fifth edition of the World Health Organization (WHO) classification of CNS tumors classifies meningiomas into three grades and fifteen subtypes [8,9,10]. The extent of surgical resection, evaluated by Simpson grading of resection degree [11], and the CNS WHO grade are the most important predictors of the recurrence risk of meningiomas, with approximately 80% being CNS WHO grade 1, which have a favorable prognosis [12]. The advances in molecular profiling have emphasized the role of genetic and epigenetic changes in characterizing meningiomas and their aggressiveness and recurrence risk [13]. Currently, there are no clinically validated liquid biopsy prognostic or diagnostic biomarkers specific for meningiomas, which would enable a non-invasive diagnosis of meningiomas and their subtypes [14].
In CNS WHO grade 2 meningiomas, the recurrence rate is approximately 50%; thus, the European Association of Neuro-oncology (EANO) guidelines recommend radiotherapy or observation of patients with CNS WHO grade 2 that have undergone gross total tumor resection [15,16,17,18]. The 5-year progression-free survival (PFS) and overall survival rates for atypical meningiomas are 48–68% and 78–91%, respectively [19,20].
CNS WHO grade 2 meningiomas represent approximately 18.3% of all meningiomas [1]. According to the latest WHO classification of CNS tumors from 2021, grade 2 meningiomas can be further divided into three subtypes, chordoid, clear cell, and atypical, with the latter being the most frequent [10]. Although the female sex is recognized as a risk factor for meningiomas, higher-grade meningiomas are more common among men [1,21,22]. Patients with grade 2 meningiomas are often younger than those with grade 1 [21]. The biological characteristics of atypical meningiomas are between benign and anaplastic meningiomas [23].
Atypical meningiomas are diagnosed based on the following criteria: a mitotic index ranging between 4 and 19 mitoses per 10 fields of 0.16 mm2 and/or brain invasion and/or at least three minor criteria, which are spontaneous necrosis, pattern-less architecture (sheeting), small cells with a high nuclear/cytoplasmic ratio, macronucleoli, and hypercellularity [8,10]. The diagnostic criteria for atypical meningiomas are presented the Table 1. Atypical meningiomas have high heterogeneity and invasiveness [23]. Magnetic resonance imaging (MRI) characteristics of atypical meningiomas are an uneven signal, irregular borders with no clear boundary with the surrounding brain tissue, large areas of oedema, a generally large neoplasm volume, or a significant increase in size over a short time [24,25]. For grade 3 meningiomas, which account for 1 to 5% of all meningiomas and are more prevalent among men, the diagnostic criteria comprise a mitotic index of 20 or more mitoses per 10 fields of 0.16 mm2, frank anaplasia (sarcoma, carcinoma, or melanoma-like appearance), or papillary/rhabdoid histology. Histologic subtypes of grade 3 meningiomas are anaplastic, papillary, and rhabdoid meningiomas. TERT promoter mutations or homozygous deletion of cyclin-dependent kinase inhibitors A and B (CDKN2A/B) are molecular features of grade 3 meningiomas [10,26].

Table 1.
Diagnostic criteria for atypical (WHO grade 2) meningioma: fulfilling either 1 of 2 major criteria or 3 of 5 minor criteria.
In cases of atypical meningiomas, the tumor has to be removed as completely as possible, including the dura and the base of the tumor as well as the invaded skull [27]. Grade 2 meningiomas are most often found within the convexity, whereas those in the skull base, however rare, are often smaller, less aggressive and, in the majority of cases, located in the sphenoid locations [28,29,30]. Gross total resection is linked with a longer overall survival, and postoperative radiotherapy is offered to patients with subtotal resection of atypical meningiomas [20,31]. The role of adjuvant radiotherapy in atypical meningiomas after gross total resection (GTR) remains controversial, with some studies demonstrating durable local control without adjuvant radiotherapy, while others advocate radiotherapy in cases of atypical meningiomas, even with GTR [32,33,34].
2. Histopathological Features
Brain invasion, characterized by the infiltration of tumor cells into the brain parenchyma without intervening leptomeninges, is, according to the fifth WHO classification of CNS tumors, enough to classify a meningioma as atypical [10]; however, studies have shown that atypical meningiomas diagnosed on the sole presence of brain invasion and lacking other criteria have a recurrence rate similar to that of CNS WHO grade 1 meningiomas [35,36,37]. In cases of tumor reappearance, other features associated with a higher risk of recurrence, such as a high Ki-67 labeling index, incomplete surgical removal, or occurrence in the context of neurofibromatosis type 2 (NF2), were present, suggesting that the relapse was independent of brain invasion [36]. Brain invasion in otherwise benign meningiomas is associated with a lower probability of recurrence compared to the high mitotic activity present in an atypical meningioma [8]. A meta-analysis of 25 studies including 3560 atypical meningiomas found no difference in the progression risk between tumors with a mitotic index of four or more mitoses per ten high-power fields (HPFs) or below that cut-off [38]. In comparison, a study with 200 atypical meningiomas discovered that a cut-off of six mitoses per ten HPFs was connected to recurrent cases [15]. Other studies have suggested that eight mitoses per ten HPFs are associated with progression in cases of atypical meningiomas [8,39]. Some propose the Ki-67 index as an alternative to the mitotic index to evaluate the proliferation of meningiomas [8]. In a cohort of 99 atypical meningiomas, a Ki-67 index above 7.5% was recognized as a significant predictor of shorter relapse-free survival (RFS) [38]. As the Ki-67 index can be affected by inter-observer variability, no universally accepted threshold has been established to distinguish between high and low proliferation to recognize atypical meningiomas with a greater risk of recurrence [8]. A high Ki-67 index is associated with increased neoplasm malignancy and an average index of 3.8% is mostly found in benign meningiomas and 7.2% in atypical meningiomas; thus, high Ki-67 expression is linked with relapse after surgery (Table 2) [40].

Table 2.
Factors for more likely recurrence of atypical meningiomas after surgery.
Atypical meningiomas, diagnosed based on minor atypical criteria, have a significantly lower risk of recurrence [15,41]. In cases of atypical meningiomas with major and minor criteria, in some studies, sheeting, macronucleoli, and spontaneous necrosis were recognized as indicators of recurrence [15,42], whereas an analysis of 262 atypical meningiomas and a meta-analysis of 25 studies found that spontaneous necrosis was the only significant prognostic factor among minor criteria for progression [38].
Immunocytochemistry staining enables the distinction of atypical meningiomas from gliomas, neurogenic tumors, mesenchymal tumors, and some metastatic tumors with epithelial membrane antigen (EMA), with vimentin being the preferred diagnostic marker for meningiomas [43,44]. Most meningiomas express EMA with a decreased expression index in high-risk meningiomas (WHO grade 2 and 3) and vimentin is also present in the majority of cases of meningiomas, especially in high-risk ones (grade 2 and 3) [23]. Somatostatin receptors (SSTRs), present in meningiomas, are involved in tumorigenesis, with SSTR2A being an independent prognostic factor regarding meningioma recurrence and with higher SSTR2A expression being linked to more aggressive meningiomas [45,46,47]. Furthermore, the expression of progesterone receptors in meningiomas is also linked to recurrence, with higher rates of progesterone receptors being associated with a better prognosis and a limited chance of recurrence or malignant transformation [48]. Additionally, high p53 expression and an elevated Ki-67 index are also features of more aggressive meningiomas [49].
3. Cytogenetic Features
3.1. Copy Number Alterations
The progressive accumulation of chromosomal losses and gains leads to meningioma progression, and monosomy of chromosome 22q is believed to be an early event in the pathogenesis of meningiomas in both NF2-mutated and non-NF2-mutated meningiomas [50,51,52,53,54,55]. 22q loss is present in many patients with an established NF2 mutation, and the somatic mutation is present in approximately 47% of sporadic meningiomas [56]. In WHO grade 2 meningiomas, additional chromosomal copy number aberrations (CNAs), such as a loss of 1p, 14q, 18q, 10q, and 6q and a gain of 20q, 12q, 15q, 1q, 9q, and 17q, are present (Table 2) [50,57]. A loss of chromosomal locations 1p and 14q has been linked to high-grade meningiomas, and 1p loss is recognized as the second most common chromosomal event after 22q loss and is associated with higher rates of tumor recurrence and progression [58,59,60].
In grade 1 meningiomas, which later progressed, cytogenetic abnormalities associated with grades 2 and 3 were present, and thus, CNAs could be used for the identification of low-grade meningiomas with a higher risk of recurrence and progression [59,61]. A study where the whole-genome CNAs were analyzed in patients with atypical meningiomas discovered that 3.5 CNAs were linked to recurrence [62]. Specific chromosomal CNAs in atypical meningiomas could be used as prognostic markers of progression and recurrence [8]. 10q loss, found using next-generation sequencing (NGS), was recognized as an indicator of a more aggressive meningioma [63]. Additionally, 1p loss was the most significant predictor of RFS [64]. A greater number of chromosomal anomalies are associated with a higher-grade meningioma [65]. Changes in genes HIST1H1C and CTGF, located on chromosome 6, are linked with meningioma recurrence and a loss of heterozygosity at certain sites of chromosome 10, and can be indicators of a worse prognosis in cases of meningiomas [66].
3.2. Molecular Models for Risk Assessment
Based on this knowledge, a molecular model for risk assessment was proposed [8]. Meningiomas, bare psammomatous, angiomatous, secretory, and clear cell, should be assessed for 1p deletion, and cases lacking this CNA are to be considered low risk of progression; however, when 1p deletion is present, analysis for 6q and 14q losses has to be conducted, and with two losses, a meningioma is considered high risk [64]. This risk stratification model was successful in categorizing the recurrence risk in cases of atypical meningiomas [67]. In cases of atypical meningiomas, an association was discovered between 1p and 10q loss and shorter RFS, with 7p and 18 losses also being recognized as negative prognostic factors [68]. It has therefore been suggested that cases of atypical meningiomas without 1p deletion can be managed conservatively [64,68]. A heterozygous deletion of cyclin-dependent kinase inhibitors A and B (CDKN2A/B) was also associated with a shorter time of meningioma recurrence, and it importantly correlates with shorter RFS in surgically resected atypical meningiomas [41,69].
3.3. Cytogenetic Features in Post-Radiation Meningiomas
Meningiomas represent the most common neoplasm type to appear after radiotherapy, with a tendency to be more aggressive than their sporadic counterparts, and a loss of 1p was found to play an important role in the development of radiation-induced meningiomas, followed by changes in chromosomal locations 9p, 19q, and 22q [70,71,72,73]. Ionizing radiation is the sole environmental risk factor linked with the development of meningiomas and there seems to be a genetic susceptibility to radiation-induced meningiomas [74].
4. Molecular Genetics and Classification of Meningiomas
The latest CNS WHO classification has not changed the criteria for diagnosing atypical meningiomas; however, for the first time, specific genetic alterations have been included in the grading, such as TERT promoter (pTERT) mutations and/or CDKN2A/B homozygous deletions (HDs), which are associated with meningioma recurrence and progression [12,69,75,76,77,78,79]. A meta-analysis of 677 patients with WHO grade 2 meningiomas found pTERT mutations in 7.9% and another analysis of 63 atypical meningiomas found pTERT mutations in only 1.6% [68]. So far, pTERT mutations have often been present in secondary atypical meningiomas, suggesting that primary and secondary atypical meningiomas have different molecular pathways [80]. Similarly, a study of 183 atypical meningiomas identified CDKN2A/B HD in a mere 4% of WHO grade 2 meningiomas [76].
Mutations in the genes AKT1, SMARCB1, and SMO (Table 2) are rare in cases of atypical meningiomas, and mutations in the genes KLF4 and TRAF7 are associated with an indolent clinical behavior, thus making these meningiomas have a low risk of progression [54,68,80,81,82]. SMO, a G-coupled receptor, is important in the Hedgehog signaling pathway, which is crucial in promoting angiogenesis and tumor progression, and is linked with larger meningiomas and a higher recurrence rate than that in AKT1-mutated meningiomas [83,84,85]. Mutations in PIK3CA (Table 2), which encodes for a catalytic subunit of phosphatidylinositol 3-kinase (PI3K), can be found in cases of atypical meningiomas and often co-occur with TRAF7 mutations, especially in cases of skull base meningiomas [86,87]. Changes in SUFU also lead to the dysregulation of the Hedgehog signaling pathway, which is important for meningioma growth and development, and they often co-occur with PTEN and ARID1A mutations (Table 2) [52,88].
4.1. The Role of NF2 Gene Mutations in Meningiomas
NF2 gene mutation is associated with the early development of meningiomas [89,90,91] and is recognized as the most common gene mutation in cases of atypical meningiomas (Table 2), present in up to 75% of tumors, making a meningioma 3.78 times more likely to be atypical than without an NF2 gene alteration [68,80,92]. NF2 loss-of-function mutation is caused by a double-hit mechanism in meningiomas, either by a germline mutation and a second hit with a somatic mutation in syndromic cases or with a somatic single-nucleotide variation, or by an insertion/deletion mutation and an overlapping chromosome 22 deletion, which is commonly seen in sporadic cases [93]. Neurofibromatosis type 2, now termed NF2-related schwannomatosis, caused by a mutation in the NF2 gene, results in the loss of function of Merlin, a tumor suppressor protein, which leads to the overexpression of YAP1 (yes-associated protein 1) [94,95,96]. The transcriptional co-activator YAP1 has an important role in tissue development and homeostasis, and the activation of YAP1 is linked with the loss of function of a tumor suppressor NF2/Merlin, which enables tumor growth, invasion, and resistance to apoptosis [97,98,99,100,101,102,103]. YAP1 fusion meningiomas resemble low-grade NF2 mutant meningiomas; based on their upregulated genes and their downregulated genes, they resemble high-grade NF2 mutant meningiomas [94,100]. Hyperactive YAP1, a consequence of the YAP1 fusion protein binding to the TEA domain, leads to tumor cell proliferation and invasion, playing an oncogenic role in meningioma tumorigenesis [98,104,105].
4.2. Epigenetic Modifications
The presence of immunohistochemical loss of histone H3 trimethylation at lysine 27 (H3K27me3), a DNA epigenetic modification, could be used as an indicator of an increased risk of tumor recurrence and negative prognosis, and it is associated with grade 2 meningiomas with shorter disease-free survival (Table 2) [8,106,107]. Additionally, cases of atypical meningiomas after stereotactic surgery can imply that these tumors are more resilient to radiation therapy [108]. In a recent study of paired samples of primary meningiomas and relapsed cases, H3K27me3 loss was present in 35% of relapses and 25% of cases following radiotherapy, thus linking the epigenetic modification to adjuvant treatment and tumor reoccurrence [108,109]. The role of H3K27me3 immunohistochemical loss needs to be further investigated, and in some studies, the results of staining are ambiguous and difficult to interpret in a clinical setting [109]. The Table 2 lists the most important factors for the recurrence of atypical meningiomas.
Patel et al. identified three classes of meningiomas (type A, B, and C), based on bulk RNA sequencing and whole-exome sequencing, with type C having the highest proliferative index, the shortest RFS, and an increased expression of FOXM1, leading to a loss of the repressive DREAM complex [81]. Vasudevan et al. also divided meningiomas into two types, with increased expression of FOXM1 being an indicator of an aggressive meningioma, and in primary atypical meningiomas, FOXM1 was often upregulated [80,110].
4.3. Genomic Analysis-Based Meningioma Division
Aggressive meningiomas can be divided into three groups based on genomic analysis of their NF2 status: NF2 mutant, NF2 agnostic, and NF2 wild-type. NF2 mutant are mostly associated with men and mutations in CDKN2A/B, whereas NF2 agnostic are linked with TERT and TP53 mutations [52].
Genome-wide DNA methylation profiling on 497 meningiomas performed by Sahm et al. further divided meningiomas into six methylation patterns for the prediction of progression and recurrence [111]. MC ben-1 to 3 had a benign course, MC int-A and B had an intermediate behavior, and MC mal was associated with an aggressive course. Atypical meningiomas were generally classified as MC int-A and MC int-B; however, some were also classified as MC ben-1 (96,97). Nassiri et al. used copy number variation analysis, somatic point mutations, methylation profiles, and messenger RNA abundance of 121 meningiomas, creating four molecular subtypes, immunogenic, benign NF2 wild-type, hypermetabolic, and proliferative, allowing them to better predict the outcomes compared to the 2016 WHO classification of CNS tumors [112]. According to the methylation analysis conducted by Olar et al., two groups of meningiomas were distinguished with different prognoses; a higher proportion of copy number aberrations was present in the unfavorable prognosis group, including losses of 1p and 14q [113].
4.4. Integrated Molecular–Morphological Grading System of Meningiomas
Combining the DNA methylation patterns, chromosomal CNAs, and WHO grade, Maas et al. created an integrated molecular–morphological grading system of meningiomas, where each meningioma was scored based on their WHO grade (0–2 points), methylation class (0–4 points), and chromosomal loss of 1p, 14q, and 6q (0–3 points). Meningiomas scoring 0–2 points were classified as low risk, with 3–5 points as intermediate risk and those with 6–9 points as high risk [64]. By using the integrated molecular–morphological grading system, researchers did not need to evaluate pTERT and/or CDKN2A/B HD to estimate the recurrence risk [64,114].
Driver et al. also developed a grading system comprising mitotic counts, CDKN2A/B deletion, and chromosomal CNAs, where each CNA among 1p, 3p, 4p/q, 6p/q, 10p/q, 14q, 18p/q, and 19p/q deletions and CDKN2A/B deletions gained one point; one point was given for a mitotic index of 4 to 19 mitoses/1.6 mm2 and two points for a mitotic index of 20 or more mitoses/1.6 mm2. Meningiomas with 0–2 points were classified as grade 1, 2–3 points as grade 2, and 4 or more points as grade 3. By using the grading system, they were able to more accurately predict the risk of recurrence compared to the 2007 and 2016 WHO grading system [64,115].
Choudhury et al., in 2022, conducted DNA methylation profiling on 565 meningiomas, dividing them into three groups. The first one was merlin-intact meningiomas, characterized by a gain of function in chromosome 5, a loss of function in chromosome 6p, and intact NF2 expression, which had the best survival among the three groups. Second was an immune-enriched meningioma, characterized by a gain of function in 6p and a loss in 22q, with lymphocytes exhibiting exhaustion markers. The last group was hypermitotic meningiomas, with the worst overall survival, characterized by an upregulation of FOXM1 expression [116,117].
5. Management
Initial treatment of atypical meningiomas comprises surgery with a maximal safe resection [118,119]. Simpson grades 1 to 3 are considered GTS and grades 4 to 5 a subtotal resection, with grade 0 representing complete tumor removal, with the removal at 2 to 3 cm from the tumor insertion site with good results [120,121]. The surgical approach has to be wide enough to allow for meningioma exposure with its dural attachments whilst enabling the surgeon to visualize the surrounding structures to disrupt the blood flow and minimize brain retraction and manipulation [122]. Small and asymptomatic meningiomas, believed to be benign, can be monitored [123]. Around a fifth of meningiomas display aggressive tendencies, and radiation therapy can be utilized as well as repeated surgery [119]. Following gross total resection of an atypical meningioma, the 5-year recurrence rate is 29–58%, with the 5-year survival rate being 79–91% and the 10-year survival rate being 53% [124,125,126,127].
Surgical resection is linked with improved neurological function; however, GTR is not always attainable, depending on the meningioma’s location and the incorporation of nerves and blood vessels [43,128,129]. Radiation therapy is most often indicated as an adjuvant therapy when subtotal resection of a WHO grade 2 meningioma is performed, and it has to be personalized, based on the neoplasm’s size, proximity to critical structures, and prior radiation to the same site, with the goal being a reduction in tumor proliferation and control over its progress [16,130,131].
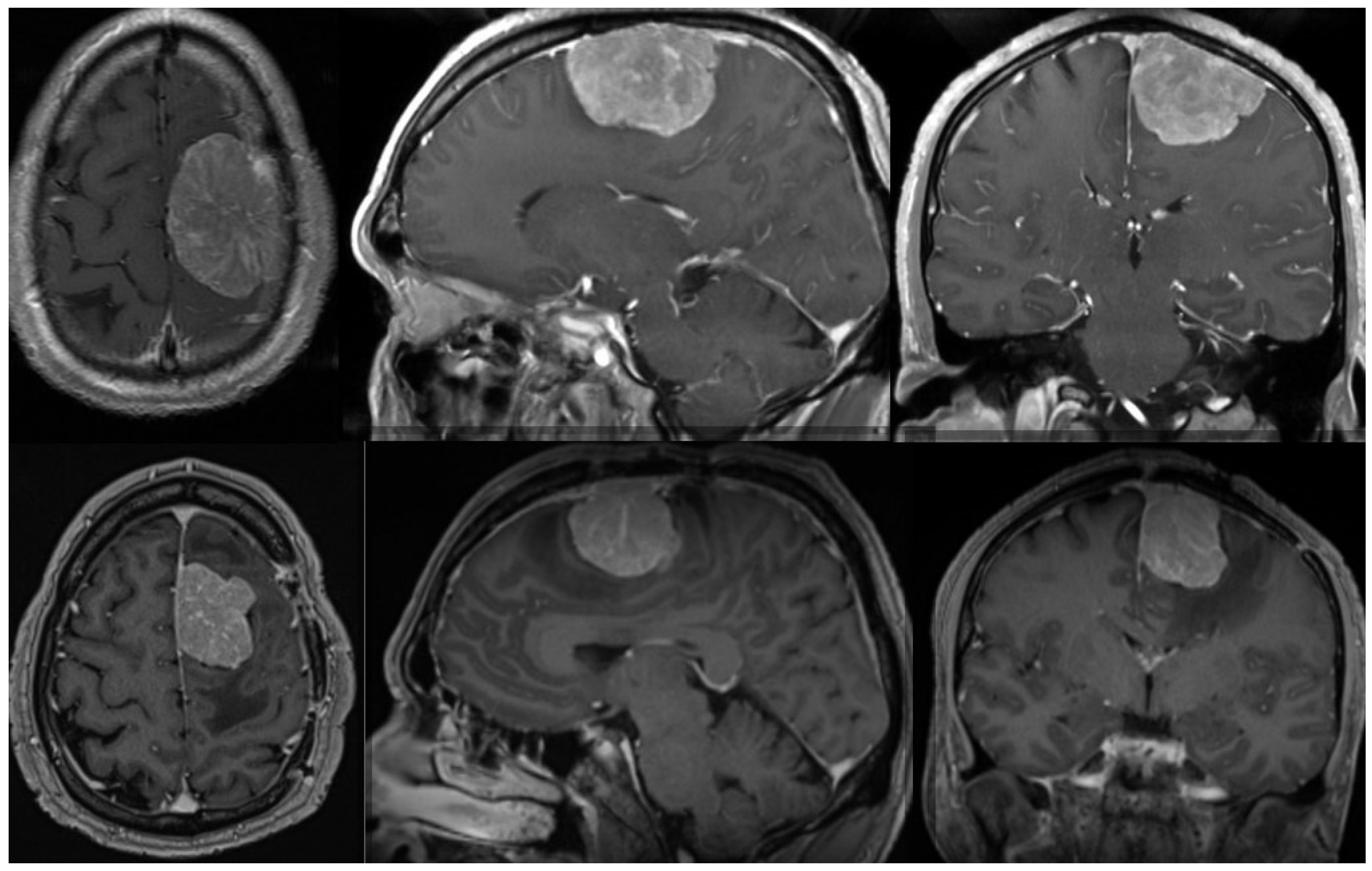
Radiation therapy can be delivered as conventional fractioned photon radiotherapy, stereotactic radiotherapy, stereotactic radiosurgery or fractional stereotactic radiation therapy, intensity-modulated photon radiation therapy, and particle therapy with photons or carbon ions [119,123,132]. External beam radiation therapy is recommended for grade 3 meningiomas, whereas small grade 2 meningiomas can be treated with stereotactic radiosurgery, especially when their diameter is less than 3 cm and they are more than 3 mm from radiosensitive tissues [125,133,134]. In cases of atypical meningiomas with residual tissue, stereotactic radiosurgery is generally used with doses ranging from 12 to 20 Gy [131,135,136]; however, a study conducted by Kano et al. discovered that patients who received less than 20 Gy had a PFS of 29% at 5 years, whereas those who received 20 Gy had a PFS of 63% [135]. In a phase II trial, intensity-modulated photon radiotherapy with doses ranging from 54 to 60 Gy in 30 fractions was administered to patients with incompletely resected atypical meningiomas, which demonstrated that the aforementioned treatment modality was safe and effective and required further research [137]. The administration of particle radiation therapy with either protons or carbon ions enables higher radiation doses, which could improve local tumor control; however, larger prospective studies are still required [138]. Figure 1 shows one example of a patient with an atypical meningioma treated surgically and with radiotherapy.
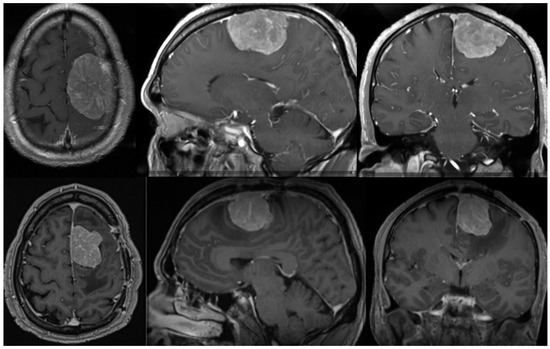
Figure 1.
Preoperative (upper row) MR of the brain (axial, sagittal, and coronal view after contrast enhancement) showing a 51-year-old male with an atypical meningioma. The patient was operated on and the tumor was removed (Simpson grade 2 resection). The tumor exhibited brain invasiveness and 7 mitotic figures/10 high-power fields. The wall of the superior sagittal sinus, where the tumor was attached, was only coagulated and the sinus was preserved. The patient had no tumor recurrence 3 months after surgery and an 11 mm large recurrence was present after 9 months. (Lower row) The lower row shows the substantial recurrence of the tumor after 15 months. The patient was successfully reoperated on (Simpson grade 2 resection) and received adjuvant radiotherapy on the tumor bed. He had no obvious recurrence three months after the second surgery. The tumor was histologically identical after the second surgery. Genetic analysis was performed after both surgeries, revealing a mutation in the NF2 gene in both cases. No other chromosomal or important genetic abnormalities were detected.
The role of radiation in the treatment of patients with atypical meningiomas remains controversial and is often based on physician preference since the literature on this topic is sparse, with the majority of conducted studies being small and retrospective with low power and often inconsistent results [126,139]. Arguments in favor of adjuvant radiation are the reduction in the recurrence risk, the increased time to recurrence, a smaller tumor burden in cases of recurrence, and an improvement in disease-specific survival [130,136,140]. On the other hand, the arguments against adjuvant radiotherapy are that it does not reduce the recurrence risk and exposes patients to unnecessary radiation with potential harm [131,141]. Some studies on the effects of adjuvant radiotherapy demonstrated lower recurrence rates, but the results were not statistically significant [126,140], whereas other studies showed no difference in recurrence rates to those of active monitoring [121,131,142]. Hasan et al. conducted a meta-analysis of 14 retrospective studies comparing patients with atypical meningiomas undergoing GTR alone with those that received GTR and adjuvant radiotherapy and discovered cases of recurrence that occurred 8 months later in the group treated with adjuvant radiotherapy [130]. Graffeo et al. also conducted a meta-analysis of seven studies including their data, comparing recurrence rates between patients with atypical meningiomas treated with GTR alone and those that after GTR received radiotherapy, and discovered a trend of lower 5-year recurrence rates and improved overall survival in patients in the radiation group; however, the differences were not statistically significant [126].
A phase II trial (RTOG 0539) was a prospective study comparing the outcomes of recurrence in atypical meningiomas after GTR with adjuvant radiotherapy using a dose of 54 Gy. In the study, the 3-year PFS was 93.8%, which was significantly higher than in historical controls, and the recurrence rate at 3 years was 4.1%, with a 96% overall survival rate [143]. Another prospective phase II trial (EORTC 22042–26042) compared the results after GTR of atypical meningiomas alone and those treated with radiotherapy using a high dose of 60 Gy, and after 3 years, the PFS was 90% and the overall survival rate was 96.4% [144].
The optimal radiation dose is illusive, with doses ranging from 50 to 60 Gy administered in 1.8–2.0 Gy fractions to the tumor bed and residual tumor tissue with a margin ranging from 0.5 to 2.0 cm [123,130]. Atypical meningiomas are mostly treated with 54 Gy; however, doses of up to 70 Gy have been used and several retrospective studies showed that higher doses may improve patients’ outcomes [145].
Systemic therapy has limited effects on meningiomas and should be considered when all surgical and radiotherapy options have been explored, since several studies conducted so far have shown that chemotherapeutic agents, namely temozolomide, doxorubicin, and vincristine, were not effective against meningiomas [123,146,147,148]. Hydroxyurea can be used in patients with atypical meningiomas when postoperative radiotherapy cannot be applied as there were no differences in the PFS period between hydroxyurea chemotherapy and radiotherapy after surgery [149]. It was discovered that hydroxyurea was linked with longer PFS of patients with atypical meningiomas after stereotactic radiotherapy than with only conservative treatment, and even following GTR, hydroxyurea led to stabilization or even shrinking of the atypical meningioma [149,150].
Interferon-alfa, an immunomodulating agent, can be administered in cases of recurrent meningiomas where repeat surgery is not an option, since several studies have demonstrated its effects of stabilization of tumor growth, with a slight improvement in PFS at 12 weeks, but with no effect on the overall survival [123,151].
Meningiomas often overexpress growth factor receptors, such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and epidermal growth factor (EGF); therefore, several studies using monoclonal antibodies or small-molecule kinase inhibitors have been conducted with limited or no success [147,152,153,154]. Anti-VEGF molecules and mTOR inhibitors have shown potential in the treatment of high-grade and recurrent meningiomas where surgical and/or radiotherapy have been less effective [119]. VEGF plays an important role in the development of the peritumoral oedema in meningiomas, and some authors have found a connection between high levels of VEGF and recurrence [155], whereas others linked VEGF to histological grade [156]. However, some did not find a correlation between the two factors, and it currently stands that VEGF should not be used as a marker of histological grade [157,158,159]. Oedema, caused by VEGF, is related to increased morbidity, and thus, anti-VEGF therapy, such as bevacizumab, has been trialed; however, the results so far have been disappointing [152,154,160,161,162]. Sunitinib, a small-molecule kinase with different targets, has been tested on atypical meningiomas, and PFS after 6 months improved from 5–30% to 42% [152]. The mTOR protein complex, a part of the PI3K complex, is crucial for meningioma development, and the mTOR inhibitor everolimus has demonstrated an increase in PFS and a decrease in tumor growth rate [163,164]. Meningiomas often express progesterone receptors; however, high-grade meningiomas tend to express estrogen receptors, but currently, anti-estrogenic agents have not demonstrated a strong effect [119,165]. Aggressive meningiomas with high relapse rates exhibit overexpression of somatostatin receptors, but inhibitors have shown little effect so far [166]. In the future, microRNAs could also be included in meningioma treatment, since a high expression of miR-190a and a low expression of miR-29c-3p and miR-219–5p have been linked with higher recurrence rates, and miR-21 has been upregulated in cases of grade 2 and 3 meningiomas [167].
6. Clinical Application of Cytogenetic Features of Atypical Meningiomas
Deng et al. created recommendations for the molecular diagnosis and treatment of meningiomas with the help of the Group of Neuro-Oncology, the Society of Neurosurgery, the Chinese Medical Association, neuropathologists, and evidence-based experts, based on identifying and analyzing relevant studies already available [168]. Based on the literature, they recommended radiation therapy in cases of meningiomas with pTERT mutations, which are associated with radiation sensitivity in de novo high-grade meningiomas. In all other cytogenetic features, the role of radiation therapy was unknown. Additionally, the researchers recommend that the combination therapy of everolimus with bevacizumab or octreotide could be considered for PI3K-activated meningiomas, and that pharmacotherapy using bevacizumab targeting VEGF could only be considered if no further local treatment option exists. Sunitinib can be used in cases of 22q and 1p deletion [119,168]. The authors suggest that meningiomas with 22q deletion, 18q deletion, NF2 mutation, and MO or SUFU mutation should be followed annually for 5 years, cases with 1p deletion, 14q deletion, AKT1 or PIK3CA mutation, H3K27me3-negative meningiomas, and TIMP3 or TP73p methylation-positive meningiomas should be followed every 6 months for 5 years, meningiomas with pTERT mutations should be followed every 3 to 6 months indefinitely, and meningiomas with CDKN2A/B HDs should be followed every 6 months indefinitely [168].
7. Conclusions
Despite considerable progress in understanding molecular and genetic alterations in meningiomas, their clinical application in the treatment of atypical meningiomas is still low. The diagnostic criteria for those tumors are becoming more refined, but they have little impact on current clinical management strategies. Surgery remains the most efficient treatment option. There are currently no specific guidelines for when adjuvant radiotherapy should be introduced. Future prospective studies are expected to produce more relevant information about the clinical usefulness of molecular and genetic diagnostics in atypical meningiomas.
Author Contributions
Conceptualization, J.R. and H.R.; resources, J.R. and H.R.; writing—original draft preparation, J.R. and H.R.; writing—review and editing, J.R. and H.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro-Oncol. 2022, 24 (Suppl. S5), v1–v95. [Google Scholar]
- Zouaoui, S.; Darlix, A.; Rigau, V.; Mathieu-Daudé, H.; Bauchet, F.; Bessaoud, F.; Fabbro-Peray, P.; Trétarre, B.; Figarella-Branger, D.; Taillandier, L.; et al. Descriptive epidemiology of 13,038 newly diagnosed and histologically confirmed meningiomas in France: 2006–2010. Neurochirurgie 2018, 64, 15–21. [Google Scholar] [CrossRef]
- James, Z.; Makwana, M.; Hayhurst, C. De Novo Skull Base Atypical Meningioma: Incidence and Outcome. J. Neurol. Surg. Part B Skull Base. 2023, 84, 113–118. [Google Scholar] [CrossRef]
- Nobee, A.; Xu, M.; Seth, A.; Rong, Y. Atypical Intraparenchymal Meningioma with YAP1-MAML2 Fusion in a Young Adult Male: A Case Report and Mini Literature Review. Int. J. Mol. Sci. 2023, 24, 12814. [Google Scholar] [CrossRef]
- Shibuya, M. Pathology and Molecular Genetics of Meningioma: Recent Advances. Neurol. Med. Chir. 2015, 55, 14–27. [Google Scholar] [CrossRef]
- Yamashima, T.; Sakuda, K.; Tohma, Y.; Yamashita, J.; Oda, H.; Irikura, D.; Eguchi, N.; Beuckmann, C.T.; Kanaoka, Y.; Urade, Y.; et al. Prostaglandin D Synthase (β-Trace) in Human Arachnoid and Meningioma Cells: Roles as a Cell Marker or in Cerebrospinal Fluid Absorption, Tumorigenesis, and Calcification Process. J. Neurosci. 1997, 17, 2376–2382. [Google Scholar] [CrossRef]
- Yasuda, K.; Cline, C.; Vogel, P.; Onciu, M.; Fatima, S.; Sorrentino, B.P.; Thirumaran, R.K.; Ekins, S.; Urade, Y.; Fujimori, K.; et al. Drug Transporters on Arachnoid Barrier Cells Contribute to the Blood–Cerebrospinal Fluid Barrier. Drug Metab. Dispos. 2013, 41, 923–931. [Google Scholar] [CrossRef]
- Marastoni, E.; Barresi, V. Atypical meningioma: Histopathological, genetic, and epigenetic features to predict recurrence risk. Histol. Histopathol. 2024, 39, 293–302. [Google Scholar]
- Marastoni, E.; Barresi, V. Meningioma Grading beyond Histopathology: Relevance of Epigenetic and Genetic Features to Predict Clinical Outcome. Cancers 2023, 15, 2945. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D. The recurrence of intracranial meningiomas after surgical treatment. J. Neurol. Neurosurg. Psychiatry 1957, 20, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bent, M.J. Interobserver variation of the histopathological diagnosis in clinical trials on glioma: A clinician’s perspective. Acta Neuropathol. 2010, 120, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Korte, B.; Mathios, D. Innovation in Non-Invasive Diagnosis and Disease Monitoring for Meningiomas. Int. J. Mol. Sci. 2024, 25, 4195. [Google Scholar] [CrossRef] [PubMed]
- Fioravanzo, A.; Caffo, M.; Di Bonaventura, R.; Gardiman, M.P.; Ghimenton, C.; Ius, T.; Maffeis, V.; Marti-ni, M.; Nicolato, A.; Pallini, R.; et al. A Risk Score Based on 5 Clinico-Pathological Variables Predicts Recurrence of Atypical Meningiomas. J. Neuropathol. Exp. Neurol. 2020, 79, 500–507. [Google Scholar] [CrossRef]
- Goldbrunner, R.; Stavrinou, P.; Jenkinson, M.D.; Sahm, F.; Mawrin, C.; Weber, D.C.; Preusser, M.; Minniti, G.; Lund-Johansen, M.; Lefranc, F.; et al. EANO guideline on the diagnosis and management of meningiomas. Neuro-Oncology 2021, 23, 1821–1834. [Google Scholar] [CrossRef]
- Corona, A.M.; Di, L.; Shah, A.H.; Crespo, R.; Eichberg, D.G.; Lu, V.M.; Luther, E.M.; Komotar, R.J.; Ivan, M.E. Current experimental therapies for atypical and malignant meningiomas. J. Neuro-Oncol. 2021, 153, 203–210. [Google Scholar] [CrossRef]
- Marosi, C.; Hassler, M.; Roessler, K.; Reni, M.; Sant, M.; Mazza, E.; Vecht, C. Meningioma. Crit. Rev. Oncol. Hematol. 2008, 67, 153–171. [Google Scholar] [CrossRef]
- Zhu, H.; Bi, W.L.; Aizer, A.; Hua, L.; Tian, M.; Den, J.; Tang, H.; Chen, H.; Wang, Y.; Mao, Y.; et al. Efficacy of adjuvant radiotherapy for atypical and anaplastic meningioma. Cancer Med. 2019, 8, 13–20. [Google Scholar] [CrossRef]
- Durand, A.; Labrousse, F.; Jouvet, A.; Bauchet, L.; Kalamaridès, M.; Menei, P.; Deruty, R.; Moreau, J.J.; Fèvre-Montange, M.; Guyotat, J. WHO grade II and III meningiomas: A study of prognostic factors. J. Neuro-Oncol. 2009, 95, 367–375. [Google Scholar] [CrossRef]
- Apra, C.; Peyre, M.; Kalamarides, M. Current treatment options for meningioma. Expert. Rev. Neurother. 2018, 18, 241–249. [Google Scholar] [CrossRef]
- Magill, S.T.; Young, J.S.; Chae, R.; Aghi, M.K.; Theodosopoulos, P.V.; McDermott, M.W. Relationship between tumor location, size, and WHO grade in meningioma. Neurosurg. Focus 2018, 44, E4. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ning, B.; Hua, X.; Liang, Z.; Ye, J.; Yu, F.; Xu, Z.; Chen, J. Atypical meningioma: A retrospective analysis of six cases and literature review. Transl. Cancer Res. 2021, 10, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Gottschling, S.; Mawrin, C.; Prell, J.; Spielmann, R.P.; Wienke, A.; Fiedler, E. Diffusion-Weighted Imaging in Meningioma: Prediction of Tumor Grade and Association with Histopathological Parameters. Transl. Oncol. 2015, 8, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Nagar, V.A.; Ye, J.R.; Ng, W.H.; Chan, Y.H.; Hui, F.; Lee, C.K.; Lim, C.C.T. Diffusion-Weighted MR Imaging: Diagnosing Atypical or Malignant Meningiomas and Detecting Tumor Dedifferentiation. Am. J. Neuroradiol. 2008, 29, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Yarabarla, V.; Mylarapu, A.; Han, T.J.; McGovern, S.L.; Raza, S.M.; Beckham, T.H. Intracranial meningiomas: An update of the 2021 World Health Organization classifications and review of management with a focus on radiation therapy. Front. Oncol. 2023, 13, 1137849. [Google Scholar] [CrossRef] [PubMed]
- Paldor, I.; Awad, M.; Sufaro, Y.Z. Review of controversies in management of non-benign meningioma. J. Clin. Neurosci. 2016, 31, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, F.; Mariniello, G.; Guadagno, E.; Barbato, M.; Corvino, S.; Del Basso De Caro, M. WHO grade, proliferation index, and progesterone receptor expression are different according to the location of meningioma. Acta Neurochir. 2019, 161, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Meling, T.R.; Da Broi, M.; Scheie, D.; Helseth, E. Meningiomas: Skull base versus non-skull base. Neurosurg. Rev. 2019, 42, 163–173. [Google Scholar] [CrossRef]
- Wang, Y.C.; Chuang, C.C.; Wei, K.C.; Hsu, Y.H.; Hsu, P.W.; Lee, S.T.; Wu, C.-T.; Tseng, C.-K.; Wang, C.-C.; Chen, Y.-L.; et al. Skull base atypical meningioma: Long term surgical outcome and prognostic factors. Clin. Neurol. Neurosurg. 2015, 128, 112–116. [Google Scholar] [CrossRef]
- Buttrick, S.; Shah, A.H.; Komotar, R.J.; Ivan, M.E. Management of Atypical and Anaplastic Meningiomas. Neurosurg. Clin. N. Am. 2016, 27, 239–247. [Google Scholar] [CrossRef]
- Aizer, A.A.; Bi, W.L.; Kandola, M.S.; Lee, E.Q.; Nayak, L.; Rinne, M.L.; Norden, A.D.; Beroukhim, R.; Reardon, D.A.; Wen, P.Y.; et al. Extent of resection and overall survival for patients with atypical and malignant meningioma. Cancer 2015, 121, 4376–4381. [Google Scholar] [CrossRef] [PubMed]
- Aizer, A.A.; Arvold, N.D.; Catalano, P.; Claus, E.B.; Golby, A.J.; Johnson, M.D.; Al-Mefty, O.; Wen, P.Y.; Reardon, D.A.; Lee, E.Q.; et al. Adjuvant radiation therapy, local recurrence, and the need for salvage therapy in atypical meningioma. Neuro-Oncology 2014, 16, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Pisćević, I.; Villa, A.; Milićević, M.; Ilić, R.; Nikitović, M.; Cavallo, L.M.; Grujičić, D. The Influence of Adjuvant Radiotherapy in Atypical and Anaplastic Meningiomas: A Series of 88 Patients in a Single Institution. World Neurosurg. 2015, 83, 987–995. [Google Scholar] [CrossRef]
- Pizem, J.; Velnar, T.; Prestor, B.; Mlakar, J.; Popovic, M. Brain invasion assessability in meningiomas is related to meningioma size and grade, and can be improved by extensive sampling of the surgically removed meningioma specimen. Clin. Neuropathol. 2014, 33, 354–363. [Google Scholar]
- Baumgarten, P.; Gessler, F.; Schittenhelm, J.; Skardelly, M.; Tews, D.S.; Senft, C.; Dunst, M.; Imoehl, L.; Plate, K.H.; Wagner, M.; et al. Brain invasion in otherwise benign meningiomas does not predict tumor recurrence. Acta Neuropathol. 2016, 132, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Biczok, A.; Jungk, C.; Egensperger, R.; Von Deimling, A.; Suchorska, B.; Tonn, J.C.; Herold-Mende, C.; Schichor, C. Microscopic brain invasion in meningiomas previously classified as WHO grade I is not associated with patient outcome. J. Neuro-Oncol. 2019, 145, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Chun, S.W.; Dho, Y.S.; Seo, Y.; Lee, J.H.; Won, J.K.; Kim, J.W.; Park, C.-K.; Park, S.-H.; Kim, Y.H. Histopathological predictors of progression-free survival in atypical meningioma: A single-center retrospective cohort and meta-analysis. Brain Tumor Pathol. 2022, 39, 99–110. [Google Scholar] [CrossRef]
- Kwon, S.M.; Kim, J.H.; Kim, Y.H.; Hong, S.H.; Cho, Y.H.; Kim, C.J.; Nam, S.J. Clinical Implications of the Mitotic Index as a Predictive Factor for Malignant Transformation of Atypical Meningiomas. J. Korean Neurosurg. Soc. 2022, 65, 297–306. [Google Scholar] [CrossRef]
- Devaprasath, A.; Chacko, G. Diagnostic validity of the Ki-67 labeling index using the MIB-1 monoclonal antibody in the grading of meningiomas. Neurol. India 2003, 51, 336–340. [Google Scholar]
- Barresi, V.; Ammendola, S.; Simbolo, M.; Pedron, S.; Caffo, M.; Scarpa, A. Atypical meningiomas with an immunohistochemical profile consistent with hypermetabolic or proliferative molecular groups show high mitotic index, chromosomal instability, and higher recurrence risk. Virchows Arch. 2023, 483, 97–104. [Google Scholar] [CrossRef]
- Bertero, L.; Dea, G.D.; Osella-Abate, S.; Botta, C.; Castellano, I.; Morra, I.; Pollo, B.; Calatozzolo, C.; Patri-arca, S.; Mantovani, C.; et al. Prognostic Characterization of Higher-Grade Meningiomas: A Histopathological Score to Predict Progression and Outcome. J. Neuropathol. Exp. Neurol. 2019, 78, 248–256. [Google Scholar] [PubMed]
- Rogers, L.; Barani, I.; Chamberlain, M.; Kaley, T.J.; McDermott, M.; Raizer, J.; Schiff, D.; Weber, D.C.; Wen, P.Y.; Vogelbaum, M.A. Meningiomas: Knowledge base, treatment outcomes, and uncertainties. A RANO review. J. Neurosurg. 2015, 122, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Jung, S.; Moon, K.S.; Pei, J.; Lee, K.H.; Jin, S.G.; Li, S.Y.; Ryu, H.H. Immunohistochemical profile of the dural tail in intracranial meningiomas. Acta Neurochir. 2014, 156, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Tollefsen, S.; Jarmund, A.; Ytterhus, B.; Salvesen, Ø.; Mjønes, P.; Torp, S. Somatostatin Receptors in Human Meningiomas—Clinicopathological Aspects. Cancers 2021, 13, 5704. [Google Scholar] [CrossRef]
- Durand, A.; Champier, J.; Jouvet, A.; Labrousse, F.; Honnorat, J.; Guyotat, J.; Fèvre-Montange, M. Expression of c-Myc, neurofibromatosis Type 2, somatostatin receptor 2 and erb-B2 in human meningiomas: Relation to grades or histotypes. Clin. Neuropathol. 2008, 27, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Fodi, C.; Skardelly, M.; Hempel, J.M.; Hoffmann, E.; Castaneda, S.; Tabatabai, G.; Honegger, J.; Tatagiba, M.; Schittenhelm, J.; Behling, F. The immunohistochemical expression of SSTR2A is an independent prognostic factor in meningioma. Neurosurg. Rev. 2022, 45, 2671–2679. [Google Scholar] [CrossRef]
- Mnango, L.; Mwakimonga, A.; Ngaiza, A.I.; Yahaya, J.J.; Vuhahula, E.; Mwakigonja, A.R. Expression of Progesterone Receptor and Its Association with Clinicopathological Characteristics in Meningiomas: A Cross-Sectional Study. World Neurosurg. X. 2021, 12, 100111. [Google Scholar] [CrossRef]
- Marcos, D.S.; Paiva Neto, M.A.; Góes, P.; Oshima, C.T.F.; Silva, M.S.; Stávale, J.N. Grade I meningiomas with atypical characteristics: A worse prognosis. Arq. Neuropsiquiatr. 2018, 76, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.G.; Boström, J.; Wolter, M.; Baudis, M.; Collins, V.P.; Reifenberger, G.; Lichter, P. Analysis of genomic alterations in benign, atypical, and anaplastic meningiomas: Toward a genetic model of meningioma progression. Proc. Natl. Acad. Sci. USA 1997, 94, 14719–14724. [Google Scholar] [CrossRef]
- Zang, K.D. Meningioma: A cytogenetic model of a complex benign human tumor, including data on 394 karyotyped cases. Cytogenet. Cell Genet. 2001, 93, 207–220. [Google Scholar] [CrossRef]
- Williams, E.A.; Santagata, S.; Wakimoto, H.; Shankar, G.M.; Barker, F.G.; Sharaf, R.; Reddy, A.; Spear, P.; Alexander, B.M.; Ross, J.S.; et al. Distinct genomic subclasses of high-grade/progressive meningiomas: NF2-associated, NF2-exclusive, and NF2-agnostic. Acta Neuropathol. Commun. 2020, 8, 171. [Google Scholar] [CrossRef] [PubMed]
- Yuzawa, S.; Nishihara, H.; Tanaka, S. Genetic landscape of meningioma. Brain Tumor Pathol. 2016, 33, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Clark, V.E.; Erson-Omay, E.Z.; Serin, A.; Yin, J.; Cotney, J.; Özduman, K.; Avşar, T.; Li, J.; Murray, P.B.; Henegariu, O.; et al. Genomic Analysis of Non-NF2 Meningiomas Reveals Mutations in TRAF7, KLF4, AKT1, and SMO. Science 2013, 339, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Choy, W.; Kim, W.; Nagasawa, D.; Stramotas, S.; Yew, A.; Gopen, Q.; Parsa, A.T.; Yang, I. The molecular genetics and tumor pathogenesis of meningiomas and the future directions of meningioma treatments. Neurosurg. Focus 2011, 30, E6. [Google Scholar] [CrossRef] [PubMed]
- Hansson, C.M.; Buckley, P.G.; Grigelioniene, G.; Piotrowski, A.; Hellström, A.R.; Mantripragada, K.; Jarbo, C.; Mathiesen, T.; Dumanski, J.P. Comprehensive genetic and epigenetic analysis of sporadic meningioma for macro-mutations on 22q and micro-mutations within the NF2 locus. BMC Genom. 2007, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.L.; Greenwald, N.F.; Abedalthagafi, M.; Wala, J.; Gibson, W.J.; Agarwalla, P.K.; Horowitz, P.; Schu-macher, S.E.; Esaulova, E.; Mei, Y.; et al. Genomic landscape of high-grade meningiomas. Npj Genom. Med. 2017, 2, 15. [Google Scholar] [CrossRef]
- Mawrin, C.; Perry, A. Pathological classification and molecular genetics of meningiomas. J. Neuro-Oncol. 2010, 99, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.X.; Banerjee, R.; Scheithauer, B.W.; Lohse, C.M.; Kleinschmidt-Demasters, B.K.; Perry, A. Chromosome 1p and 14q FISH Analysis in Clinicopathologic Subsets of Meningioma: Diagnostic and prognostic Implications. J. Neuropathol. Exp. Neurol. 2001, 60, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Lamszus, K. Meningioma pathology, genetics, and biology. J. Neuropathol. Exp. Neurol. 2004, 63, 275–286. [Google Scholar] [CrossRef]
- Al-Mefty, O.; Kadri, P.A.; Pravdenkova, S.; Sawyer, J.R.; Stangeby, C.; Husain, M. Malignant progression in meningioma: Documentation of a series and analysis of cytogenetic findings. J. Neurosurg. 2004, 101, 210–218. [Google Scholar] [CrossRef]
- Aizer, A.A.; Abedalthagafi, M.; Linda Bi, W.; Horvath, M.C.; Arvold, N.D.; Al-Mefty, O.; Lee, E.Q.; Nayak, L.; Rinne, M.L.; Norden, A.D.; et al. A prognostic cytogenetic scoring system to guide the adjuvant management of patients with atypical meningioma. Neuro-Oncology 2016, 18, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Barresi, V.; Simbolo, M.; Fioravanzo, A.; Piredda, M.; Caffo, M.; Ghimenton, C.; Pinna, G.; Longhi, M.; Nicolato, A.; Scarpa, A. Molecular Profiling of 22 Primary Atypical Meningiomas Shows the Prognostic Significance of 18q Heterozygous Loss and CDKN2A/B Homozygous Deletion on Recurrence-Free Survival. Cancers 2021, 13, 903. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.L.N.; Stichel, D.; Hielscher, T.; Sievers, P.; Berghoff, A.S.; Schrimpf, D.; Sill, M.; Euskirchen, P.; Blume, C.; Patel, A.; et al. Integrated Molecular-Morphologic Meningioma Classification: A Multicenter Retrospective Analysis, Retrospectively and Prospectively Validated. J. Clin. Oncol. 2021, 39, 3839–3852. [Google Scholar] [CrossRef] [PubMed]
- Pfisterer, W.K.; Hank, N.C.; Preul, M.C.; Hendricks, W.P.; Pueschel, J.; Coons, S.W.; Scheck, A.C. Diagnostic and prognostic significance of genetic regional heterogeneityin meningiomas. Neuro-Oncology 2004, 6, 290–299. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Pham, M.H.; Pease, M.; Zada, G.; Giannotta, S.L.; Wang, K.; Mack, W.J. A review of epigenetic and gene expression alterations associated with intracranial meningiomas. Neurosurg. Focus 2013, 35, E5. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Li, H.; Chen, R.; Yang, H.; Zou, Y.; Ke, C.; Chen, J.; Yu, J. Integration of molecular pathology with histopathology to accurately evaluate the biological behaviour of WHO grade 2 meningiomas and patient prognosis. J. Neuro-Oncol. 2022, 160, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Vaubel, R.A.; Kumar, R.; Weiskittel, T.M.; Jenkins, S.; Dasari, S.; Uhm, J.H.; Lachance, D.H.; Brown, P.D.; Van Gompel, J.J.; Jenkins, R.B.; et al. Genomic markers of recurrence risk in atypical meningioma following gross total resection. Neuro-Oncol. Adv. 2023, 5, vdad004. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.B.; English, C.W.; Chen, W.C.; Athukuri, P.; Bayley, J.C.; Brandt, V.L.; Shetty, A.; Hadley, C.C.; Choudhury, A.; Lu, H.-C.; et al. Even heterozygous loss of CDKN2A/B greatly accelerates recurrence in aggressive meningioma. Acta Neuropathol. 2023, 145, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Neglia, J.P.; Robison, L.L.; Stovall, M.; Liu, Y.; Packer, R.J.; Hammond, S.; Yasui, Y.; Kasper, C.E.; Mertens, A.C.; Donaldson, S.S.; et al. New Primary Neoplasms of the Central Nervous System in Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study. JNCI J. Natl. Cancer Inst. 2006, 98, 1528–1537. [Google Scholar] [CrossRef]
- Broniscer, A.; Ke, W.; Fuller, C.E.; Wu, J.; Gajjar, A.; Kun, L.E. Second neoplasms in pediatric patients with primary central nervous system tumors: The St. Jude Children’s Research Hospital experience. Cancer 2004, 100, 2246–2252. [Google Scholar] [CrossRef]
- Shoshan, Y.; Chernova, O.; Jeun, S.S.; Somerville, R.P.; Israel, Z.; Barnett, G.H.; Cowell, J.K. Radiation-Induced Meningioma: A Distinct Molecular Genetic Pattern? J. Neuropathol. Exp. Neurol. 2000, 59, 614–620. [Google Scholar] [CrossRef]
- Yamanaka, R.; Hayano, A.; Kanayama, T. Radiation-Induced Meningiomas: An Exhaustive Review of the Literature. World Neurosurg. 2017, 97, 635–644.e8. [Google Scholar] [CrossRef]
- Flint-Richter, P.; Sadetzki, S. Genetic predisposition for the development of radiation-associated meningioma: An epidemiological study. Lancet Oncol. 2007, 8, 403–410. [Google Scholar] [CrossRef]
- Sahm, F.; Schrimpf, D.; Olar, A.; Koelsche, C.; Reuss, D.; Bissel, J.; Kratz, A.; Capper, D.; Schefzyk, S.; Hielscher, T.; et al. TERT Promoter Mutations and Risk of Recurrence in Meningioma. J. Natl. Cancer Inst. 2016, 108, djv377. [Google Scholar] [CrossRef] [PubMed]
- Sievers, P.; Hielscher, T.; Schrimpf, D.; Stichel, D.; Reuss, D.E.; Berghoff, A.S.; Neidert, M.C.; Wirsching, H.-G.; Mawrin, C.; Ketter, R.; et al. CDKN2A/B homozygous deletion is associated with early recurrence in meningiomas. Acta Neuropathol. 2020, 140, 409–413. [Google Scholar] [CrossRef]
- Krimpenfort, P.; Snoek, M.; Lambooij, J.P.; Song, J.Y.; Van Der Weide, R.; Bhaskaran, R.; Teunissen, H.; Adams, D.J.; De Wit, E.; Berns, A. A natural WNT signaling variant potently synergizes with Cdkn2ab loss in skin carcinogenesis. Nat. Commun. 2019, 10, 1425. [Google Scholar] [CrossRef]
- Guyot, A.; Duchesne, M.; Robert, S.; Lia, A.S.; Derouault, P.; Scaon, E.; Lemnos, L.; Salle, H.; Durand, K.; Labrousse, F. Analysis of CDKN2A gene alterations in recurrent and non-recurrent meningioma. J. Neuro-Oncol. 2019, 145, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Peyre, M.; Stemmer-Rachamimov, A.; Clermont-Taranchon, E.; Quentin, S.; El-Taraya, N.; Walczak, C.; Volk, A.; Niwa-Kawakita, M.; Karboul, N.; Giovannini, M.; et al. Meningioma progression in mice triggered by Nf2 and Cdkn2ab inactivation. Oncogene 2013, 32, 4264–4272. [Google Scholar] [CrossRef] [PubMed]
- Harmancı, A.S.; Youngblood, M.W.; Clark, V.E.; Coşkun, S.; Henegariu, O.; Duran, D.; Erson-Omay, E.Z.; Kaulen, L.D.; Lee, T.I.; Abraham, B.J.; et al. Integrated genomic analyses of de novo pathways underlying atypical meningiomas. Nat. Commun. 2017, 8, 14433. [Google Scholar] [CrossRef]
- Patel, A.J.; Wan, Y.W.; Al-Ouran, R.; Revelli, J.P.; Cardenas, M.F.; Oneissi, M. Molecular profiling predicts meningioma recurrence and reveals loss of DREAM complex repression in aggressive tumors. Proc. Natl. Acad. Sci. USA 2019, 116, 21715–21726. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.S.; Hielscher, T.; Ricken, G.; Furtner, J.; Schrimpf, D.; Widhalm, G.; Rajky, U.; Marosi, C.; Hainfellner, J.A.; Von Deimling, A.; et al. Prognostic impact of genetic alterations and methylation classes in meningioma. Brain Pathol. 2022, 32, e12970. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gómez, A.; Molnar, C.; Holguín, H.; Mayor, F.; De Celis, J.F. The cell biology of Smo signalling and its relationships with GPCRs. Biochim. Biophys. Acta BBA Biomembr. 2007, 1768, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Boetto, J.; Bielle, F.; Sanson, M.; Peyre, M.; Kalamarides, M. SMO mutation status defines a distinct and frequent molecular subgroup in olfactory groove meningiomas. Neuro-Oncology 2017, 19, 345–351. [Google Scholar]
- Brastianos, P.K.; Horowitz, P.M.; Santagata, S.; Jones, R.T.; McKenna, A.; Getz, G.; Ligon, K.L.; Palescan-dolo, E.; Van Hummelen, P.; Ducar, M.D.; et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat. Genet. 2013, 45, 285–289. [Google Scholar] [CrossRef]
- Karakas, B.; Bachman, K.E.; Park, B.H. Mutation of the PIK3CA oncogene in human cancers. Br. J. Cancer 2006, 94, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Abedalthagafi, M.; Bi, W.L.; Aizer, A.A.; Merrill, P.H.; Brewster, R.; Agarwalla, P.K.; Listewnik, M.L.; Di-as-Santagata, D.; Thorner, A.R.; Van Hummelen, P.; et al. Oncogenic PI3K mutations are as common as AKT1 and SMO mutations in meningioma. Neuro-Oncology 2016, 18, 649–655. [Google Scholar] [CrossRef]
- Laurendeau, I.; Ferrer, M.; Garrido, D.; D’Haene, N.; Ciavarelli, P.; Basso, A.; Vidaud, M.; Bieche, I.; Salmon, I.; Szijan, I. Gene Expression Profiling of the Hedgehog Signaling Pathway in Human Meningiomas. Mol. Med. 2010, 16, 262–270. [Google Scholar] [CrossRef]
- Goutagny, S.; Bah, A.B.; Henin, D.; Parfait, B.; Grayeli, A.B.; Sterkers, O.; Kalamarides, M. Long-term follow-up of 287 meningiomas in neurofibromatosis type 2 patients: Clinical, radiological, and molecular features. Neuro-Oncology 2012, 14, 1090–1096. [Google Scholar] [CrossRef]
- Bachir, S.; Shah, S.; Shapiro, S.; Koehler, A.; Mahammedi, A.; Samy, R.N.; Zuccarello, M.; Schorry, E.; Sengupta, S. Neurofibromatosis Type 2 (NF2) and the Implications for Vestibular Schwannoma and Meningioma Pathogenesis. Int. J. Mol. Sci. 2021, 22, 690. [Google Scholar] [CrossRef]
- Plotkin, S.R.; Messiaen, L.; Legius, E.; Pancza, P.; Avery, R.A.; Blakeley, J.O.; Babovic-Vuksanovic, D.; Ferner, R.; Fisher, M.J.; Friedman, J.M.; et al. Updated diagnostic criteria and nomenclature for neurofibromatosis type 2 and schwannomatosis: An international consensus recommendation. Genet. Med. 2022, 24, 1967–1977. [Google Scholar] [CrossRef]
- Halabi, R.; Dakroub, F.; Haider, M.Z.; Patel, S.; Amhaz, N.A.; Reslan, M.A.; Eid, A.H.; Mechref, Y.; Dar-wiche, N.; Kobeissy, F.; et al. Unveiling a Biomarker Signature of Meningioma: The Need for a Panel of Genomic, Epigenetic, Proteomic, and RNA Biomarkers to Advance Diagnosis and Prognosis. Cancers 2023, 15, 5339. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Lee, Y.S. Molecular characteristics of meningiomas. J. Pathol. Transl. Med. 2020, 54, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Sievers, P.; Chiang, J.; Schrimpf, D.; Stichel, D.; Paramasivam, N.; Sill, M.; Gayden, T.; Casalini, B.; Reuss, D.E.; Dalton, J.; et al. YAP1-fusions in pediatric NF2-wildtype meningioma. Acta Neuropathol. 2020, 139, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Guo, S.; Liu, D.; Chu, J.; Li, Y.; Wang, X.; Zhang, X.; Song, C.; Huang, Q. Pediatric meningioma with a Novel MAML2-YAP1 fusion variant: A case report and literature review. BMC Pediatr. 2022, 22, 694. [Google Scholar] [CrossRef]
- Esposito, S.; Marucci, G.; Antonelli, M.; Miele, E.; Modena, P.; Giagnacovo, M.; Massimino, M.; Biassoni, V.; Taddei, M.; Schiariti, M.; et al. Interhemispheric Pediatric Meningioma, YAP1 Fusion-Positive. Diagnostics 2022, 12, 2367. [Google Scholar] [CrossRef]
- Yagi, R.; Chen, L.F.; Shigesada, K.; Murakami, Y.; Ito, Y. A WW domain-containing Yes-associated protein (YAP) is a novel transcriptional co-activator. EMBO J. 1999, 18, 2551–2562. [Google Scholar] [CrossRef]
- Szulzewsky, F.; Holland, E.C.; Vasioukhin, V. YAP1 and its fusion proteins in cancer initiation, progression and therapeutic resistance. Dev. Biol. 2021, 475, 205–221. [Google Scholar] [CrossRef]
- Huang, J.; Wu, S.; Barrera, J.; Matthews, K.; Pan, D. The Hippo Signaling Pathway Coordinately Regulates Cell Proliferation and Apoptosis by Inactivating Yorkie, the Drosophila Homolog of YAP. Cell 2005, 122, 421–434. [Google Scholar] [CrossRef]
- Szulzewsky, F.; Arora, S.; Arakaki, A.K.S.; Sievers, P.; Almiron Bonnin, D.A.; Paddison, P.J.; Sahm, F.; Cimino, P.J.; Gujral, T.S.; Holland, E.C. Both YAP1-MAML2 and constitutively active YAP1 drive the formation of tumors that resemble NF2 mutant meningiomas in mice. Genes Dev. 2022, 36, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.; Giancotti, F.G. Molecular insights into NF2/Merlin tumor suppressor function. FEBS Lett. 2014, 588, 2743–2752. [Google Scholar] [CrossRef]
- Loewenstern, J.; Rutland, J.; Gill, C.; Arib, H.; Pain, M.; Umphlett, M.; Kinoshita, Y.; McBride, R.; Do-novan, M.; Sebra, R.; et al. Comparative genomic analysis of driver mutations in matched primary and recurrent meningiomas. Oncotarget. 2019, 10, 3506–3517. [Google Scholar] [CrossRef]
- Pemov, A.; Dewan, R.; Hansen, N.F.; Chandrasekharappa, S.C.; Ray-Chaudhury, A.; Jones, K.; Luo, W.; Heiss, J.D.; Mullikin, J.C.; Chittiboina, P.; et al. Comparative clinical and genomic analysis of neurofibromatosis type 2-associated cranial and spinal meningiomas. Sci. Rep. 2020, 10, 12563. [Google Scholar] [CrossRef] [PubMed]
- Laraba, L.; Hillson, L.; De Guibert, J.G.; Hewitt, A.; Jaques, M.R.; Tang, T.T.; Post, L.; Ercolano, E.; Rai, G.; Yang, S.-M.; et al. Inhibition of YAP/TAZ-driven TEAD activity prevents growth of NF2-null schwannoma and meningioma. Brain 2023, 146, 1697–1713. [Google Scholar] [CrossRef]
- Szulzewsky, F.; Arora, S.; Hoellerbauer, P.; King, C.; Nathan, E.; Chan, M.; Cimino, P.J.; Ozawa, T.; Ka-wauchi, D.; Pajtler, K.W.; et al. Comparison of tumor-associated YAP1 fusions identifies a recurrent set of functions critical for oncogenesis. Genes Dev. 2020, 34, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Behling, F.; Fodi, C.; Gepfner-Tuma, I.; Kaltenbach, K.; Renovanz, M.; Paulsen, F.; Skardelly, M.; Honeg-ger, J.; Tatagiba, M.; International Consortium on Meningiomas; et al. H3K27me3 loss indicates an increased risk of recurrence in the Tübingen meningioma cohort. Neuro-Oncology 2021, 23, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.M.; Hielscher, T.; Liechty, B.; Silverman, J.; Zagzag, D.; Sen, R.; Wu, P.; Golfinos, J.G.; Reuss, D.; Neidert, M.C.; et al. Loss of histone H3K27me3 identifies a subset of meningiomas with increased risk of recurrence. Acta Neuropathol. 2018, 135, 955–963. [Google Scholar] [CrossRef]
- Ammendola, S.; Rizzo, P.C.; Longhi, M.; Zivelonghi, E.; Pedron, S.; Pinna, G.; Sala, F.; Nicolato, A.; Scarpa, A.; Barresi, V. The Immunohistochemical Loss of H3K27me3 in Intracranial Meningiomas Predicts Shorter Progression-Free Survival after Stereotactic Radiosurgery. Cancers 2022, 14, 1718. [Google Scholar] [CrossRef]
- Hua, L.; Ren, L.; Wu, Q.; Deng, J.; Chen, J.; Cheng, H.; Wang, D.; Chen, H.; Xie, Q.; Wakimoto, H.; et al. Loss of H3K27me3 expression enriches in recurrent grade 1&2 meningiomas and maintains as a biomarker stratifying progression risk. J. Neuro-Oncol. 2023, 161, 267–275. [Google Scholar]
- Vasudevan, K.; Saad, H.; Oyesiku, M.N. The Role of Three-Dimensional Endoscopy in Pituitary Adenoma Surgery. Neurosurg. Clin. N. Am. 2019, 30, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Sahm, F.; Schrimpf, D.; Stichel, D.; Jones, D.T.W.; Hielscher, T.; Schefzyk, S.; Okonechnikov, K.; Koelsche, C.; Reuss, D.E.; Capper, D.; et al. DNA methylation-based classification and grading system for meningioma: A multicentre, retrospective analysis. Lancet Oncol. 2017, 18, 682–694. [Google Scholar] [CrossRef]
- Nassiri, F.; Liu, J.; Patil, V.; Mamatjan, Y.; Wang, J.Z.; Hugh-White, R.; Macklin, A.M.; Khan, S.; Singh, O.; Karimi, S.; et al. A clinically applicable integrative molecular classification of meningiomas. Nature 2021, 597, 119–125. [Google Scholar] [CrossRef]
- Olar, A.; Wani, K.M.; Wilson, C.D.; Zadeh, G.; DeMonte, F.; Jones, D.T.W.; Pfister, S.M.; Sulman, E.P.; Al-dape, K.D. Global epigenetic profiling identifies methylation subgroups associated with recurrence-free survival in meningioma. Acta Neuropathol. 2017, 133, 431–444. [Google Scholar] [CrossRef]
- Hielscher, T.; Sill, M.; Sievers, P.; Stichel, D.; Brandner, S.; Jones, D.T.W.; Von Deimling, A.; Sahm, F.; Maas, S.L.N. Clinical implementation of integrated molecular-morphologic risk prediction for meningioma. Brain Pathol. 2023, 33, e13132. [Google Scholar] [CrossRef]
- Driver, J.; Hoffman, S.E.; Tavakol, S.; Woodward, E.; Maury, E.A.; Bhave, V.; Greenwald, N.F.; Nassiri, F.; Aldape, K.; Zadeh, G.; et al. A molecularly integrated grade for meningioma. Neuro-Oncology 2022, 24, 796–808. [Google Scholar] [CrossRef]
- Choudhury, A.; Magill, S.T.; Eaton, C.D.; Prager, B.C.; Chen, W.C.; Cady, M.A.; Seo, K.; Lucas, C.-H.G.; Casey-Clyde, T.J.; Vasudevan, H.N.; et al. Meningioma DNA methylation groups identify biological drivers and therapeutic vulnerabilities. Nat. Genet. 2022, 54, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.L.; Nayak, L.; Meredith, D.M.; Driver, J.; Du, Z.; Hoffman, S.; Li, Y.; Lee, E.Q.; Beroukhim, R.; Rinne, M.; et al. Activity of PD-1 blockade with nivolumab among patients with recurrent atypical/anaplastic meningioma: Phase II trial results. Neuro-Oncology 2022, 24, 101–113. [Google Scholar] [CrossRef]
- Islim, A.I.; Kolamunnage-Dona, R.; Mohan, M.; Moon, R.D.C.; Crofton, A.; Haylock, B.J.; Rathi, N.; Brod-belt, A.R.; Mills, S.J.; Jenkinson, M.D. A prognostic model to personalize monitoring regimes for patients with incidental asymptomatic meningiomas. Neuro-Oncology 2020, 22, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Ferrarotto, R.; Curcio, A.; Metro, L.; Pasqualetti, F.; Gaviani, P.; Barresi, V.; Angileri, F.F.; Caffo, M. Novel Advances in Treatment of Meningiomas: Prognostic and Therapeutic Implications. Cancers 2023, 15, 4521. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.; Gilbert, M.; Vogelbaum, M.A. Intracranial meningiomas of atypical (WHO grade II) histology. J. Neuro-Oncol. 2010, 99, 393–405. [Google Scholar] [CrossRef]
- Palma, L.; Cantore, G. Long-term prognosis for atypical and malignant meningiomas: A study of 71 surgical cases. Neurosurg. Focus 1997, 2, e3. [Google Scholar] [CrossRef]
- Morales-Valero, S.F.; Van Gompel, J.J.; Loumiotis, I.; Lanzino, G. Craniotomy for anterior cranial fossa meningiomas: Historical overview. Neurosurg. Focus 2014, 36, E14. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.A.; Huang, L.; Ramanathan, D.; Lopez-Gonzalez, M.; Pillai, P.; De Los Reyes, K.; Kumal, M.; Boling, W. Review of Atypical and Anaplastic Meningiomas: Classification, Molecular Biology, and Management. Front. Oncol. 2020, 10, 565582. [Google Scholar] [CrossRef] [PubMed]
- Perry, A. Unmasking the secrets of meningioma: A slow but rewarding journey. Surg. Neurol. 2004, 61, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Buerki, R.A.; Horbinski, C.M.; Kruser, T.; Horowitz, P.M.; James, C.D.; Lukas, R.V. An Overview of Meningiomas. Future Oncol. 2018, 14, 2161–2177. [Google Scholar] [CrossRef] [PubMed]
- Graffeo, C.S.; Leeper, H.E.; Perry, A.; Uhm, J.H.; Lachance, D.J.; Brown, P.D.; Ma, D.J.; Gompel, J.J.V.; Giannini, C.; Johnson, D.R.; et al. Revisiting Adjuvant Radiotherapy After Gross Total Resection of World Health Organization Grade II Meningioma. World Neurosurg. 2017, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Demchuk, A.M.; Menon, B.K.; Eesa, M.; Rempel, J.L.; Thornton, J.; Roy, D.; Jovin, T.G.; Willin-sky, R.A.; Sapkota, B.L.; et al. Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke. N. Engl. J. Med. 2015, 372, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Englot, D.J.; Magill, S.T.; Han, S.J.; Chang, E.F.; Berger, M.S.; McDermott, M.W. Seizures in supratentorial meningioma: A systematic review and meta-analysis. J. Neurosurg. 2016, 124, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Magill, S.T.; Englot, D.J.; Baal, J.D.; Wagle, S.; Rick, J.W.; McDermott, M.W. Factors Associated with Pre- and Postoperative Seizures in 1033 Patients Undergoing Supratentorial Meningioma Resection. Neurosurgery 2017, 81, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Young, M.; Albert, T.; Shah, A.H.; Okoye, C.; Bregy, A.; Lo, S.S.; Ishkanian, F.; Komotar, R.J. The Role of Adjuvant Radiotherapy After Gross Total Resection of Atypical Meningiomas. World Neurosurg. 2015, 83, 808–815. [Google Scholar] [CrossRef]
- Attia, A.; Chan, M.D.; Mott, R.T.; Russell, G.B.; Seif, D.; Daniel Bourland, J.; Deguzman, A.F.; Ellis, T.L.; McMullen, K.P.; Munley, M.T.; et al. Patterns of failure after treatment of atypical meningioma with gamma knife radiosurgery. J. Neuro-Oncol. 2012, 108, 179–185. [Google Scholar] [CrossRef]
- Jimenez, R.B.; Alexander, B.M.; Mahadevan, A.; Niemierko, A.; Rajakesari, S.; Arvold, N.D.; Floyd, S.R.; Oh, K.S.; Loeffler, J.S.; Shih, H.A. The impact of different stereotactic radiation therapy regimens for brain metastases on local control and toxicity. Adv. Radiat. Oncol. 2017, 2, 391–397. [Google Scholar] [CrossRef]
- Cohen-Inbar, O.; Lee C chia Sheehan, J.P. The Contemporary Role of Stereotactic Radiosurgery in the Treatment of Meningiomas. Neurosurg. Clin. N. Am. 2016, 27, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.K.; Niranjan, A.; McInerney, J.; Kondziolka, D.; Flickinger, J.C.; Lunsford, L.D. Stereotactic radiosurgery providing long-term tumor control of cavernous sinus meningiomas. J. Neurosurg. 2002, 97, 65–72. [Google Scholar] [CrossRef]
- Kano, H.; Takahashi, J.A.; Katsuki, T.; Araki, N.; Oya, N.; Hiraoka, M.; Hashimoto, N. Stereotactic radiosurgery for atypical and anaplastic meningiomas. J. Neuro-Oncol. 2007, 84, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.E.; Lee, J.Y.K.; Omalu, B.; Flickinger, J.C.; Kondziolka, D.; Lunsford, L.D. The effect of radiosurgeryduring management of aggressive meningiomas. Surg. Neurol. 2003, 60, 298–305. [Google Scholar] [CrossRef]
- Rogers, C.L.; Won, M.; Vogelbaum, M.A.; Perry, A.; Ashby, L.S.; Modi, J.M.; Alleman, A.M.; Galvin, J.; Fogh, S.E.; Youssef, E.; et al. High-risk Meningioma: Initial Outcomes from NRG Oncology/RTOG 0539. Int. J. Radiat. Oncol. 2020, 106, 790–799. [Google Scholar] [CrossRef]
- Madani, I.; Lomax, A.J.; Albertini, F.; Trnková, P.; Weber, D.C. Dose-painting intensity-modulated proton therapy for intermediate- and high-risk meningioma. Radiat. Oncol. 2015, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Hammouche, S.; Clark, S.; Wong, A.H.L.; Eldridge, P.; Farah, J.O. Long-term survival analysis of atypical meningiomas: Survival rates, prognostic factors, operative and radiotherapy treatment. Acta Neurochir. 2014, 156, 1475–1481. [Google Scholar] [CrossRef]
- Komotar, R.J.; Iorgulescu, J.B.; Raper, D.M.S.; Holland, E.C.; Beal, K.; Bilsky, M.H.; Brennan, C.W.; Tabar, V.; Sherman, J.H.; Yamada, Y.; et al. The role of radiotherapy following gross-total resection of atypical meningiomas: Clinical article. J. Neurosurg. 2012, 117, 679–686. [Google Scholar] [CrossRef]
- Pasquier, D.; Bijmolt, S.; Veninga, T.; Rezvoy, N.; Villa, S.; Krengli, M.; Weber, D.C.; Baumert, B.G.; Canyilmaz, E.; Yalman, D.; et al. Atypical and Malignant Meningioma: Outcome and Prognostic Factors in 119 Irradiated Patients. A Multicenter, Retrospective Study of the Rare Cancer Network. Int. J. Radiat. Oncol. 2008, 71, 1388–1393. [Google Scholar] [CrossRef]
- Jääskeläinen, J.; Haltia, M.; Laasonen, E.; Wahlström, T.; Valtonen, S. The growth rate of intracranial meningiomas and its relation to histology. An analysis of 43 patients. Surg. Neurol. 1985, 24, 165–172. [Google Scholar] [CrossRef]
- Jenkinson, M.D.; Javadpour, M.; Haylock, B.J.; Young, B.; Gillard, H.; Vinten, J.; Bulbeck, H.; Das, K.; Far-rell, M.; Looby, S.; et al. The ROAM/EORTC-1308 trial: Radiation versus Observation following surgical resection of Atypical Meningioma: Study protocol for a randomised controlled trial. Trials 2015, 16, 519. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.C.; Ares, C.; Villa, S.; Peerdeman, S.M.; Renard, L.; Baumert, B.G.; Lucas, A.; Veninga, T.; Pica, A.; Jefferies, S.; et al. Adjuvant postoperative high-dose radiotherapy for atypical and malignant meningioma: A phase-II parallel non-randomized and observation study (EORTC 22042-26042). Radiother. Oncol. 2018, 128, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.; Zhang, P.; Vogelbaum, M.A.; Perry, A.; Ashby, L.S.; Modi, J.M.; Alleman, A.M.; Galvin, J.; Brachman, D.; Jenrette, J.M.; et al. Intermediate-risk meningioma: Initial outcomes from NRG Oncology RTOG 0539. J. Neurosurg. 2018, 129, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Sioka, C.; Kyritsis, A.P. Chemotherapy, hormonal therapy, and immunotherapy for recurrent meningiomas. J. Neuro-Oncol. 2009, 92, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Norden, A.D.; Raizer, J.J.; Abrey, L.E.; Lamborn, K.R.; Lassman, A.B.; Chang, S.M.; Yung, W.K.A.; Gilbert, M.R.; Fine, H.A.; Mehta, M.; et al. Phase II trials of erlotinib or gefitinib in patients with recurrent meningioma. J. Neuro-Oncol. 2010, 96, 211–217. [Google Scholar] [CrossRef]
- Chamberlain, M.C.; Tsao-Wei, D.D.; Groshen, S. Salvage chemotherapy with CPT-11 for recurrent meningioma. J. Neuro-Oncol. 2006, 78, 271–276. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.H.; Kim, Y.Z. The Clinical Outcome of Hydroxyurea Chemotherapy after Incomplete Resection of Atypical Meningiomas. Brain Tumor Res. Treat. 2017, 5, 77–86. [Google Scholar] [CrossRef]
- Abdel Karim, K.; El Shehaby, A.; Emad, R.; Reda, W.; El Mahdy, M.; Ghali, R.; Nabeel, A. Role of hydroxyurea as an adjuvant treatment after Gamma knife radiosurgery for atypical (WHO grade II) meningiomas. J. Egypt Natl. Cancer Inst. 2018, 30, 69–72. [Google Scholar] [CrossRef]
- Chamberlain, M.C.; Glantz, M.J. Interferon-α for recurrent World Health Organization grade 1 intracranial meningiomas. Cancer 2008, 113, 2146–2151. [Google Scholar] [CrossRef]
- Kaley, T.J.; Wen, P.; Schiff, D.; Ligon, K.; Haidar, S.; Karimi, S.; Lassman, A.B.; Nolan, C.P.; DeAngelis, L.M.; Gavrilovic, I.; et al. Phase II trial of sunitinib for recurrent and progressive atypical and anaplastic meningioma. Neuro-Oncology 2015, 17, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Raizer, J.J.; Abrey, L.E.; Lassman, A.B.; Chang, S.M.; Lamborn, K.R.; Kuhn, J.G.; Yung, W.K.A.; Gilbert, M.R.; Aldape, K.D.; Wen, P.Y.; et al. A phase I trial of erlotinib in patients with nonprogressive glioblastoma multiforme postradiation therapy, and recurrent malignant gliomas and meningiomas. Neuro-Oncology 2010, 12, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Raizer, J.J.; Grimm, S.A.; Rademaker, A.; Chandler, J.P.; Muro, K.; Helenowski, I.; Rice, L.; McCarthy, K.; Johnston, S.K.; Mrugala, M.M.; et al. A phase II trial of PTK787/ZK 222584 in recurrent or progressive radiation and surgery refractory meningiomas. J. Neuro-Oncol. 2014, 117, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Caffo, M.; Pino, M.A.; Caruso, G.; Tomasello, F. Antisense Molecular Therapy in Cerebral Gliomas. J. Anal. Oncol. 2012, 1, 135–144. Available online: https://neoplasiaresearch.com/pms/index.php/jao/article/view/92 (accessed on 15 July 2024).
- Sakuma, T.; Nakagawa, T.; Ido, K.; Takeuchi, H.; Sato, K.; Kubota, T. Expression of vascular endothelial growth factor-A and mRNA stability factor HuR in human meningiomas. J. Neuro-Oncol. 2008, 88, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Barresi, V.; Tuccari, G. Increased ratio of vascular endothelial growth factor to semaphorin3A is a negative prognostic factor in human meningiomas. Neuropathology 2010, 30, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Baxter, D.S.; Orrego, A.; Rosenfeld, J.V.; Mathiesen, T. An audit of immunohistochemical marker patterns in meningioma. J. Clin. Neurosci. 2014, 21, 421–426. [Google Scholar] [CrossRef]
- Winter, R.C.; Antunes, A.C.M.; Oliveira, F.H.D. The relationship between vascular endothelial growth factor and histological grade in intracranial meningioma. Surg. Neurol. Int. 2020, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Kshettry, V.R.; Selman, W.R.; Bambakidis, N.C. Peritumoral brain edema in intracranial meningiomas: The emergence of vascular endothelial growth factor–directed therapy. Neurosurg. Focus 2013, 35, E2. [Google Scholar] [CrossRef]
- Salokorpi, N.; Yrjänä, S.; Tuominen, H.; Karttunen, A.; Heljasvaara, R.; Pihlajaniemi, T.; Heikkinen, E.; Koivukangas, J. Expression of VEGF and collagen XVIII in meningiomas: Correlations with histopathological and MRI characteristics. Acta Neurochir. 2013, 155, 989–996. [Google Scholar] [CrossRef]
- Shih, K.C.; Chowdhary, S.; Rosenblatt, P.; Weir, A.B.; Shepard, G.C.; Williams, J.T.; Shastry, M.; Burris, H.A.; Hainsworth, J.D. A phase II trial of bevacizumab and everolimus as treatment for patients with refractory, progressive intracranial meningioma. J. Neuro-Oncol. 2016, 129, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Pachow, D.; Andrae, N.; Kliese, N.; Angenstein, F.; Stork, O.; Wilisch-Neumann, A.; Kirches, E.; Mawrin, C. mTORC1 Inhibitors Suppress Meningioma Growth in Mouse Models. Clin. Cancer Res. 2013, 19, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Graillon, T.; Sanson, M.; Campello, C.; Idbaih, A.; Peyre, M.; Peyrière, H.; Basset, N.; Autran, D.; Roche, C.; Kalamarides, M.; et al. Everolimus and Octreotide for Patients with Recurrent Meningioma: Results from the Phase II CEVOREM Trial. Clin. Cancer Res. 2020, 26, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Wolfsberger, S.; Doostkam, S.; Boecher-Schwarz, H.G.; Roessler, K.; Van Trotsenburg, M.; Hainfellner, J.A.; Knosp, E. Progesterone-receptor index in meningiomas: Correlation with clinico-pathological parameters and review of the literature. Neurosurg. Rev. 2004, 27, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Norden, A.D.; Ligon, K.L.; Hammond, S.N.; Muzikansky, A.; Reardon, D.A.; Kaley, T.J.; Batchelor, T.T.; Plotkin, S.R.; Raizer, J.J.; Wong, E.T.; et al. Phase II study of monthly pasireotide LAR (SOM230C) for recurrent or progressive meningioma. Neurology 2015, 84, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Zhi, F.; Zhou, G.; Wang, S.; Shi, Y.; Peng, Y.; Shao, N.; Guan, W.; Qu, H.; Zhang, Y.; Wang, Q.; et al. A microRNA expression signature predicts meningioma recurrence. Int. J. Cancer. 2013, 132, 128–136. [Google Scholar] [CrossRef]
- Deng, J.; Hua, L.; Bian, L.; Chen, H.; Chen, L.; Cheng, H.; Dou, C.; Geng, D.; Hong, T.; Ji, H.; et al. Molecular diagnosis and treatment of meningiomas: An expert consensus (2022). Chin. Med. J. 2022, 135, 1894–1912. (In English) [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).