Abstract
Cutaneous squamous cell carcinoma (cSCC) is the second-most-prevalent malignancy in humans. A delayed diagnosis of cSCC leads to heightened invasiveness and positive surgical margins. Bowen’s disease (BD) represents an early form of cSCC and presents as a small erythematous, photo-distributed, psoriasiform plaque. Although certain dermoscopy features in BD are quite characteristic, histopathology remains the gold standard for diagnosis and provides a severity-scoring system that assists in guiding appropriate treatment strategies. The classification of precancerous lesions of the vulva and penis has undergone multifarious transformations due to variations in clinical and histopathological characteristics. Presently, erythroplasia of Queyrat is categorized as a clinical variant of penile intraepithelial neoplasia (PeIN). The diagnoses of vulvar intraepithelial neoplasia (VIN) and PeIN present significant challenges and typically necessitate one or more biopsies, potentially guided by dermoscopy. Aceto-white testing demonstrates a notably high negative predictive value for genital precancerous lesions. Histopathological examination represents the gold-standard diagnosis in VIN and PeIN, while p16 and p53 immunostainings alongside HPV testing provide crucial diagnostic clues. The histopathologic features, degree of differentiation, and associations with lichen planus, lichen sclerosus, and HPV guide the selection of conservative treatments or surgical excision.
1. Introduction
Cutaneous squamous cell carcinoma (cSCC) ranks as the second-most-prevalent neoplasm in humans following basal cell carcinoma, exhibiting an escalating incidence. The probability of cSCC onset is notably elevated, reaching up to 11% within the Caucasian demographic [1,2]. Additionally, cSCC stands as the second-leading cause of skin cancer fatality. Moreover, it is deemed the predominant contributor to skin cancer-related mortalities among the elderly, surpassing both melanoma and adnexal carcinomas. Notably, the propensity for metastasis is accentuated in elderly males and typically manifests 12–24 months after the treatment of the primary lesion. A delayed diagnosis of cSCC precipitates a 4.7-fold escalation in invasiveness and a three-fold increase in positive surgical margins [3,4,5].
Bowen’s disease represents an in situ cSCC, with a 3–5% risk of progressing into invasive SCC [6]. While SCC ranks as the most prevalent cancer of the vulva and penis, its incidence remains low in high-income nations. The treatment of invasive forms frequently results in disfigurement and inflicts a profound psychological toll on patients [7]. Consecutively, the timely diagnosis of penile (PeIN, penile intraepithelial neoplasia) and vulvar (VIN, vulvar intraepithelial neoplasia) incipient SCC remains a desideratum. Notably, glans and prepuce Bowen’s disease, historically referred to as erythroplasia of Queyrat, bears a transformation risk into invasive SCC of up to 33% [8,9].
This manuscript is intended to emphasize the significance of diagnosis in early cSCC and to address the principal diagnostic and management challenges associated with Bowen’s disease, VIN, PeIN, and erythroplasia of Queyrat.
2. Bowen’s Disease (BD)
BD typically affects individuals over 60 and is uncommon in those under 30 and black individuals. Immunocompromised individuals are at risk of developing BD at a younger age. Most studies indicate a slight female predominance. The incidence is higher among Caucasians (1.42/1000). In BD, the term “SCC in situ” denotes an intraepithelial lesion characterized by the clonal proliferation of atypical keratinocytes occupying the full thickness of the epidermis [10,11,12]. Lesions are generally solitary. The morphology of BD varies depending on the age of the lesion, the site of origin, and the degree of keratinization. BD is often referred to as the “lull before the storm”, indicating its role as a precursor to overt cSCC [13]. Progression to the invasive and metastatic form occurs over a long period in only 3% to 5% of cases [14].
The primary etiological factors of BD are exposure to ultraviolet light, immunosuppression, and human papillomavirus (HPV) infections. While BD commonly occurs in photo-exposed areas of the skin, it can also affect other regions [11]. Its pathogenesis encompasses various forms of radiation, including ionizing radiation, in addition to ultraviolet (UV) radiation [11,13]. Cumulative exposure to ultraviolet (UV) light radiation causes DNA damage and immunosuppression, which promotes the clonal expansion of cells with p53 mutations [15]. Furthermore, exposure to certain toxic substances, such as arsenic, and infection with oncogenic strains of HPV also contribute to the development of BD [11,13,16]. Most HPV-positive lesions regarding the skin in BD are commonly found on the distal extremities (such as periungual sites), with HPV type-16 often being detected [17,18].
Clinically, BD is classically described as a small, erythematous, scaly plaque that enlarges erratically over time [12]. The overlying scale can be white or yellow, either easily removable or adherent. When the scale is removed, it does not cause bleeding and exposes an erythematous, moist surface [11,19]. The margins of the lesion are well-defined, and the affected area is slightly raised above the normal skin level. The surface is generally flat but can sometimes become hyperkeratotic or crusted. The presence of an ulcer often indicates cSCC, except for palmar superficial lesions, which may result from repeated friction [19].
The primary differential diagnoses include infiltrating cSCC, superficial basal cell carcinoma, nummular eczema, condylomata acuminata, psoriasis, lichen planus, lichen simplex chronicus, lichen planus-like keratosis, porokeratosis, chronic discoid cutaneous lupus erythematosus, Paget disease and seborrheic keratosis [10,20].
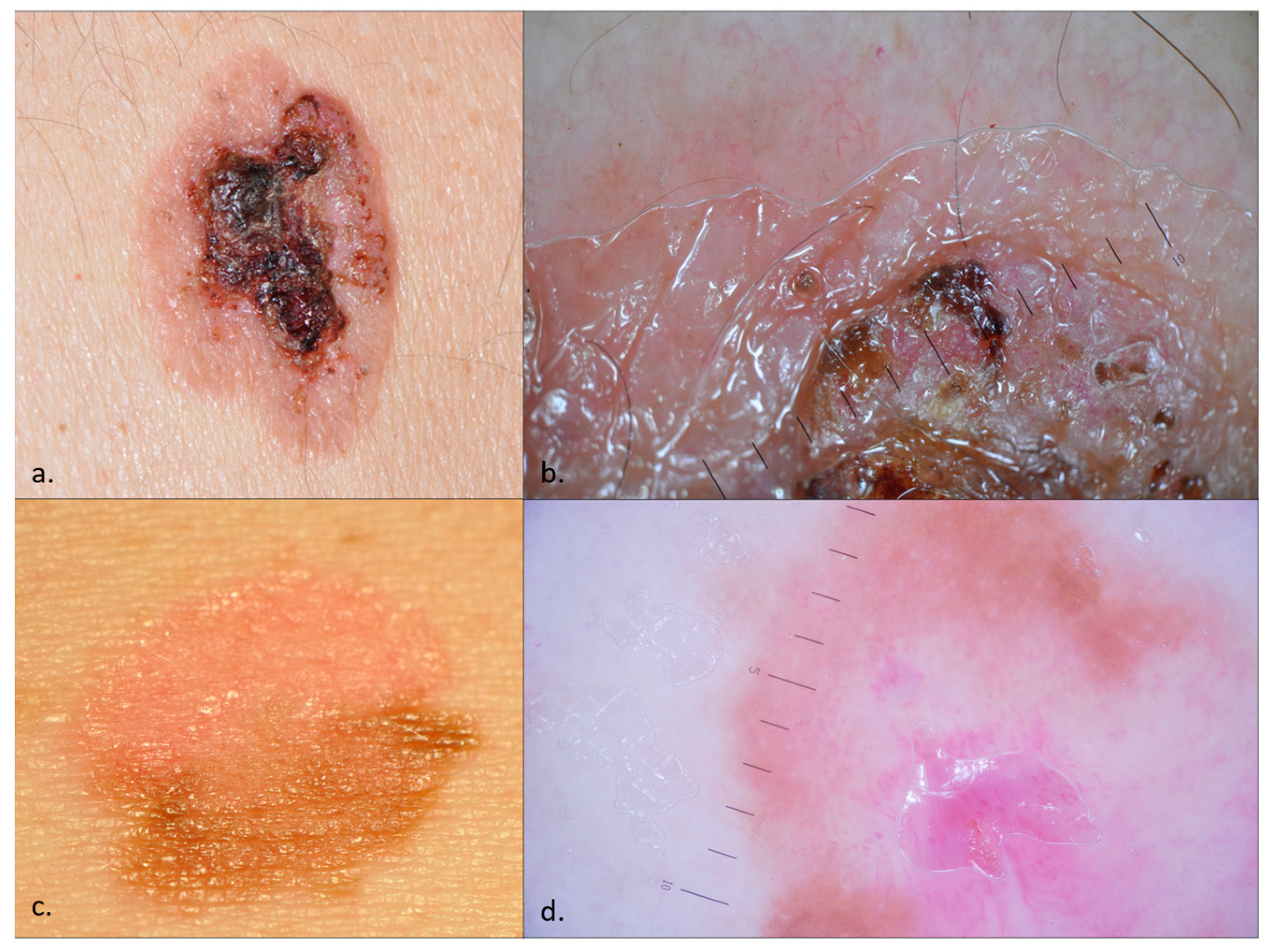
Dermoscopy can assist in the diagnostic process, as the clinical presentations may pose significant diagnostic difficulties (Figure 1). The dermoscopic features of BD were first detailed by Zalaudek et al. in 2004. Since then, multiple studies have been conducted on the dermoscopic characteristics of BD, consistently identifying glomerular vessels, a scaly surface, small brown globules, and structureless grey to brown pigmentation as its primary features [15,17,21,22]. BD can be categorized into three types: (a) classic BD—irregular vascular pattern, whitish scaling, and a pinkish network; (b) pigmented BD—unstructured pigmentation, pigmented stripes, and crust formation; and (c) fragmentarily pigmented BD—a combination of characteristics from both classic and pigmented BD [14,23]. Pigmented BD manifests as heterogeneous brown plaques that may appear keratotic or verrucous. The most common differential diagnoses are seborrheic keratosis, solar lentigo, pigmented actinic keratosis, melanocytic nevus, and melanoma [16,23,24,25].
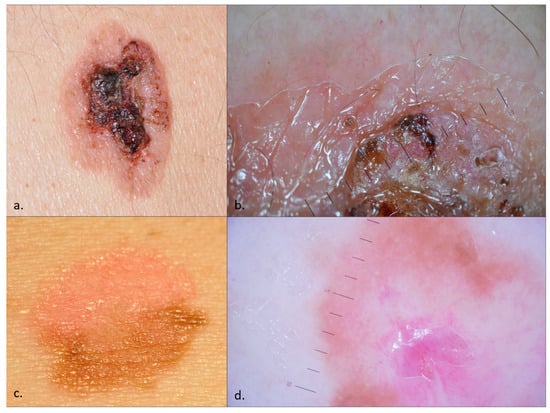
Figure 1.
Bowen disease (BD)—clinical images (a,c) and dermoscopy (b,d). An ulcerated plaque with white scales, hematic crusts, and few vessels in the periphery mimicking a cSCC, whose pathology examination revealed an ulcerated, non-pigmented BD (a,b). While ulceration and crusting often characterize cSCC transformation, histopathological examination confirmed the absence of SCC in this lesion; An erythematous and pigmented plaque with fine scales, prominent glomerular vessels, and pigmentation, suggestive of fragmentarily pigmented BD (c,d).
Histopathology remains the gold standard for diagnosis, revealing hyperkeratosis, parakeratosis, acanthosis with elongation and thickening of rete ridges, absence of the granular layer, and full-thickness keratinocyte atypia without breaching the dermo–epidermal junction [18,23]. The keratinocytes exhibit intense mitotic activity, pleomorphism, large nuclei, and loss of maturity and polarity, giving the epidermis a “windblown” appearance. Two types of giant cells in the epidermis are recognized: one with a keratinocyte engulfing a dyskeratotic cell, and another with multiple central nuclei surrounded by dyskeratotic tonofilaments. The dermis shows moderate lymphocytic infiltrates, the occasional vacuolization of upper dermal cells, and secondary amyloid deposition. BD may also involve sebaceous or mucinous metaplasia [11,26,27]. Histologic variants include psoriasiform, atrophic, acantholytic, epidermolytic, and other patterns like verrucous-hyperkeratotic, orthokeratotic, mucinous, sebaceous, papillated, irregular (highly pleomorphic), pigmented, pagetoid, and clear cell, some of which can be associated with HPV infection [14,23,26].
A grading system for BD (Table 1) helps stratify patients based on the severity of their condition. This scoring system allows for a standardized assessment of BD severity and aids in guiding appropriate treatment strategies and follow-up care. Adjustments and validations by clinical studies may further refine this system to ensure its efficacy and accuracy [11,17,19,21,23,25,28,29,30,31,32,33].

Table 1.
Bowen’s disease (BD) severity scoring system.
BD is characterized by distinct immunohistochemical profiles that aid in its differentiation from other dermatological conditions. Keratinocyte nuclei in BD show a diffuse pattern of staining for proliferating cell nuclear antigen (PCNA), and cytokeratin 10 (CK10) is universally expressed. The overexpression of p16 reflects disrupted G1/S checkpoint control, useful for distinguishing BD from actinic keratosis (AK) and seborrheic keratosis. A distinct p53 and p16 staining pattern in basal epidermal keratinocytes is instrumental in distinguishing BD from AK. Unlike BD, Paget’s disease expresses CK7, while BD of the nipple stains positive for cytokeratin 5/6 and negative for CK7 [34,35,36]. Markers such as Ki-67 and p27 provide additional diagnostic clarity; Ki-67 shows a diffuse pattern in BD, differentiating it from AK, and p27 indicates cellular latency, distinguishing BD from cSCC [36,37]. Positive immunostaining for lumican and the expression of CK14, associated with tumor progression, further assist in the histopathological evaluation [35].
3. Vulvar Intraepithelial Neoplasia (VIN)
Throughout the years, the terminology associated with vulvar precancerous lesions has witnessed diverse transformations attributable to disparities in clinical and histopathological aspects. A consensus on the terminology was achieved by the International Society for the Study of Vulvovaginal Diseases (ISSVD) in 2015. The evolution of the terminology for vulvar precancerous lesions dates back to the description of erythroplakiform dyskeratosis in 1922 [38,39]. Subsequent nomenclature variations included Bowen dermatosis (1929), carcinoma in situ (1943), vulvar atypia (1972), Bowenoid atypia (1973), hyperplastic dystrophy with atypia (1976), and Bowenoid papulosis (1979) [40].
The term vulvar intraepithelial neoplasia (VIN) was formalized in 1982 and underwent further subclassification over time [41,42]. Initially, VIN was categorized as squamous VIN, encompassing subtypes 1–3 representing varying degrees of atypia, along with non-squamous VIN of the melanoma or Paget’s disease type. The designation “differentiated VIN” was introduced in 1986, based on histopathologic considerations. In 2004, the ISSVD Group redefined the classification of VIN into two groups, delineated by histopathologic and pathogenic aspects: VIN of the usual type, induced by HPV, subdivided into basaloid, warty, mixed, and VIN of the differentiated type, non-HPV-infected. VIN 1 disappears due to a skin reaction to HPV infection rather than a precancerous lesion [41].
In 2012, the grading of VIN from 1-2-3 was replaced by a standardized terminology, which replaced VIN, CIN (cervical intraepithelial neoplasia), and PeIN (penile intraepithelial neoplasia), with the term “SIL” (squamous intraepithelial lesion) [43]. This term characterizes the group of precancerous lesions of the lower genital tract and anogenital tract caused by HPV. The SIL system comprises LSIL (low-grade SIL) and HSIL (high-grade SIL) [44,45]. There have been multiple debates focusing on the pathological significance of vulvar LSIL lesions, with arguments against their treatment. From 2014 onwards, diagnostic and treatment protocols have been premised on classifying vulvar precancerous lesions, encompassing LSIL, HSIL, and differentiated VIN (simplex) [40]. In summary, vulvar LSIL (VLSIL, formerly known as VIN 1), denotes flat condyloma, an HPV-related vulvar lesion. Vulvar HSIL (VHSIL, previously referred to as VIN 2 and VIN 3) signifies the usual type of VIN, whereas differentiated VIN indicates vulvar lesions unrelated to HPV, often associated with vulvar dermatoses, particularly lichen sclerosus.
Multiple cohort studies have underscored an elevated prevalence of VHSIL, with an incidence of 3.8 per 100,000 women per year. This rate surpasses the incidence of differentiated VIN. Concurrently, an increased manifestation of VHSIL has been documented, escalating from 2.39 to 3.26 per 100,000 females between 1991 and 2011. These findings underscore the significance of this issue from a public health perspective [46]. This variance is partly attributable to the heightened frequency of HPV infections before the era of widespread vaccination, the relatively young age of women falling into this category, and the enhanced discernibility upon the manifestation of a vulvar lesion [47]. In contrast, diagnosing differentiated VIN poses greater challenges for both clinicians and pathologists, as it is more commonly observed in older women with concomitant lichen sclerosus, with an average age of 68 years [48]. According to Virnig et al., there was a 411% increase in the prevalence of vulvar carcinoma in situ among women aged 40–49 from 1973 to 2000 [49], with the highest reported frequency comprising women aged 20 to 35 years [50]. There has been no apparent change in the incidence of VSCC over time. This stability can be attributed to the increased early detection of pre-invasive lesions and the subsequent expedited treatment [51].
Differentiated VIN (dVIN) has a higher propensity to progress to vulvar SCC than HSIL (43.2%, respectively 9.7%). Moreover, patients with dVIN pose a higher risk of developing recurring vulvar SCC [46,52]. VHSIL is linked to the presence of high-risk HPV in 80% of cases. Among these cases, HPV 16 is predominant, accounting for 80%, followed by HPV 33, which is present in 6% of cases [53]. In another study, the majority of LSILs were associated with low-risk HPV types 6 and 11, comprising over 90% of cases [54].
Immunosuppression is a critical factor in the development of VHSIL, with HPV playing a pathogenic role that is similar to other preinvasive lesions of the lower reproductive tract, including the cervix and vagina [55]. Additionally, multiple low-risk and high-risk HPV strains can coexist in the same lesion [44,56,57]. Vulvar condyloma acuminatum is primarily attributed to low-risk HPV 6 and 11 and is not considered to pose a theoretical risk of neoplastic development. Nevertheless, several studies have indicated an indirect association with anogenital, head, and neck cancers due to co-infection with high-risk strains [57,58]. Individuals with compromised immune systems may rarely experience a malignant transformation of anogenital warts, especially in Buschke–Löwenstein tumors [59,60].
Additional risk factors identified in the literature for VHSIL encompass smoking, the number of sexual partners, and immunosuppression resulting from HIV infection, coinfection with herpes simplex virus (HSV), human T-lymphotropic virus type I (HTLV-I) infection, or chronic illnesses [61,62,63,64]. Upon diagnosing a vulvar lesion, it is essential to conduct a comprehensive evaluation that encompasses not only the vulva but also the anal region, vagina, and cervix, as these areas frequently coexist. For instance, in 60% of cases with VaIN (vaginal intraepithelial neoplasia) or VIN, synchronous or pre-existing CIN is identified. It is noteworthy that 10% of patients with CIN 3 may exhibit squamous intraepithelial neoplasm in other sites. Additionally, HPV is detected in more than 94% of VaIN 2/3. These considerations bear significant importance in understanding the recurrence of lesions [65,66,67,68].
The distinct etiology and epidemiology of the two forms of intraepithelial neoplasia also signify their differing pathogenesis. VHSIL is linked with HPV lesions and exhibits a heightened incidence of other anogenital cancers due to their common embryologic origin [67,68,69,70]. Consequently, VHSILs are frequently found to be multicentric and multifocal, thereby accounting for metachronous and synchronous occurrences of cancer across the vulva, anus, cervix, and vagina [70].
Lesions resulting from high-risk HPV are attributable to an immune escape mechanism, as previously shown in other sexually transmitted disorders [71]. This mechanism involves HPV replication within host cells without the production of viral antigens that would typically trigger an immune response. Hence, cytolysis and inflammation are absent due to reduced levels of IFN type-1 induced by the E6 and E7 oncogenes [72]. The downregulation of cytotoxic T cells in the local environment is attributed to the decreased activity of HLA class I, facilitated by the E5 protein. This dynamic allows for the immune system to tolerate the silent viral replication, along with the tumor microenvironment, ultimately contributing to the progression of preneoplastic lesions [73,74]. Less than 5% of VHSILs progress to vulvar cancer, while approximately 9.7% of women under 35 years of age who have been treated for a previous VHSIL develop this condition [70,75].
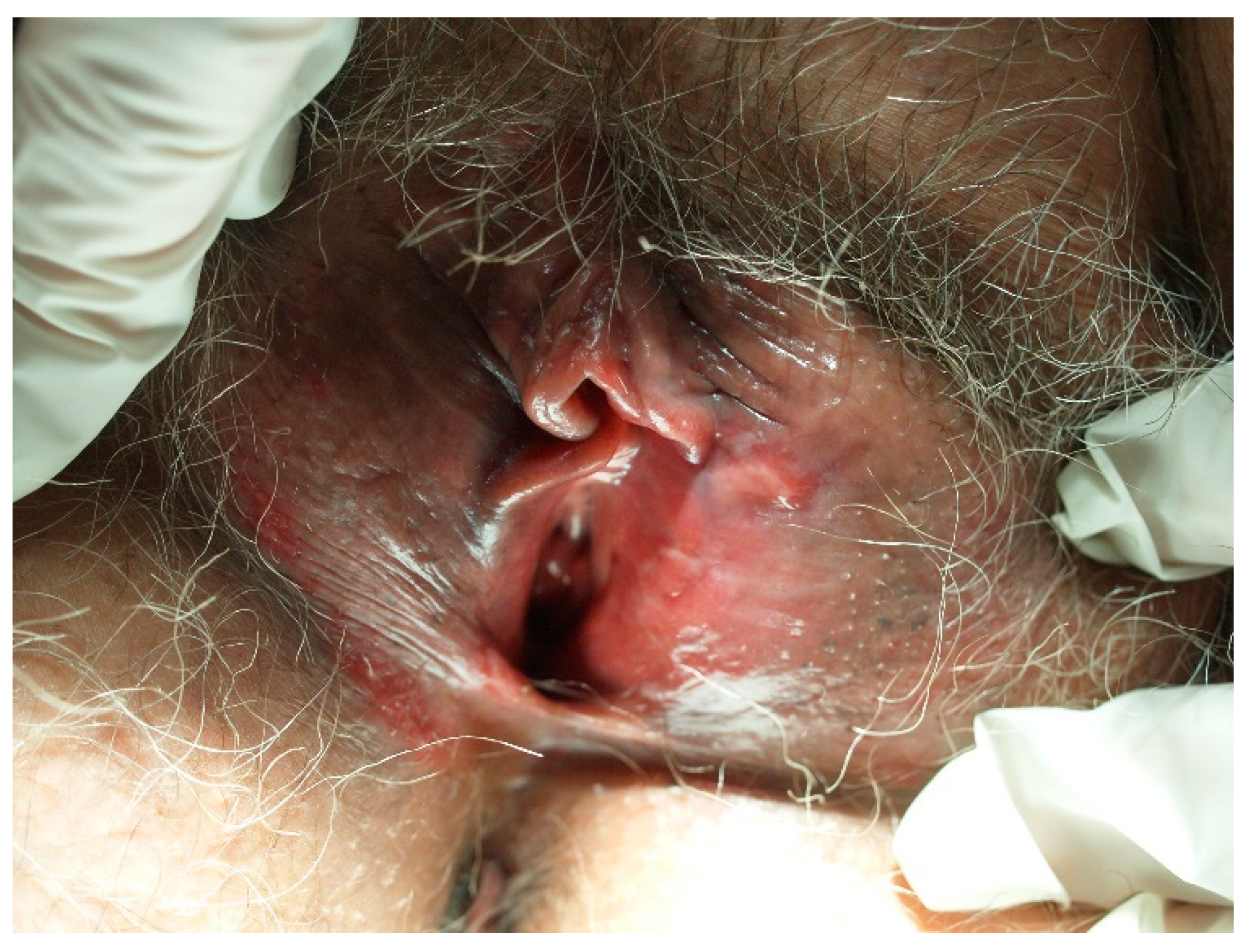
The majority (80%) of keratinized vulvar cancers are preceded by differentiated vulvar intraepithelial neoplasia (dVIN). It is believed that dVIN develops as a result of aberrant cellular functions instigated by genetic oxidative stress. dVIN lesions typically manifest as single, site-specific growths and are often linked to persistent inflammatory conditions, such as lichen sclerosus or lichen planus (Figure 2). Notably, these lesions exhibit an aggressive progression toward SCC. The association of dVIN with lichen sclerosus frequently leads to poorly defined lesions that are resistant to topical therapies. Lichen sclerosus associated with dVIN often precedes the development of vulvar cancer, with the risk of transformation increasing with age [52,61,62,63,76,77,78]. Gallio et al. observed that of the 76 patients with dVIN, more than 80% presented associated lichen sclerosus, while no instances of lichen planus were reported. This observation is in line with the conflicting data on the correlation between lichen planus and VIN [52].
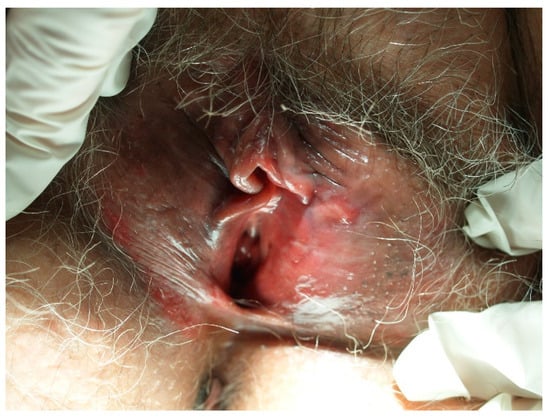
Figure 2.
Vulvar intraepithelial neoplasia (VIN) developed on lichen sclerosus in a postmenopausal patient.
Alongside the conventional TP53 mutagenic pathway [79], the presence of NOTCH1 mutation leading to the loss of the tumor suppressor function and the involvement of the HRAS oncogene in the RTK/RAS/PI(3)K pathway have been demonstrated to contribute to the pathogenesis of dVIN cancers through somatic mutations and aberrant cell proliferation [48,70,80,81].
The clinical presentation of VIN lesions is diverse and lacks pathognomonic features. The presence of abnormal cervical cytology or a positive high-risk HPV test necessitates a thorough examination of the vulvo-perianal region, the pubis, and the lymph nodes, as screening tests are not standardized. VIN lesions may be asymptomatic. However, patients frequently report experiencing superficial dyspareunia or vulvar pain, along with symptoms such as burning, itching, discharge, or bleeding, which may indicate the potential invasion of the lesion. In clinical practice, it is not uncommon to observe various lesion patterns within a single patient. These lesions may exhibit pigmentation and appear white, gray, red, warty, flat, or raised. During the examination, it is imperative to meticulously note the number, shape, topography, size, thickness, and color of each lesion [48,82,83], as presented in Table 2 [83,84,85,86].

Table 2.
Clinical presentations of VIN and main differentials with vulvar lesions.
dVIN lesions are often found in patients with a history of vulvar cancer or the proximity of a vulvar SCC. The lesions can be recognized as white or gray discolorations with a bumpy surface, elevated nodules, or faint thick plaques [48].
Cytology obtained directly from vulvar lesions is hindered by the thick keratinization layer of the vulvar skin, leading to inefficiency. Despite its low specificity, vulvar aceto-whitening demonstrates nearly 100% sensitivity. In a study involving 344 women, Likes et al. established that vulvoscopy, combined with the application of 3–5% acetic acid, exhibits a notably high negative predictive value [87]. This implies that the absence of an aceto-whitening lesion correlates with the absence of a precancerous lesion. Nevertheless, the application of acetic acid to the vulvar area should be performed exclusively by experienced professionals.
In instances of multifocal lesions, vulvar mapping is deemed essential, and dermoscopy serves to assist the clinician in minimizing redundant biopsies. Consequently, efforts are being made to establish dermatoscopic criteria for successful differential diagnosis, both in distinguishing between the severity grades of the lesions and in differentiation from other conditions. In the absence of standardized criteria for assessing the dermatoscopic appearance of vulvar lesions, various authors have published case series utilizing dermoscopy to advocate for the adoption of this practice (Table 3).

Table 3.
Dermoscopy characteristics of VIN.
The differential diagnosis of primary dermoscopy structures/features in VIN is of utmost importance: dotted vessels (extramammary Paget disease, vulvar psoriasis), glomerular vessels (BD), dotted and linear vessels (vulvar lichen sclerosus), cerebriform pattern (vulvar seborrheic keratosis) and parallel pigmented dots with well-demarcated borders (pigmented BD, lentigo) [91,92,93,94,95]. However, there is currently no consensus regarding the dermoscopy diagnosis of VIN. The histopathology examination represents the gold-standard diagnosis in VIN. Table 4 and Table 5 present the pathology features of VHSIL and dVIN [48,82,85,96,97,98].

Table 4.
VIN histopathology characteristics.

Table 5.
Morphologic subsets of dVIN and VHSIL.
4. Penile Intraepithelial Neoplasia (PeIN)
The incidence of penile cancer in Europe and North America is low (<1/106). Most forms of penile cancer are SCC, classified into 12 histopathological forms, among which the keratinized, conventional form predominates [99,100]. Penile SCC is associated with lack of circumcision, low socioeconomic status, smoking, phimosis, poor hygiene, and sexually transmitted infections, especially human immunodeficiency virus, and human papillomavirus [101,102]. Its most common localizations are the glans, mucosal surface of the foreskin, and coronal sulcus of the penis [103].
The terminology associated with in situ penile SCC is intricate. Presently, the term erythroplasia of Queyrat (EQ) is used synonymously with Bowen’s disease of the penile mucosa and holds heightened clinical significance. However, it should be differentiated from the BD of the penile shaft and other non-mucosal genital regions, which should be stratified according to Table 1. Over time, the classification of penile lesions has undergone significant revisions, with the contemporary preference being the concept of penile intraepithelial neoplasia (PeIN), akin to cervical intraepithelial neoplasia (CIN) [104,105]. Consequently, EQ/Bowen’s disease of the penile mucosa is currently regarded as a form of PeIN. Although some authors classify Bowenoid papulosis as a PeIN, it typically follows a benign course and may warrant classification as a distinct entity [106,107].
Lichen planus and lichen sclerosus are the primary pre-existing penile dermatoses strongly associated with PeIN. Additional risk factors consist of preputial diseases (phimosis, paraphimosis, and adherent prepuce), prior penile surgery, use of immunosuppressive drugs, presence of genital warts, history of organ transplantation, and phototherapy. Notably, the uncircumcised state remains the predominant risk factor for PeIN [108,109].
Any enduring brown-gray, red, or white penile papules, plaques, or macules should prompt the suspicion of PeIN. PeIN typically presents as an asymptomatic condition, although pain, bleeding, crusting, and pruritus may manifest. In circumcised men, PeIN may exhibit scales atop a fully keratinized glans. Clinical examination of a suspected penile lesion includes the application of 3–5% acetic acid, although its specificity for PeIN is limited, as only 20% of PeIN lesions yield a positive reaction to acetic acid [110,111].
In clinical practice, it is often difficult to differentiate different forms of PeIN from other dermatoses, and the diagnosis is even more difficult in the case of PeIN lesions developed on pre-existing, chronic, penile lesions. Infectious (herpes progenitalis, fungi, syphilis), inflammatory (seborrheic dermatitis, lichen planus, Zoon’s balanitis, fixed drug eruption, psoriasis, balanitis circinate, etc.), and neoplastic (e.g., extramammary Paget’s disease) dermatoses have to be ruled out [112,113,114,115].
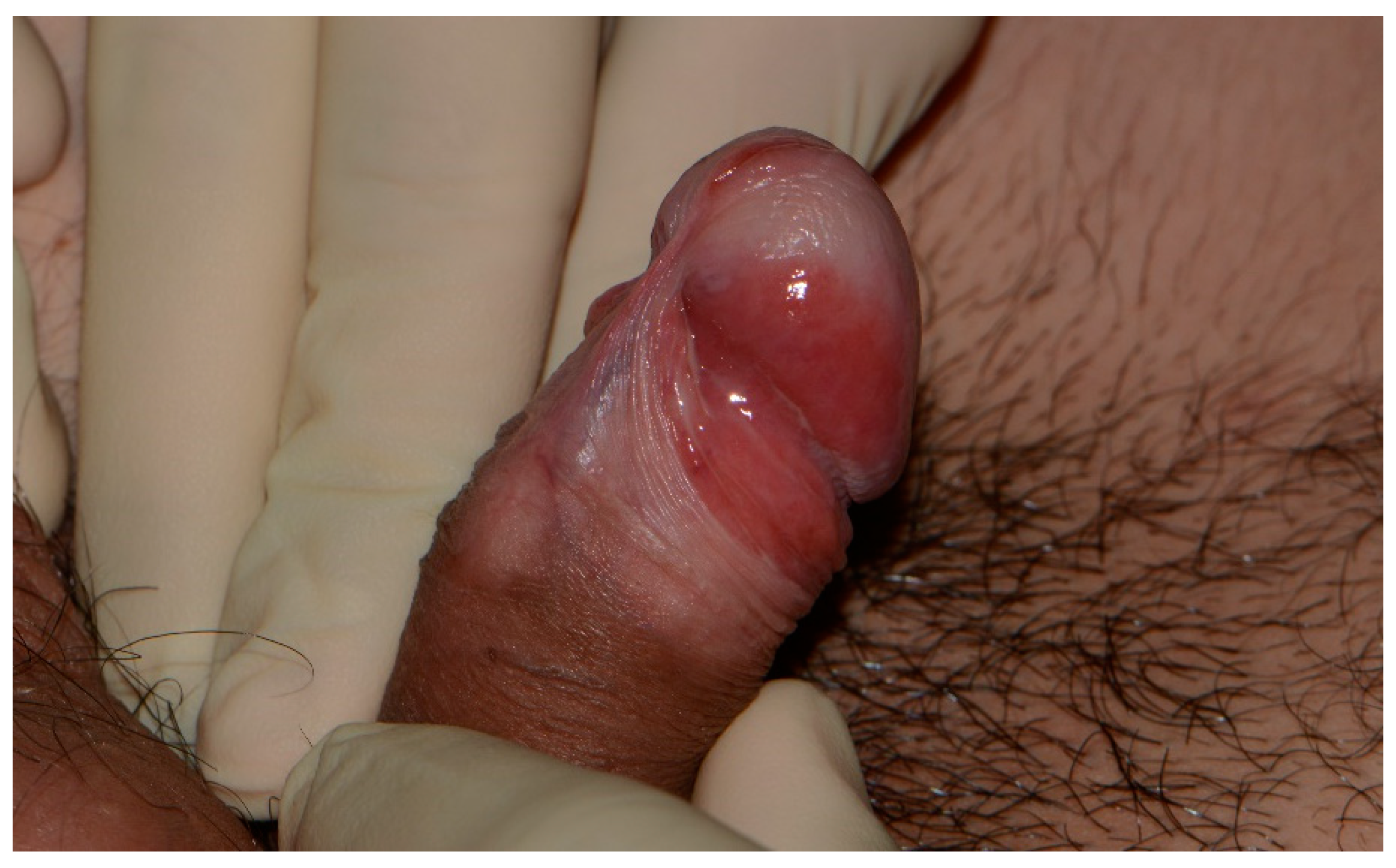
EQ typically manifests as slowly progressive, well-defined, slightly elevated, erythematous plaques on the glans penis, coronal sulcus, and prepuce, predominantly affecting uncircumcised men. Histopathological examination often reveals penile intraepithelial neoplasia (PeIN) and may be managed through local resection, ablative laser treatment, and topical agents such as 5-fluorouracil and TLR-7 agonist (imiquimod). Glansectomy is associated with the lowest recurrence rate [116,117,118]. Unlike the discernible appearance of EQ (Figure 3), the emergence of PeIN on pre-existing skin lesions like lichen planus and lichen sclerosus may pose diagnostic challenges.
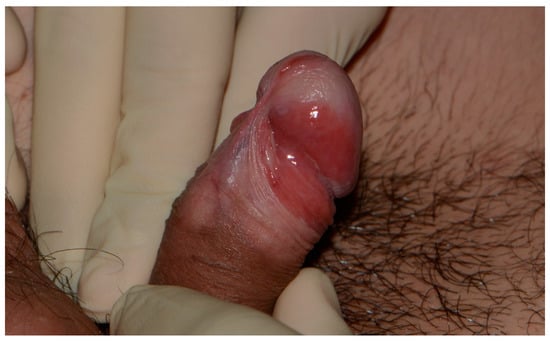
Figure 3.
Erythroplasia of Queyrat involving the glans, coronal sulcus, and prepuce. Glans etiolation, frenulum sclerosis, and the waxy pallor of the distal prepuce are suggestive of an associated lichen sclerosus.
Genital lichen planus has a higher incidence in men, affecting 25% of individuals diagnosed with lichen planus. It manifests with annular (most commonly on the glans), reticular, erosive, bullous, and atrophic appearances. Progressive developments may include PeIN, penile SCC, stenosis, and synechiae [119]. Noteworthy changes in a lichenoid clinical lesion such as ulceration, pain, or the growth of a mass may indicate the potential development of PeIN or SCC [120].
Penile lichen sclerosus predominantly affects men aged 30–40, with an estimated incidence rate of up to 0.3%. It commonly involves the meatus, foreskin, penile shaft, and glans penis and is frequently a disease of the uncircumcised, due to the chronic exposure of the epithelium to urine (urinary dribbling/microincontinence). Initially, it manifests as an erythematous plaque, progressing to atrophy and depigmentation, often presenting a pearly appearance, and demonstrating proclivity toward scarring and erosion [121]. The contentious association between lichen sclerosus and HPV and Borrelia burgdorferi infections is subject to ongoing debate. Studies have not found a significant correlation between lichen sclerosus and HPV infection, with the association with HPV-16 considered more incidental than causal. Additionally, most research did not yield evidence of Borrelia DNA in skin biopsy specimens. Treatments for penile lichen sclerosus encompass surgical interventions and conservative modalities such as topical steroids, intralesional steroids, and platelet-rich plasma injections. Of note, it recurs in skin grafts. Left untreated, lichen sclerosus may advance to PeIN or SCC. Its malignant transformation risk is variable, estimated between 4 and 13.4%, while 12% of penile SCC is due to lichen sclerosus. Notably, differentiated PeIN prevalence surpasses that of undifferentiated subtypes among lichen sclerosus patients [121,122,123]. Pseudoepitheliomatous keratotic and micaceous balanitis (PEKMB) represents a chronic and unstable form of lichen sclerosus with a significant potential to develop into an SCC. It clinically presents as silvery scales on the glans in patients with an undiagnosed or treatment-refractory lichen sclerosus [124].
HPV-associated PeIN is associated with the viral genome integration into the human genome, which leads to oncogene overexpression and malignant transformation [125], as in VIN. HPV types 6 and 11 are more frequent in condyloma lesions and subtypes 6, 11, and 18 in the PeINs. However, less than 1% of the penile HPV progress to PeIN, with a median time from infection of 12.7 months [126].
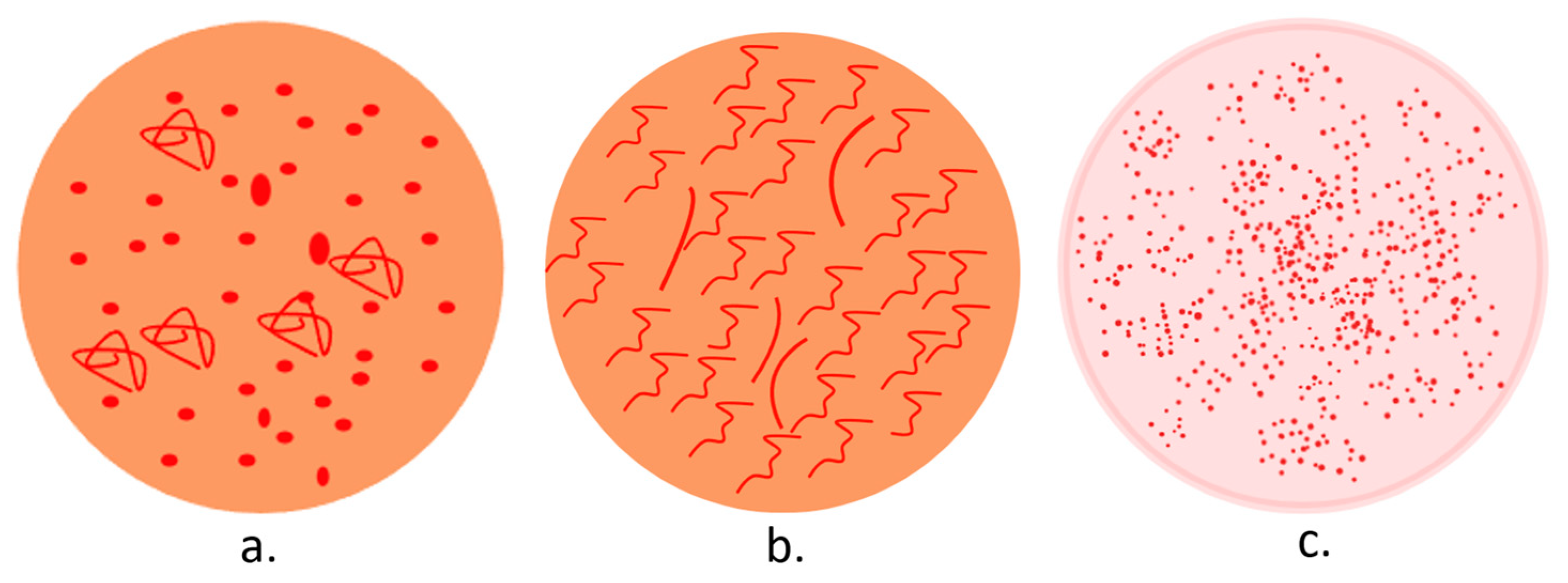
A dermatoscopic examination of any penile lesion suspected to be PeIN can provide numerous diagnostic clues. In addition, it can facilitate a dermoscopy-guided biopsy and differential diagnoses. In dermoscopy (Figure 4), PeIN displays an orange structureless area and a vascular pattern, comprising nonhomogeneous dotted (predominantly) and glomerular vessels. Its main differential, Zoon balanitis, presents an orange structureless area and linear-curved vessels, while glans psoriasis reveals diffuse and homogeneous dotted vessels on an erythematous background [127,128,129].
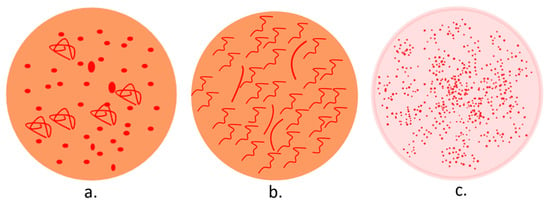
Figure 4.
Dermoscopy patterns in PeIN (a), Zoon balanitis (b), and penile psoriasis (c).
From a histopathologic point of view, PeIN represents a dysplastic modification of the epithelium that does not affect the basement membrane, which remains intact [106]. Nowadays, PeIN is classified into differentiated and undifferentiated morphologic subtypes, which correlate with HPV infection status [130]. Table 6 displays the main PeIN subtypes according to pathology features and HPV infection [112,124,130,131,132,133].

Table 6.
Histopathology characteristics of PeIN.
5. Current Treatments in BD, VIN, and PeIN
The effective management of BD involves the utilization of a diverse range of treatment modalities customized to the specific characteristics of the lesions, the patient’s health condition, and their individual preferences. Commonly employed topical therapies, such as 5-fluorouracil and imiquimod, demonstrate efficacy in targeting atypical keratinocytes. Furthermore, curettage, electrodesiccation, and cryotherapy are viable options for treating small, superficial lesions, while surgical excision with histological margin control stands as the gold standard for ensuring the complete removal of larger or recurrent lesions. Laser therapy is a non-invasive alternative suitable for cosmetically sensitive areas, while radiotherapy is specifically reserved for challenging lesion locations or patients ineligible for surgery [29,32,33,134,135,136].
The objective of VIN treatment is the prevention of recurrent invasive cancer and the enhancement of the patient’s quality of life through the amelioration of symptoms and functionality of the vulvar region. Additionally, careful consideration of a low-risk treatment approach is essential [137]. The treatments for dVIN and VHSIL share similarities, yet each possesses distinct nuances in their methodologies. The loop electrosurgical excision procedure and cold-knife surgical excision are highly recommended for both VIN subtypes, preferably with 4–5 mm deep and lateral margins. Considering the higher recurrence rate in dVIN, wide surgical excision becomes warranted. Following dVIN excision, topical high-potency steroids should be applied to the remaining lichen sclerosus lesions. As for alternative therapies, topical imiquimod and 5-fluorouracil are particularly relevant for VHSIL [70,138,139,140]. The presence of HPV-16 infection, together with small lesions, correlates with a favorable therapeutic outcome and a reduced likelihood of recurrence after topical imiquimod therapy. Conversely, multifocal lesions and dVIN are indicative of a less-favorable response to imiquimod treatment [141,142]. Of note, CO2 laser therapy should be avoided on hair-bearing skin in VIN patients [142].
In the management of PeIN, circumcision plays a significant role, as it facilitates the removal of potentially neoplastic tissue and may enhance the subsequent application of topical treatments. Local therapy options include topicals like 5-fluorouracil, imiquimod, cryosurgery, and CO2 lasers. Combining local treatments has been associated with lower recurrence rates than monotherapy, akin to other precancerous/cancerous dermatoses. Surgical excision with penile sparing is recommended for relapsing/non-responsive lesions [143,144,145]. However, the surgical management of PeIN may result in a recurrence in up to 30% of cases. Circumcision commonly resolves all preputial PeIN. Following surgical intervention for PeIN of the glans, recurrence rates are as follows: 25% after wide local excision, 4% after Mohs surgery, 5% after total glans resurfacing, and 10% after glansectomy [146]. In a study conducted by Kravvas et al., it was revealed that among 345 patients diagnosed with PeIN, 8.7% exhibited concomitant SCC. Furthermore, 58% of these patients were found to have an association with HPV, while 12% showed indications of lichen sclerosus. Interestingly, 29.4% of the patients displayed a co-occurrence of both HPV and lichen sclerosus. The study’s findings suggest that a sole reliance on topical treatments is unsatisfactory, with less than 15% of patients demonstrating the potential for successful treatment using topical agents alone [147].
6. Conclusions
The clinical, histological, and immunohistochemical profile of BD, including markers such as PCNA, CK10, CK14, p16, p53, Ki-67, and p27, provide critical insights for its diagnosis and differentiation from other skin lesions. Diagnosing VIN and PeIN poses considerable challenges and typically entails one or more biopsies, potentially guided by dermoscopy and aceto-white testing. The vascular pattern against an orange background facilitates the differentiation of PeIN from other penile dermatoses, whereas dermoscopy in VIN often exhibits less distinct characteristics. Immunohistochemical and HPV tests assist in guiding the choice of therapies for the treatment of VIN and PeIN. The management of these cases is further complicated by the frequent association with lichen sclerosus, which perturbs the local anatomy and is linked to an elevated risk of recurrence following surgical excision.
The early diagnosis of BD, VIN, and PeIN is crucial due to their potential for malignant transformation and significant impact on patient outcomes. Detecting these early SCCs at an initial stage enables timely and effective treatment, reducing the risk of progression to invasive SCC. Early intervention not only improves prognosis but also minimizes the likelihood of metastasis and associated complications.
Author Contributions
Conceptualization, L.G.S., F.S., S.C.D. and O.S.; writing—original draft preparation, L.G.S., F.S. and S.C.D.; writing—review and editing, L.G.S. and O.S.; visualization, L.G.S. and O.S.; supervision, O.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Muzic, J.G.; Schmitt, A.R.; Wright, A.C.; Alniemi, D.T.; Zubair, A.S.; Olazagasti Lourido, J.M.; Sosa Seda, I.M.; Weaver, A.L.; Baum, C.L. Incidence and Trends of Basal Cell Carcinoma and Cutaneous Squamous Cell Carcinoma: A Population-Based Study in Olmsted County, Minnesota, 2000 to 2010. Mayo Clin. Proc. 2017, 92, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L.; Weinstock, M.A. Nonmelanoma skin cancer in the United States: Incidence. J. Am. Acad. Dermatol. 1994, 30, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Brougham, N.D.; Tan, S.T. The incidence and risk factors of metastasis for cutaneous squamous cell carcinoma—Implications on the T-classification system. J. Surg. Oncol. 2014, 110, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Taccioli, F.; Blessent, C.G.; Paganelli, A.; Fagioli, F.; Chester, J.M.; Kaleci, S.; Costantini, M.; Ferrari, B.; Fiorentini, C.; De Santis, G.; et al. Delay in Cutaneous Squamous Cell Carcinoma Diagnosis Due to Interrupted Services Is Associated with Worse Prognoses and Modified Surgical Approaches. Cancers 2023, 16, 1469. [Google Scholar] [CrossRef]
- Sajin, M.; Luchian, M.C.; Hodorogea Prisăcaru, A.; Dumitru, A.; Pătraşcu, O.M.; Costache, D.; Dumitrescu, D.; Oproiu, A.M.; Simionescu, O.; Costache, M. Trichilemmal carcinoma—A rare cutaneous malignancy: Report of two cases. Rom. J. Morphol. Embryol. 2014, 55 (Suppl. S2), 687–691. [Google Scholar] [PubMed]
- Pai, K.; Shetty, S.; Padmapriya, J.; Pai, S.; Rao, L. Acantholytic Variant of Bowen’s Disease with Micro-invasive Squamous Cell Carcinoma: A Case Report of a Unique Variant. Indian J. Dermatol. 2014, 59, 635. [Google Scholar] [CrossRef] [PubMed]
- WHO. Classification of Female Genital Tumours and Tumours of the Urinary System and Male Genitals, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2022. [Google Scholar]
- Young, T.K.; Gutierrez, D.; Zampella, J.G. An Overview of Penile and Scrotal Dermatoses. Urology 2020, 142, 14–21. [Google Scholar] [CrossRef]
- Shimizu, A.; Takahashi, A.; Ishikawa, O. Bowen’s disease involving the urethra. J. Dermatol. 2005, 32, 210–213. [Google Scholar]
- Ferrándiz, C.; Malvehy, J.; Guillén, C.; Ferrándiz-Pulido, C.; Fernández-Figueras, M. Precancerous Skin Lesions. Precáncer cutáneo. Actas Dermosifiliogr. 2017, 108, 31–41. [Google Scholar] [CrossRef]
- Palaniappan, V.; Karthikeyan, K. Bowen’s Disease. Indian Dermatol. Online J. 2022, 13, 177–189. [Google Scholar] [CrossRef]
- Dandale, A.; Mantri, M.D.; Thakkar, V.; Dhurat, R.S.; Ghate, S. Bowen’s disease: An unusual clinical presentation. Indian Dermatol. Online J. 2014, 5, 526–528. [Google Scholar] [CrossRef]
- Shabbir, M.; Minhas, S.; Muneer, A. Diagnosis and management of premalignant penile lesions. Ther. Adv. Urol. 2011, 3, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Neagu, T.P.; Ţigliş, M.; Botezatu, D.; Enache, V.; Cobilinschi, C.O.; Vâlcea-Precup, M.S.; GrinŢescu, I.M. Clinical, histological and therapeutic features of Bowen’s disease. Rom. J. Morphol. Embryol. 2017, 58, 33–40. [Google Scholar]
- Grossman, D.; Leffell, D.J. The molecular basis of nonmelanoma skin cancer: New understanding. Arch. Dermatol. 1997, 133, 1263–1270. [Google Scholar] [CrossRef]
- Lellis, R.F.; Veasey, J.V.; Gonçalves, R.D.J. Pigmented Bowen’s disease associated with high-risk HPV simulating melanoma of the hand. An. Bras. Dermatol. 2017, 92, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, H.; Gharaei Nejad, K.; Azimi, S.Z.; Rafiei, R.; Mesbah, A. Bowen’s Disease Associated With Two Human Papilloma Virus Types. Acta Med. Iran. 2017, 55, 594–596. [Google Scholar]
- Grundmeier, N.; Hamm, H.; Weissbrich, B.; Lang, S.C.; Bröcker, E.B.; Kerstan, A. High-risk human papillomavirus infection in Bowen’s disease of the nail unit: Report of three cases and review of the literature. Dermatology 2011, 223, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Kossard, S.; Rosen, R. Cutaneous Bowen’s disease. An analysis of 1001 cases according to age, sex, and site. J. Am. Acad. Dermatol. 1992, 27, 406–410. [Google Scholar] [CrossRef]
- Barrutia, L.; Martínez-García, G.; Santamarina-Albertos, A.; Garabito Solovera, E.L.; Volo, V.; Ruíz-Sánchez, D.; Manchado López, P. Differentiating pagetoid Bowen disease from Paget disease on the nipple-areola complex: Two unique, challenging cases. J. Cutan. Pathol. 2021, 48, 1416–1422. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, J.; Fang, S.; Han, S.; Song, Z. What’s new in dermoscopy of Bowen’s disease: Two new dermoscopic signs and its differential diagnosis. Int. J. Dermatol. 2017, 56, 1022–1025. [Google Scholar] [CrossRef]
- Blum, A.; Simionescu, O.; Argenziano, G.; Braun, R.; Cabo, H.; Eichhorn, A.; Kirchesch, H.; Malvehy, J.; Marghoob, A.A.; Puig, S.; et al. Dermoscopy of pigmented lesions of the mucosa and the mucocutaneous junction: Results of a multicenter study by the International Dermoscopy Society (IDS). Arch. Dermatol. 2011, 147, 1181–1187. [Google Scholar] [CrossRef]
- Fernández-Sánchez, M.; Charli-Joseph, Y.; Domínguez-Cherit, J.; Guzman-Herrera, S.; Reyes-Terán, G. Acral and Multicentric Pigmented Bowen’s Disease in HIV-Positive Patients: Report on Two Unusual Cases. Indian J. Dermatol. 2018, 63, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Idriss, M.H.; Misri, R.; Böer-Auer, A. Orthokeratotic Bowen disease: A histopathologic, immunohistochemical and molecular study. J. Cutan. Pathol. 2016, 43, 24–31. [Google Scholar] [CrossRef]
- Zalaudek, I.; Argenziano, G.; Leinweber, B.; Citarella, L.; Hofmann-Wellenhof, R.; Malvehy, J.; Puig, S.; Pizzichetta, M.A.; Thomas, L.; Soyer, H.P.; et al. Dermoscopy of Bowen’s disease. Br. J. Dermatol. 2004, 150, 1112–1116. [Google Scholar] [CrossRef]
- Mota, A.N.; Piñeiro-Maceira, J.; Alves Mde, F.; Tarazona, M.J. Pigmented Bowen’s disease. An. Bras. Dermatol. 2014, 89, 825–827. [Google Scholar] [CrossRef] [PubMed]
- Torre-Castro, J.; Nájera, L.; Salgüero, I.; Requena, L. Bowen Disease Within a Circumscribed Palmar Hypokeratosis. Am. J. Dermatopathol. 2022, 44, 961–963. [Google Scholar] [CrossRef]
- Victoria-Martínez, A.M.; Martínez-Leborans, L.; Ortiz-Salvador, J.M.; Pérez-Ferriols, A. Treatment of Bowen Disease With Photodynamic Therapy and the Advantages of Sequential Topical Imiquimod. Tratamiento de la enfermedad de Bowen con terapia fotodinámica y ventajas de la aplicación secuencial de imiquimod tópico. Actas Dermosifiliogr. 2017, 108, e9–e14. [Google Scholar] [CrossRef]
- Ball, S.B.; Dawber, R.P. Treatment of cutaneous Bowen’s disease with particular emphasis on the problem of lower leg lesions. Australas. J. Dermatol. 1998, 39, 63–70. [Google Scholar] [CrossRef]
- Cox, N.H.; Eedy, D.J.; Morton, C.A. Therapy Guidelines and Audit Subcommittee, British Association of Dermatologists. Guidelines for management of Bowen’s disease: 2006 update. Br. J. Dermatol. 2007, 156, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Arlette, J.P.; Trotter, M.J. Squamous cell carcinoma in situ of the skin: History, presentation, biology and treatment. Australas. J. Dermatol. 2004, 45, 1–11. [Google Scholar] [CrossRef]
- Sharma, A.; Birnie, A.J.; Bordea, C.; Cheung, S.T.; Mann, J.; Morton, C.A.; Salim, A.; Hasan, Z.U.; Hashme, M.; Mansour Kiaee, Z.; et al. British Association of Dermatologists guidelines for the management of people with cutaneous squamous cell carcinoma in situ (Bowen disease) 2022. Br. J. Dermatol. 2023, 188, 186–194. [Google Scholar] [CrossRef]
- O’Connell, K.A.; Okhovat, J.P.; Zeitouni, N.C. Photodynamic therapy for Bowen’s Disease (squamous cell carcinoma in situ) current review and update. Photodiagnosis Photodyn. Ther. 2018, 24, 109–114. [Google Scholar] [CrossRef]
- Nasiri, S.; Azhari, V.; Bidari-Zerehpoosh, F.; Asadi-Kani, Z.; Talebi, A. The diagnostic value of p63, p16, and p53 immunohistochemistry in distinguishing seborrheic keratosis, actinic keratosis, and Bowen’s disease. Dermatol. Ther. 2021, 34, e14817. [Google Scholar] [CrossRef]
- Goto, H.; Sugita, K.; Horie, T.; Yamamoto, O. Ultrastructural and morphological analysis during progression of Bowen disease reveals a complex interplay between hyperkeratosis, cytokeratin expression, host immunity and amyloid deposition. Eur. J. Dermatol. 2023, 33, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Bahrani, E.; Sitthinamsuwan, P.; McCalmont, T.H.; Pincus, L.B. Ki-67 and p16 Immunostaining Differentiates Pagetoid Bowen Disease From “Microclonal” Seborrheic Keratosis. Am. J. Clin. Pathol. 2019, 151, 551–560. [Google Scholar] [CrossRef]
- Alhumaidi, A. Practical immunohistochemistry of epithelial skin tumor. Indian J. Dermatol. Venereol. Leprol. 2012, 78, 698–708. [Google Scholar] [CrossRef]
- Hudelo, M.L.; Oury, C.; Cailliau, M. Dyskeratose erythroplasiforme de la muqueuse vulvaire. Bull. Soc. Franc. Dermatol. Syph. 1922, 29, 139–142. [Google Scholar]
- Wilkinson, E.J.; Cox, J.T.; Selim, M.A.; O’Connor, D.M. Evolution of terminology for human-papillomavirus-infection-related vulvar squamous intraepithelial lesions. J. Low. Genit. Tract Dis. 2015, 19, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, J.; Bogliatto, F.; Haefner, H.K.; Stockdale, C.K.; Preti, M.; Bohl, T.G.; Reutter, J.; ISSVD Terminology Committee. The 2015 International Society for the Study of Vulvovaginal Disease (ISSVD) Terminology of Vulvar Squamous Intraepithelial Lesions. Obstet. Gynecol. 2016, 127, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Sideri, M.; Jones, R.W.; Wilkinson, E.J.; Preti, M.; Heller, D.S.; Scurry, J.; Haefner, H.; Neill, S. Squamous vulvar intraepithelial neoplasia: 2004 modified terminology, ISSVD Vulvar Oncology Subcommittee. J. Reprod. Med. 2005, 50, 807–810. [Google Scholar] [CrossRef]
- Crum, C.P.; Fu, Y.S.; Levine, R.U.; Richart, R.M.; Townsend, D.E.; Fenoglio, C.M. Intraepithelial squamous lesions of the vulva: Biologic and histologic criteria for the distinction of condylomas from vulvar intraepithelial neoplasia. Am. J. Obstet. Gynecol. 1982, 144, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Darragh, T.M.; Colgan, T.J.; Thomas Cox, J.; Heller, D.S.; Henry, M.R.; Luff, R.D.; McCalmont, T.; Nayar, R.; Palefsky, J.M.; Stoler, M.H.; et al. The Lower Anogenital Squamous Terminology Standardization project for HPV-associated lesions: Background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Int. J. Gynecol. Pathol. 2013, 32, 76–115, Erratum in Int. J. Gynecol. Pathol. 2013, 32, 432. Erratum in Int. J. Gynecol. Pathol. 2013, 32, 241. [Google Scholar] [CrossRef] [PubMed]
- Srodon, M.; Stoler, M.H.; Baber, G.B.; Kurman, R.J. The distribution of low risk and high-risk types in vulvar and vaginal intraepithelial neoplasia (VIN and VaIN). Am. J. Surg. Pathol. 2006, 30, 1513–1518. [Google Scholar] [CrossRef]
- Sideri, M.; Jones, R.W.; Heller, D.S.; Haefner, H.; Neill, S.; Preti, M.; Scurry, J.; Wilkinson, E.J.; Edwards, L. Comment on the Article: Srodon M, Stoler MH, Baber GB; et al. The distribution of low and high-risk HPV types in vulvar and vaginal intraepithelial neoplasia (VIN and VaIN) Am J Surg Pathol. 2006;30:1513-1518. Am. J. Surg. Pathol. 2007, 31, 1452, author reply 1452–1454. [Google Scholar] [CrossRef] [PubMed]
- Thuijs, N.B.; van Beurden, M.; Bruggink, A.H.; Steenbergen, R.D.M.; Berkhof, J.; Bleeker, M.C.G. Vulvar intraepithelial neoplasia: Incidence and long-term risk of vulvar squamous cell carcinoma. Int. J. Cancer 2021, 148, 90–98. [Google Scholar] [CrossRef]
- Castle, P.E.; Maza, M. Prophylactic HPV vaccination: Past, present, and future. Epidemiol. Infect. 2016, 144, 449–468, Erratum in Epidemiol. Infect. 2016, 144, 2472. [Google Scholar] [CrossRef]
- Jin, C.; Liang, S. Differentiated Vulvar Intraepithelial Neoplasia: A Brief Review of Clinicopathologic Features. Arch. Pathol. Lab. Med. 2019, 143, 768–771. [Google Scholar] [CrossRef]
- Judson, P.L.; Habermann, E.B.; Baxter, N.N.; Durham, S.B.; Virnig, B.A. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet. Gynecol. 2006, 107, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Colgan, T.J. Vulvar intraepithelial neoplasia: A synopsis of recent developments. J. Low. Genit. Tract Dis. 1998, 2, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Joura, E.A.; Lösch, A.; Haider-Angeler, M.G.; Breitenecker, G.; Leodolter, S. Trends in vulvar neoplasia. Increasing incidence of vulvar intraepithelial neoplasia and squamous cell carcinoma of the vulva in young women. J. Reprod. Med. 2000, 45, 613–615. [Google Scholar]
- Gallio, N.; Preti, M.; Jones, R.W.; Borella, F.; Woelber, L.; Bertero, L.; Urru, S.; Micheletti, L.; Zamagni, F.; Bevilacqua, F.; et al. Differentiated vulvar intraepithelial neoplasia long-term follow up and prognostic factors: An analysis of a large historical cohort. Acta Obstet. Gynecol. Scand. 2024, 103, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Garland, S.M.; Joura, E.A.; Ault, K.A.; Bosch, F.X.; Brown, D.R.; Castellsagué, X.; Ferenczy, A.; Ferris, D.G.; Giuliano, A.R.; Hernandez-Avila, M.; et al. Human Papillomavirus Genotypes From Vaginal and Vulvar Intraepithelial Neoplasia in Females 15-26 Years of Age. Obstet. Gynecol. 2018, 132, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Léonard, B.; Kridelka, F.; Delbecque, K.; Goffin, F.; Demoulin, S.; Doyen, J.; Delvenne, P. A clinical and pathological overview of vulvar condyloma acuminatum, intraepithelial neoplasia, and squamous cell carcinoma. Biomed. Res. Int. 2014, 2014, 480573. [Google Scholar] [CrossRef] [PubMed]
- Maniar, K.P.; Ronnett, B.M.; Vang, R.; Yemelyanova, A. Coexisting high-grade vulvar intraepithelial neoplasia (VIN) and condyloma acuminatum: Independent lesions due to different HPV types occurring in immunocompromised patients. Am. J. Surg. Pathol. 2013, 37, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Insinga, R.P.; Liaw, K.L.; Johnson, L.G.; Madeleine, M.M. A systematic review of the prevalence and attribution of human papillomavirus types among cervical, vaginal, and vulvar precancers and cancers in the United States. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 1611–1622. [Google Scholar] [CrossRef] [PubMed]
- Garland, S.M.; Steben, M.; Sings, H.L.; James, M.; Lu, S.; Railkar, R.; Barr, E.; Haupt, R.M.; Joura, E.A. Natural history of genital warts: Analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J. Infect. Dis. 2009, 199, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Barzon, L.; Militello, V.; Pagni, S.; Franchin, E.; Dal Bello, F.; Mengoli, C.; Palù, G. Distribution of human papillomavirus types in the anogenital tract of females and males. J. Med. Virol. 2010, 82, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Venter, F.; Heidari, A.; Viehweg, M.; Rivera, M.; Natarajan, P.; Cobos, E. Giant Condylomata Acuminata of Buschke-Lowenstein Associated with Paraneoplastic Hypercalcemia. J. Investig. Med. High Impact Case Rep. 2018, 6, 2324709618758348. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Cheng, C.E.; Kroshinsky, D. Combination Systemic Fluorouracil and Radiation for the Treatment of Recalcitrant Condyloma with Associated Squamous Cell Carcinoma in an Immunocompromised 15-Year-Old Girl. Pediatr. Dermatol. 2015, 32, e148–e150. [Google Scholar] [CrossRef]
- Bornstein, J.; Kaufman, R.H.; Adam, E.; Adler-Storthz, K. Multicentric intraepithelial neoplasia involving the vulva. Clinical features and association with human papillomavirus and herpes simplex virus. Cancer 1988, 62, 1601–1604. [Google Scholar] [CrossRef]
- Sykes, P.; Smith, N.; McCormick, P.; Frizelle, F.A. High-grade vulval intraepithelial neoplasia (VIN 3): A retrospective analysis of patient characteristics, management, outcome and relationship to squamous cell carcinoma of the vulva 1989–1999. Aust. N. Z. J. Obstet. Gynaecol. 2002, 42, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Del Pino, M.; Rodriguez-Carunchio, L.; Ordi, J. Pathways of vulvar intraepithelial neoplasia and squamous cell carcinoma. Histopathology 2013, 62, 161–175. [Google Scholar] [CrossRef]
- Blas, M.M.; Alva, I.E.; Garcia, P.J.; Carcamo, C.; Montano, S.M.; Muñante, R.; Zunt, J.R. Association between human papillomavirus and human T-lymphotropic virus in indigenous women from the Peruvian Amazon. PLoS ONE 2012, 7, e44240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Xia, R.; Chen, D.; Zhang, X. Analysis of related factors of cervical intraepithelial neoplasia complicated with vaginal intraepithelial neoplasia. Clin. Transl. Oncol. 2022, 24, 902–908. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Y. Clinical characteristics, HPV involvement, and demographic risk factors in women with cervical intraepithelial neoplasia complicated by vaginal intraepithelial neoplasia. BMC Womens Health 2024, 24, 220. [Google Scholar] [CrossRef] [PubMed]
- Preti, M.; Boldorini, R.; Gallio, N.; Cavagnetto, C.; Borella, F.; Pisapia, E.; Ribaldone, R.; Bovio, E.; Bertero, L.; Airoldi, C.; et al. Human papillomavirus genotyping in high-grade vaginal intraepithelial neoplasia: A multicentric Italian study. J. Med. Virol. 2024, 96, e29474. [Google Scholar] [CrossRef]
- Hørding, U.; Junge, J.; Poulsen, H.; Lundvall, F. Vulvar intraepithelial neoplasia III: A viral disease of undetermined progressive potential. Gynecol. Oncol. 1995, 56, 276–279. [Google Scholar] [CrossRef]
- Østergård, S.; Vorbeck, C.S.; Meinert, M. Vulvar intraepithelial neoplasia. Ugeskr. Læger 2018, 180, V12170931. (In Danish) [Google Scholar]
- Preti, M.; Scurry, J.; Marchitelli, C.E.; Micheletti, L. Vulvar intraepithelial neoplasia. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 1051–1062. [Google Scholar] [CrossRef]
- Scurtu, L.G.; Jinga, V.; Simionescu, O. Fascinating Molecular and Immune Escape Mechanisms in the Treatment of STIs (Syphilis, Gonorrhea, Chlamydia, and Herpes Simplex). Int. J. Mol. Sci. 2022, 23, 3550. [Google Scholar] [CrossRef]
- Jain, M.; Yadav, D.; Jarouliya, U.; Chavda, V.; Yadav, A.K.; Chaurasia, B.; Song, M. Epidemiology, Molecular Pathogenesis, Immuno-Pathogenesis, Immune Escape Mechanisms and Vaccine Evaluation for HPV-Associated Carcinogenesis. Pathogens 2023, 12, 1380. [Google Scholar] [CrossRef]
- Sasagawa, T.; Takagi, H.; Makinoda, S. Immune responses against human papillomavirus (hpv) infection and evasion of host defense in cervical cancer. J. Infect. Chemother. 2012, 18, 807–815. [Google Scholar] [CrossRef]
- Schröer, N.; Pahne, J.; Walch, B.; Wickenhauser, C.; Smola, S. Molecular pathobiology of human cervical high-grade lesions: Paracrine stat3 activation in tumor-instructed myeloid cells drives local mmp-9 expression. Cancer Res. 2011, 71, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Kesterson, J.P.; Lele, S. Vulvar Intraepithelial Neoplasia 3 in Women Less than 35 Years. J. Low. Genit. Tract. Dis. 2009, 13, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, E.J. Normal histology and nomenclature of the vulva, and malignant neoplasms, including VIN. Dermatol. Clin. 1992, 10, 283–296. [Google Scholar] [CrossRef]
- Roma, A.A.; Hart, W.R. Progression of simplex (differentiated) vulvar intraepithelial neoplasia to invasive squamous cell carcinoma: A prospective case study confirming its precursor role in the pathogenesis of vulvar cancer. Int. J. Gynecol. Pathol. 2007, 26, 248–253. [Google Scholar] [CrossRef]
- Ayala, M.; Fatehi, M. Vulvar Intraepithelial Neoplasia. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Trietsch, M.D.; Nooij, L.S.; Gaarenstroom, K.N.; van Poelgeest, M.I. Genetic and epigenetic changes in vulvar squamous cell carcinoma and its precursor lesions: A review of the current literature. Gynecol. Oncol. 2015, 136, 143–157. [Google Scholar] [CrossRef]
- Nooij, L.S.; Ter Haar, N.T.; Ruano, D.; Rakislova, N.; van Wezel, T.; Smit, V.T.H.B.M.; Trimbos, B.J.B.M.Z.; Ordi, J.; van Poelgeest, M.I.E.; Bosse, T. Genomic Characterization of Vulvar (Pre)cancers Identifies Distinct Molecular Subtypes with Prognostic Significance. Clin. Cancer Res. 2017, 23, 6781–6789. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, A.; Samantha, S.T.; Brar, H.; Sadownik, L.A.; Proctor, L. A Systematic Review of Risk Factors for Development, Recurrence, and Progression of Vulvar Intraepithelial Neoplasia. J. Low. Genit. Tract. Dis. 2022, 26, 140–146. [Google Scholar] [CrossRef]
- Hoang, L.N.; Park, K.J.; Soslow, R.A.; Murali, R. Squamous precursor lesions of the vulva: Current classification and diagnostic challenges. Pathology 2016, 48, 291–302. [Google Scholar] [CrossRef]
- Kesić, V.; Vieira-Baptista, P.; Stockdale, C.K. Early Diagnostics of Vulvar Intraepithelial Neoplasia. Cancers 2022, 14, 1822. [Google Scholar] [CrossRef] [PubMed]
- Heller, D.S. Pigmented vulvar lesions—A pathology review of lesions that are not melanoma. J. Low. Genit. Tract. Dis. 2013, 17, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Ahuja, S.; Kalwaniya, D.S.; Shamsunder, S.; Solanki, S. Vulval premalignant lesions: A review article. Obstet. Gynecol. Sci. 2024, 67, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, A.V.; Țăpoi, D.A.; Costache, M.; Ciongariu, A.M.; Ionescu, A.I.; Liscu, H.D.; Alius, C.; Tampa, M.; Marin, A.; Furtunescu, A.R. Metastatic Nodular Melanoma with Angiosarcomatous Transdifferentiation-A Case Report and Review of the Literature. Diagnostics 2024, 14, 1323. [Google Scholar] [CrossRef] [PubMed]
- Santoso, J.T.; Likes, W. Colposcopic acetowhitening of vulvar lesion: A validity study. Arch. Gynecol. Obstet. 2015, 292, 387–390. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, V.; Magnaterra, E.; Zuccaro, B.; Magliulo, M.; Maio, V.; Muccilli, A.; Venturi, F.; Stanganelli, I.; Massi, D. Assessment of Vulvar Intraepithelial Neoplasia (VIN) Grades Based on Dermoscopic Features: A Diagnostic Study. Dermatol. Pract. Concept. 2023, 13, e2023269. [Google Scholar] [CrossRef] [PubMed]
- Barisani, A.; Dika, E.; Fanti, P.A.; De Iaco, P.; Tosti, G.; Patrizi, A.; Vaccari, S. Dermoscopic findings of vulvar intraepithelial neoplasia: A series of four cases. Br. J. Dermatol. 2017, 176, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.M.S.; Koti, V.R. Dermoscopic Aid in Diagnosing Vulval Intraepithelial Neoplasia. Clin. Dermatol. Rev. 2023, 7, 92–94. [Google Scholar] [CrossRef]
- Ronger-Savle, S.; Julien, V.; Duru, G.; Raudrant, D.; Dalle, S.; Thomas, L. Features of pigmented vulval lesions on dermoscopy. Br. J. Dermatol. 2011, 164, 54–61. [Google Scholar] [CrossRef]
- Mun, J.H.; Park, S.M.; Kim, G.W.; Song, M.; Kim, H.S.; Ko, H.C.; Kim, B.S.; Kim, M.B. Clinical and dermoscopic characteristics of extramammary Paget disease: A study of 35 cases. Br. J. Dermatol. 2016, 174, 1104–1107. [Google Scholar] [CrossRef]
- Zalaudek, I.; Argenziano, G. Dermoscopy of actinic keratosis, intraepidermal carcinoma and squamous cell carcinoma. Curr. Probl. Dermatol. 2015, 46, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.; Rosendahl, C.; Tschandl, P.; Riedl, E.; Kittler, H. Dermatoscopy of pigmented Bowen’s disease. J. Am. Acad. Dermatol. 2010, 62, 597–604. [Google Scholar] [CrossRef]
- Puig, S.; Malvehy, J. Dermoscopic findings of pigmented lesions of the mucosae. In Principles of Dermoscopy; Malvehy, J., Puig, S., Eds.; Cage Editors: San Francisco, CA, USA, 2002; pp. 289–299. [Google Scholar]
- Yang, B.; Hart, W.R. Vulvar intraepithelial neoplasia of the simplex (differentiated) type: A clinicopathologic study including analysis of HPV and p53 expression. Am. J. Surg. Pathol. 2000, 24, 429–441. [Google Scholar] [CrossRef]
- Scurry, J.; Campion, M.; Scurry, B.; Kim, S.N.; Hacker, N. Pathologic audit of 164 consecutive cases of vulvar intraepithelial neoplasia. Int. J. Gynecol. Pathol. 2006, 25, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Heller, D.S.; Day, T.; Allbritton, J.I.; Scurry, J.; Radici, G.; Welch, K.; Preti, M. ISSVD Difficult Pathologic Diagnoses Committee. Diagnostic Criteria for Differentiated Vulvar Intraepithelial Neoplasia and Vulvar Aberrant Maturation. J. Low. Genit. Tract. Dis. 2021, 25, 57–70. [Google Scholar] [CrossRef]
- Mentrikoski, M.J.; Stelow, E.B.; Culp, S.; Frierson HFJr Cathro, H.P. Histologic and immunohistochemical assessment of penile carcinomas in a North American population. Am. J. Surg. Pathol. 2014, 38, 1340–1348. [Google Scholar] [CrossRef]
- Cubilla, A.L.; Reuter, V.; Velazquez, E.; Piris, A.; Saito, S.; Young, R.H. Histologic classification of penile carcinoma and its relation to outcome in 61 patients with primary resection. Int. J. Surg. Pathol. 2001, 9, 111–120. [Google Scholar] [CrossRef]
- Pow-Sang, M.R.; Ferreira, U.; Pow-Sang, J.M.; Nardi, A.C.; Destefano, V. Epidemiology and natural history of penile cancer. Urology 2010, 76 (Suppl. S1), S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Miralles-Guri, C.; Bruni, L.; Cubilla, A.L.; Castellsagué, X.; Bosch, F.X.; de Sanjosé, S. Human papillomavirus prevalence and type distribution in penile carcinoma. J. Clin. Pathol. 2009, 62, 870–878. [Google Scholar] [CrossRef]
- Velazquez, E.F.; Soskin, A.; Bock, A.; Codas, R.; Cai, G.; Barreto, J.E.; Cubilla, A.L. Epithelial abnormalities and precancerous lesions of anterior urethra in patients with penile carcinoma: A report of 89 cases. Mod. Pathol. 2005, 18, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.T. Precancerous dermatoses: A study of 2 cases of chronic atypical proliferation. J. Cutan. Dis. Syph. 1912, 30, 241–255. [Google Scholar]
- Epstein, J.H.; Cubilla, A.L.; Humphrey, P.A. Tumors of the prostate gland, seminal vesicles, penis and scrotum. In Atlas of Tumor Pathology; Armed Forces Institute of Pathology: Washington, DC, USA, 2011; pp. 405–612. [Google Scholar]
- Velazquez, E.F.; Chaux, A.; Cubilla, A.L. Histologic classification of penile intraepithelial neoplasia. Semin. Diagn. Pathol. 2012, 29, 96–102. [Google Scholar] [CrossRef]
- Chamli, A.; Zaouak, A. Bowenoid Papulosis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kristiansen, S.; Svensson, Å.; Drevin, L.; Forslund, O.; Torbrand, C.; Bjartling, C. Risk Factors for Penile Intraepithelial Neoplasia: A Population-based Register Study in Sweden, 2000–2012. Acta Derm. Venereol. 2019, 99, 315–320. [Google Scholar] [CrossRef]
- Bunker, C.B.; Porter, W.M. Dermatoses of the Male Genitalia. In Rook’s Textbook of Dermatology, 9th ed.; Griffiths, C., Barker, J., Bleiker, T., Chalmers, R., Creamer, D., Eds.; John Wiley & Sons Inc.: Chichester, UK, 2016; pp. 111.1–111.41. [Google Scholar]
- Wikström, A.; Hedblad, M.A.; Johansson, B.; Kalantari, M.; Syrjänen, S.; Lindberg, M.; von Krogh, G. The acetic acid test in evaluation of subclinical genital papillomavirus infection: A comparative study on penoscopy, histopathology, virology and scanning electron microscopy findings. Genitourin. Med. 1992, 68, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Singhal, R.R.; Patel, T.M.; Pariath, K.A.; Vora, R.V. Premalignant male genital dermatoses. Indian J. Sex. Transm. Dis. AIDS 2019, 40, 97–104. [Google Scholar] [PubMed]
- You, H.S.; Kim, G.W.; Kim, W.J.; Mun, J.H.; Song, M.; Kim, H.S.; Ko, H.C.; Kim, B.S.; Kim, M.B. Dermatoses of the Glans Penis in Korea: A 10-Year Single Center Experience. Ann. Dermatol. 2016, 28, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.; Kittler, H.; Zalaudek, I.; Simionescu, O.; Marghoob, A.A.; Hofmann-Wellenhof, R.; Argenziano, G.; Soyer, H.P. Unklare Hautveränderung an der Glans penis führt zu unterschiedlichen dermatoskopischen Diagnosen [Unclear clinical change on the glans penis leads to different dermoscopic diagnoses]. Hautarzt 2013, 64, 768–769. [Google Scholar] [CrossRef]
- Țăpoi, D.A.; Gheorghișan-Gălățeanu, A.A.; Dumitru, A.V.; Ciongariu, A.M.; Furtunescu, A.R.; Marin, A.; Costache, M. Primary Undifferentiated/Dedifferentiated Cutaneous Melanomas-A Review on Histological, Immunohistochemical, and Molecular Features with Emphasis on Prognosis and Treatment. Int. J. Mol. Sci. 2023, 24, 9985. [Google Scholar] [CrossRef]
- Giuglea, C.; Marin, A.; Gavrila, I.; Paunescu, A.; Dobrete, N.A.; Marinescu, S.A. Basal Cell Carcinoma-A Retrospective Descriptive Study Integrated in Current Literature. Life 2023, 13, 832. [Google Scholar] [CrossRef]
- Antônio, J.R.; Antônio, C.R.; Trídico, L.A.; Alves, F.T.; Rollemberg, I. Erythroplasia of Queyrat treated with topical 5-fluorouracil. Bras Dermatol. 2016, 91 (Suppl. 1), 42–44. [Google Scholar] [CrossRef]
- Fanning, D.M.; Flood, H. Erythroplasia of Queyrat. Clin. Pract. 2012, 2, e63. [Google Scholar] [CrossRef] [PubMed]
- Scurtu, L.G.; Simionescu, O. Soluble Factors and Receptors Involved in Skin Innate Immunity—What Do We Know So Far? Biomedicines 2021, 9, 1795. [Google Scholar] [CrossRef]
- Tamanini, J.M.; Lellis, R.F.; Veasey, J.V. Genital Lichen Planus in Males: Clinical, Laboratory, and Histological Aspects of 7 Cases. Clin. Case Rep. Int. 2022, 6, 1301. [Google Scholar]
- de Oliveira Lea, M.L.; Alencar, L.R.P.J.; Santana, S.C.; de Souza, B.C.A.; Athanazio, D.A. Penile squamous cell carcinoma and lichen planus. Surg. Exp. Pathol. 2020, 3, 1. [Google Scholar] [CrossRef]
- Kravvas, G.; Shim, T.N.; Doiron, P.R.; Freeman, A.; Jameson, C.; Minhas, S.; Muneer, A.; Bunker, C.B. The diagnosis and management of male genital lichen sclerosus: A retrospective review of 301 patients. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Vladulescu, D.; Scurtu, L.G.; Simionescu, A.A.; Scurtu, F.; Popescu, M.I.; Simionescu, O. Platelet-Rich Plasma (PRP) in Dermatology: Cellular and Molecular Mechanisms of Action. Biomedicines 2023, 12, 7. [Google Scholar] [CrossRef]
- De Luca, D.A.; Papara, C.; Vorobyev, A.; Staiger, H.; Bieber, K.; Thaçi, D.; Ludwig, R.J. Lichen sclerosus: The 2023 update. Front. Med. 2023, 10, 1106318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spencer, A.; Watchorn, R.E.; Kravvas, G.; Ben-Salha, I.; Haider, A.; Francis, N.; Freeman, A.; Alnajjar, H.M.; Muneer, A.; Bunker, C.B. Pseudoepitheliomatous keratotic and micaceous balanitis: A series of eight cases. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1851–1856. [Google Scholar] [CrossRef]
- Spiess, P.E.; Dhillon, J.; Baumgarten, A.S.; Johnstone, P.A.; Giuliano, A.R. Pathophysiological basis of human papillomavirus in penile cancer: Key to prevention and delivery of more effective therapies. CA Cancer J. Clin. 2016, 66, 481–495. [Google Scholar] [CrossRef]
- Sudenga, S.L.; Ingles, D.J.; Pierce Campbell, C.M.; Lin, Y.; Fulp, W.J.; Messina, J.L.; Stoler, M.H.; Abrahamsen, M.; Villa, L.L.; Lazcano-Ponce, E.; et al. Genital HPV infection progression to external genital lesions: The HIM Study. Eur. Urol. 2015, 69, 166. [Google Scholar] [CrossRef]
- Errichetti, E.; Lacarrubba, F.; Micali, G.; Stinco, G. Dermoscopy of Zoon’s plasma cell balanitis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, e209–e210. [Google Scholar] [CrossRef]
- Chan, S.L.; Watchorn, R.E.; Panagou, E.; Panou, E.; Ong, E.L.; Heelan, K.; Haider, A.; Freeman, A.; Bunker, C.B. Dermatoscopic findings of penile intraepithelial neoplasia: Bowenoid papulosis, Bowen disease and erythroplasia of Queyrat. Australas. J. Dermatol. 2019, 60, e201–e207. [Google Scholar] [CrossRef] [PubMed]
- Lacarrubba, F.; Nasca, M.R.; Micali, G. Videodermatoscopy enhances diagnostic capability in psoriatic balanitis. J. Am. Acad. Dermatol. 2009, 61, 1084–1086. [Google Scholar] [CrossRef]
- Straub Hogan, M.M.; Spieker, A.J.; Orejudos, M.; Gheit, T.; Herfs, M.; Tommasino, M.; Sanchez, D.F.; Fernandez-Nestosa, M.J.; Pena, M.D.C.R.; Gordetsky, J.B.; et al. Pathological characterization and clinical outcome of penile intraepithelial neoplasia variants: A North American series. Mod. Pathol. 2022, 35, 1101–1109. [Google Scholar] [CrossRef]
- Sanchez, D.F.; Fernandez-Nestosa, M.J.; Cañete-Portillo, S.; Cubilla, A.L. Evolving insights into penile cancer pathology and the eighth edition of the AJCC TNM staging system. Urol. Oncol. 2022, 40, 215–222. [Google Scholar] [CrossRef]
- Chaux, A.; Velazquez, E.F.; Amin, A.; Soskin, A.; Pfannl, R.; Rodríguez, I.M.; Barreto, J.E.; Lezcano, C.; Ayala, G.; Netto, G.J.; et al. Distribution and characterization of subtypes of penile intraepithelial neoplasia and their association with invasive carcinomas: A pathological study of 139 lesions in 121 patients. Hum. Pathol. 2012, 43, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Chaux, A.; Pfannl, R.; Lloveras, B.; Alejo, M.; Clavero, O.; Lezcano, C.; Muñoz, N.; de Sanjosé, S.; Bosch, X.; Hernández-Pérez, M.; et al. Distinctive association of p16INK4a overexpression with penile intraepithelial neoplasia depicting warty and/or basaloid features: A study of 141 cases evaluating a new nomenclature. Am. J. Surg. Pathol. 2010, 34, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Verhave, B.; Goldberg, M.; Hashim, P.; Levitt, J. Treatment of Arsenic-Induced Bowen’s Disease with Topical 5-Fluorouracil. J. Drugs Dermatol. 2019, 18, 477–479. [Google Scholar] [PubMed]
- van Egmond, S.; Hoedemaker, C.; Sinclair, R. Successful treatment of perianal Bowen’s disease with imiquimod. Int. J. Dermatol. 2007, 46, 318–319. [Google Scholar] [CrossRef]
- Wang, W.E.; Chang, C.H. Successful treatment of extremely large Bowen’s disease lesion by topical photodynamic therapy and imiquimod: Using optical coherence tomography to detect early recurrence loci and validate the cure. Photodiagnosis Photodyn. Ther. 2023, 41, 103201. [Google Scholar] [CrossRef]
- Hillemanns, P.; Wang, X.; Staehle, S.; Michels, W.; Dannecker, C. Evaluation of different treatment modalities for vulvar intraepithelial neoplasia (VIN): CO2 laser vaporization, photodynamic therapy, excision and vulvectomy. Gynecol. Oncol. 2006, 100, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Fiascone, S.; Vitonis, A.F.; Feldman, S. Topical 5-Fluorouracil for Women With High-Grade Vaginal Intraepithelial Neoplasia. Obstet. Gynecol. 2017, 130, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Preti, M.; Joura, E.; Vieira-Baptista, P.; Van Beurden, M.; Bevilacqua, F.; Bleeker, M.C.G.; Bornstein, J.; Carcopino, X.; Chargari, C.; Cruickshank, M.E.; et al. The European Society of Gynaecological Oncology (ESGO), the International Society for the Study of Vulvovaginal Disease (ISSVD), the European College for the Study of Vulval Disease (ECSVD) and the European Federation for Colposcopy (EFC) consensus statements on pre-invasive vulvar lesions. Int. J. Gynecol. Cancer 2022, 32, 830–845. [Google Scholar] [CrossRef]
- Maluf, F.C.; Zibetti, G.D.; Paulino, E.; De Melo, A.C.; Racy, D.; Ferrigno, R.; Uson Junior, P.L.; Ribeiro, R.; Moretti, R.; Sadalla, J.C. Recommendations for the treatment of vulvar cancer in settings with limited resources: Report from the International Gynecological Cancer Society consensus meeting. Front. Oncol. 2022, 12, 928568. [Google Scholar] [CrossRef]
- van Poelgeest, M.I.; van Seters, M.; van Beurden, M.; Kwappenberg, K.M.; Heijmans-Antonissen, C.; Drijfhout, J.W.; Melief, C.J.; Kenter, G.G.; Helmerhorst, T.J.; Offringa, R.; et al. Detection of human papillomavirus (HPV) 16-specific CD4+ T-cell immunity in patients with persistent HPV16-induced vulvar intraepithelial neoplasia in relation to clinical impact of imiquimod treatment. Clin. Cancer Res. 2005, 11, 5273–5280. [Google Scholar] [CrossRef]
- Lawrie, T.A.; Nordin, A.; Chakrabarti, M.; Bryant, A.; Kaushik, S.; Pepas, L. Medical and surgical interventions for the treatment of usual-type vulval intraepithelial neoplasia. Cochrane Database Syst. Rev. 2016, 2016, CD011837. [Google Scholar] [CrossRef]
- Porter, W.M.; Francis, N.; Hawkins, D.; Dinneen, M.; Bunker, C.B. Penile intraepithelial neoplasia: Clinical spectrum and treatment of 35 cases. Br. J. Dermatol. 2002, 147, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Scurtu, L.G.; Petrica, M.; Grigore, M.; Avram, A.; Popescu, I.; Simionescu, O. A Conservative Combined Laser Cryoimmunotherapy Treatment vs. Surgical Excision for Basal Cell Carcinoma. J. Clin. Med. 2022, 11, 3439. [Google Scholar] [CrossRef] [PubMed]
- Del Losada, J.P.; Ferré, A.; San Román, B.; Vieira, V.; Fonseca, E. Erythroplasia of Queyrat with urethral involvement: Treatment with carbon dioxide laser vaporization. Dermatol. Surg. 2005, 31, 1454–1457. [Google Scholar] [CrossRef]
- Issa, A.; Sebro, K.; Kwok, A.; Janisch, F.; Grossmann, N.C.; Lee, E.; Lucky, M.; Oliveira, P.; Lau, M.; Parnham, A.; et al. Treatment Options and Outcomes for Men with Penile Intraepithelial Neoplasia: A Systematic Review. Eur. Urol. Focus. 2022, 8, 829–832. [Google Scholar] [CrossRef]
- Kravvas, G.; Ge, L.; Ng, J.; Shim, T.N.; Doiron, P.R.; Watchorn, R.; Kentley, J.; Panou, E.; Dinneen, M.; Freeman, A.; et al. The management of penile intraepithelial neoplasia (PeIN): Clinical and histological features and treatment of 345 patients and a review of the literature. J. Dermatolog Treat. 2022, 33, 1047–1062. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).