Line-Field Confocal Optical Coherence Tomography: A New Skin Imaging Technique Reproducing a “Virtual Biopsy” with Evolving Clinical Applications in Dermatology
Abstract
:1. Introduction
1.1. LC-OCT Technical Properties
1.2. LC-OCT Practical Properties
2. Materials and Methods
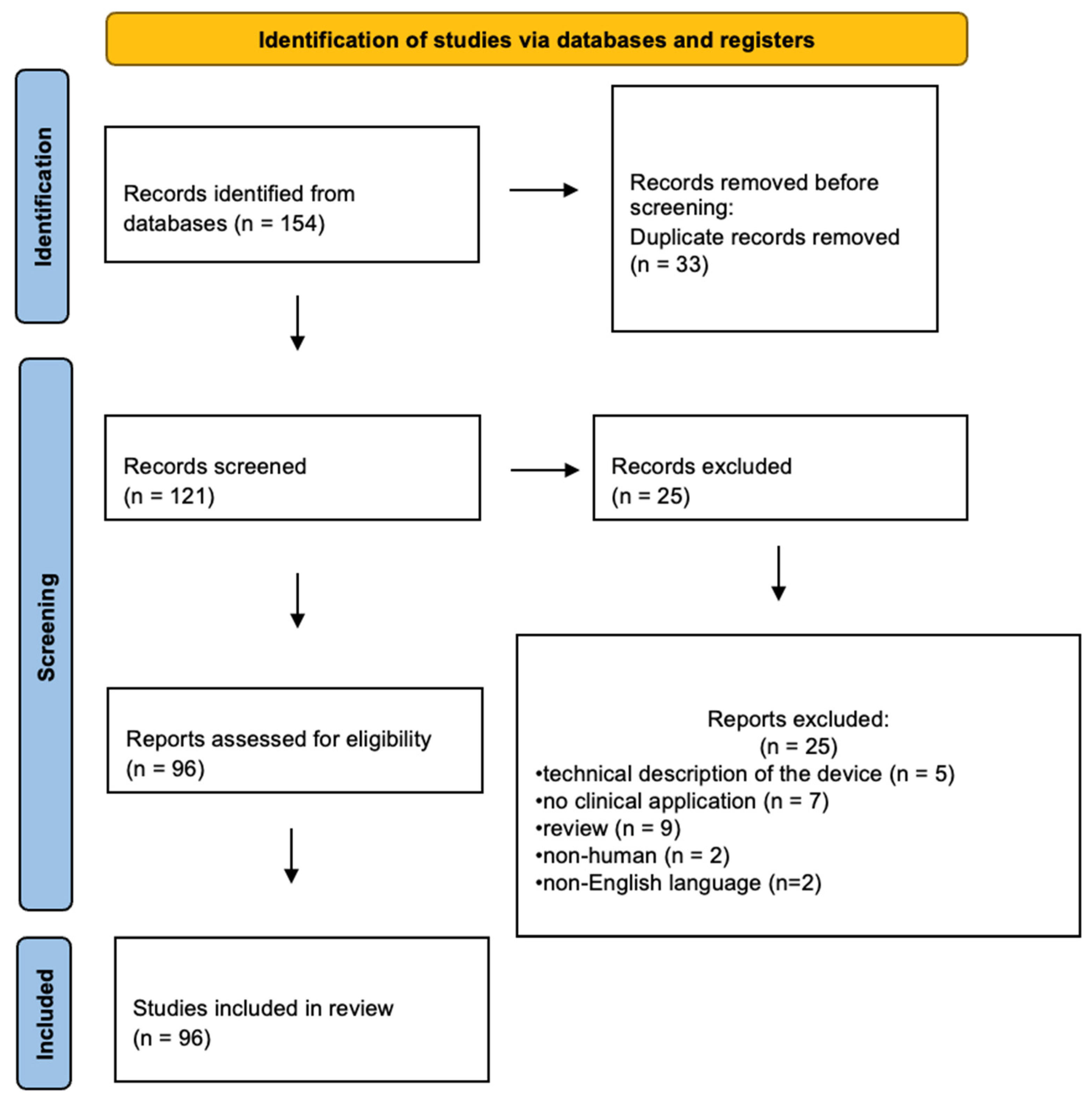
2.1. Search Methods, Types of Studies, and Participants
2.2. Data Collection and Analysis
3. Results
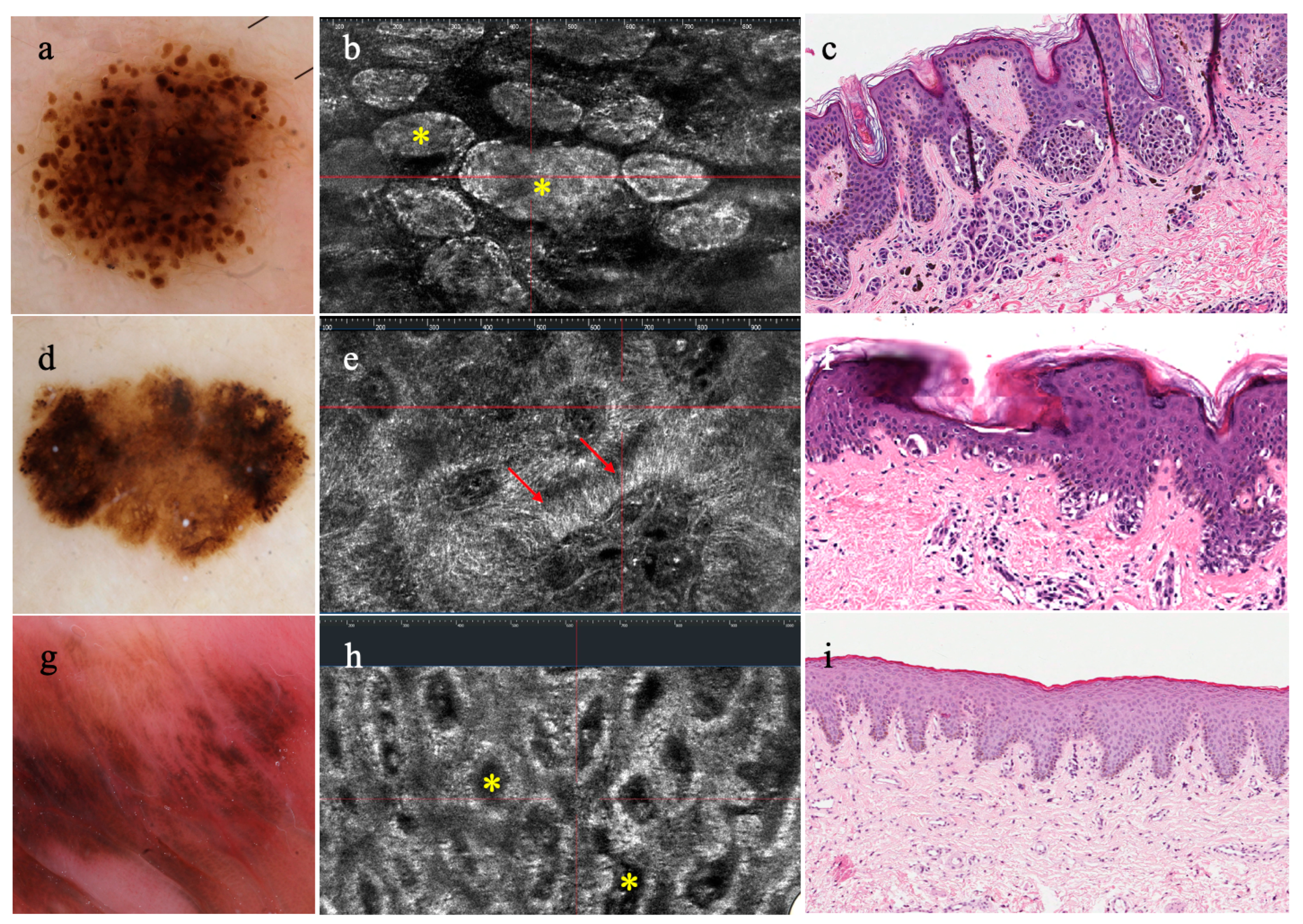
3.1. Melanocytic Lesions
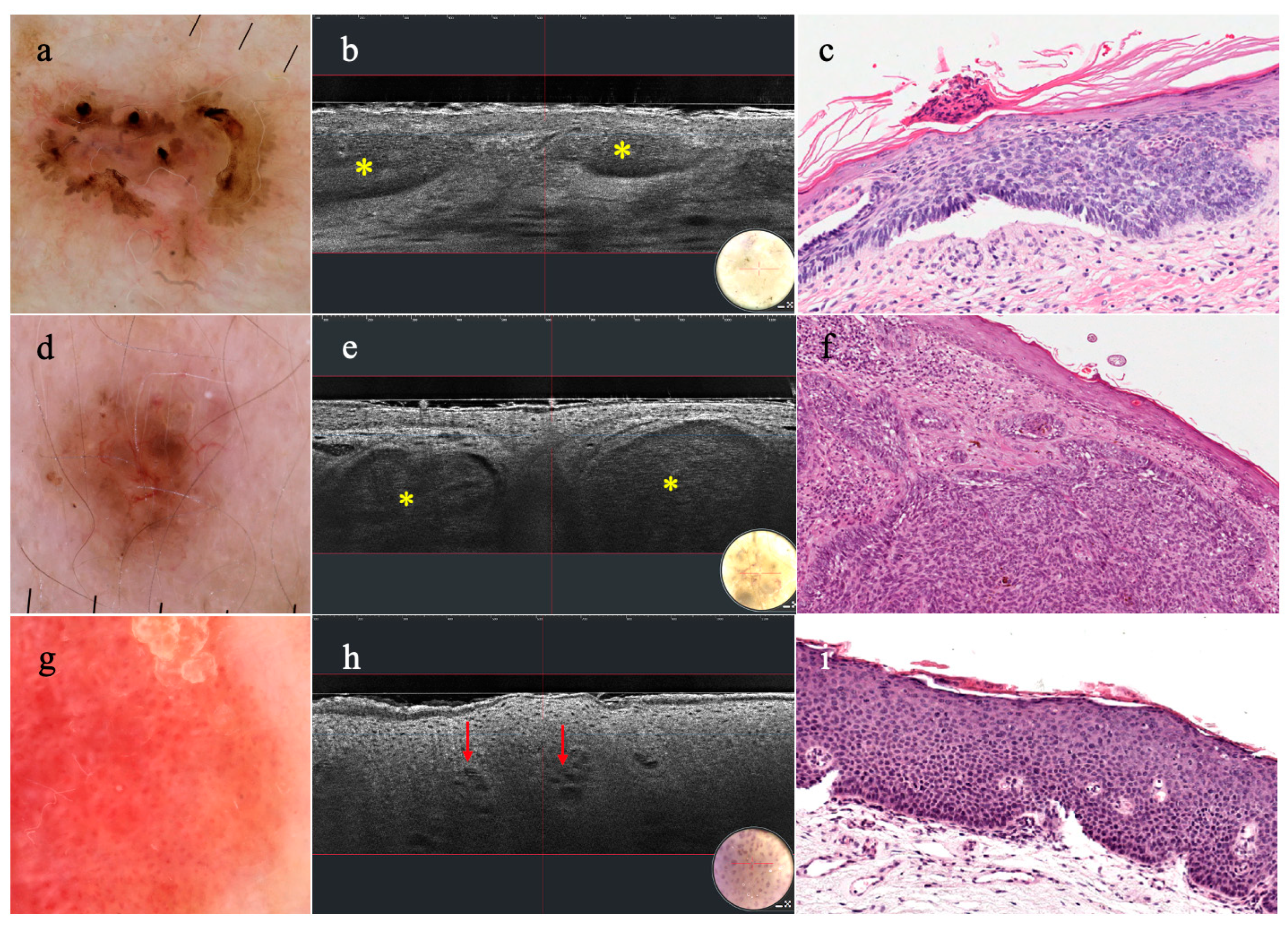
3.2. Non-Melanocytic Lesions
3.3. Inflammatory Skin Diseases
3.4. Hair and Nails
3.5. Infectious Diseases
3.6. Pediatric Population
3.7. Genetic Diseases
3.8. Skin Vascular Lesions
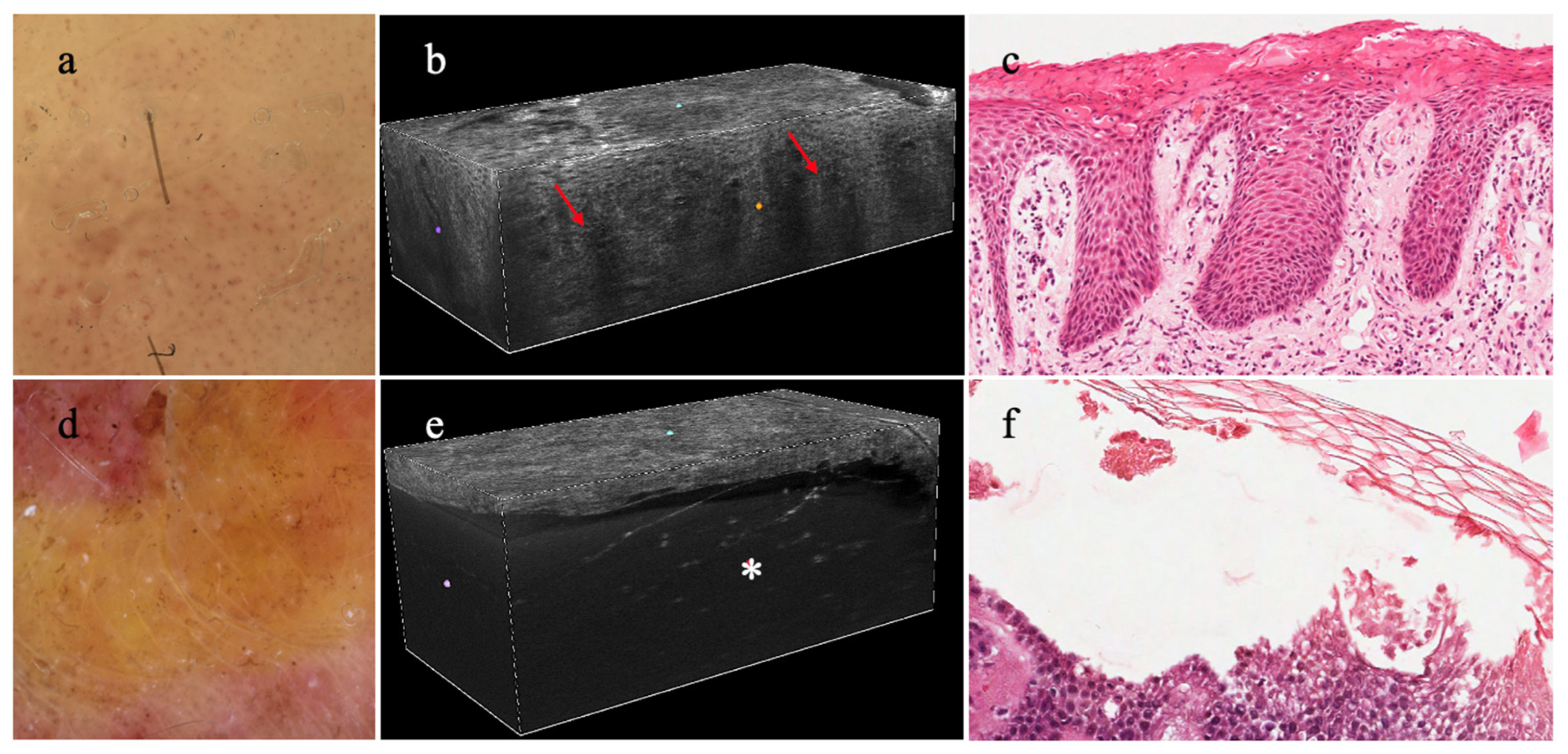
3.9. Cosmetic Applications
3.10. Others
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jartarkar, S.R.; Patil, A.; Wollina, U.; Gold, M.H.; Stege, H.; Grabbe, S.; Goldust, M. New diagnostic and imaging technologies in dermatology. J. Cosmet. Dermatol. 2021, 20, 3782–3787. [Google Scholar] [CrossRef]
- Schneider, S.L.; Kohli, I.; Hamzavi, I.H.; Council, M.L.; Rossi, A.M.; Ozog, D.M. Emerging imaging technologies in dermatology: Part I: Basic principles. J. Am. Acad. Dermatol. 2019, 80, 1114–1120. [Google Scholar] [CrossRef]
- Zavattaro, E.; Veronese, F.; Savoia, P. Editorial: Non-invasive diagnostic tools in the management of skin disorders. Front. Med. 2023, 10, 1271195. [Google Scholar] [CrossRef]
- Greenwood, J.D.; Merry, S.P.; Boswell, C.L. Skin Biopsy Techniques. Prim. Care 2022, 49, 1–22. [Google Scholar] [CrossRef]
- Elston, D.M.; Stratman, E.J.; Miller, S.J. Skin biopsy: Biopsy issues in specific diseases. J. Am. Acad. Dermatol. 2016, 74, 1–16, Erratum in J. Am. Acad. Dermatol. 2016, 75, 854. [Google Scholar] [CrossRef]
- Korfitis, C.; Gregoriou, S.; Antoniou, C.; Katsambas, A.D.; Rigopoulos, D. Skin biopsy in the context of dermatological diagnosis: A retrospective cohort study. Dermatol. Res. Pract. 2014, 2014, 734906. [Google Scholar] [CrossRef]
- Barksdale, S.K.; Oberlender, S.A.; Barnhill, R.L. “Rush” skin biopsy specimens in a tertiary medical center: Diagnostic yield and clinical utility. J. Am. Acad. Dermatol. 1998, 38, 548–554. [Google Scholar] [CrossRef]
- Mandel, V.D.; Ardigò, M. Non-Invasive Diagnostic Techniques in Dermatology. J. Clin. Med. 2023, 12, 1081. [Google Scholar] [CrossRef]
- Errichetti, E.; Stinco, G. Dermoscopy in General Dermatology: A Practical Overview. Dermatol. Ther. 2016, 6, 471–507. [Google Scholar] [CrossRef]
- Kittler, H.; Pehamberger, H.; Wolff, K.; Binder, M. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002, 3, 159–165. [Google Scholar] [CrossRef]
- Lacarrubba, F.; Ardigò, M.; Di Stefani, A.; Verzì, A.E.; Micali, G. Dermatoscopy and Reflectance Confocal Microscopy Correlations in Nonmelanocytic Disorders. Dermatol. Clin. 2018, 36, 487–501. [Google Scholar] [CrossRef]
- Waddell, A.; Star, P.; Guitera, P. Advances in the use of reflectance confocal microscopy in melanoma. Melanoma Manag. 2018, 5, MMT04. [Google Scholar] [CrossRef]
- Levine, A.; Markowitz, O. Introduction to reflectance confocal microscopy and its use in clinical practice. JAAD Case Rep. 2018, 4, 1014–1023. [Google Scholar] [CrossRef]
- Levine, A.; Wang, K.; Markowitz, O. Optical Coherence Tomography in the Diagnosis of Skin Cancer. Dermatol. Clin. 2017, 35, 465–488. [Google Scholar] [CrossRef]
- Wan, B.; Ganier, C.; Du-Harpur, X.; Harun, N.; Watt, F.; Patalay, R.; Lynch, M.D. Applications and future directions for optical coherence tomography in dermatology. Br. J. Dermatol. 2021, 184, 1014–1022. [Google Scholar] [CrossRef]
- Dubois, A.; Levecq, O.; Azimani, H.; Siret, D.; Barut, A.; Suppa, M.; del Marmol, V.; Malvehy, J.; Cinotti, E.; Rubegni, P.; et al. Line-field confocal optical coherence tomography for high-resolution noninvasive imaging of skin tumors. J. Biomed. Opt. 2018, 23, 106007. [Google Scholar] [CrossRef]
- Ogien, J.; Tavernier, C.; Fischman, S.; Dubois, A. Line-field confocal optical coherence tomography (LC-OCT): Principles and practical use. Ital. J. Dermatol. Venerol. 2023, 158, 171–179. [Google Scholar] [CrossRef]
- Suppa, M.; Palmisano, G.; Tognetti, L.; Lenoir, C.; Cappilli, S.; Fontaine, M.; Cano, C.O.; Diet, G.; Perez-Anker, J.; Schuh, S.; et al. Line-field confocal optical coherence tomography in melanocytic and non-melanocytic skin tumors. Ital. J. Dermatol. Venerol. 2023, 158, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Donelli, C.; Rubegni, P.; Tognetti, L.; Lacarrubba, F.; DI Stefani, A.; Cappilli, S.; Suppa, M.; Perrot, J.-L.; Cinotti, E. Inflammatory and infectious skin diseases in line-field confocal optical coherence tomography: State of the art. Ital. J. Dermatol. Venerol. 2023, 158, 190–196. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Lenoir, C.; Perez-Anker, J.; Diet, G.; Tognetti, L.; Cinotti, E.; Trépant, A.; Rubegni, P.; Puig, S.; Perrot, J.; Malvehy, J.; et al. Line-field confocal optical coherence tomography of benign dermal melanocytic proliferations: A case series. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e399–e401. [Google Scholar] [CrossRef]
- Di Stefani, A.; Cappilli, S.; Cuffaro, G.; Fionda, B.; Pagliara, M.M.; Paradisi, A.; Ricci, C.; Rossi, E.; Sammarco, M.G.; Schinzari, G.; et al. Line-Field Confocal Optical Coherence Tomography Evaluation of Eyelid Skin Lesions. Diagnostics 2023, 13, 3590. [Google Scholar] [CrossRef]
- Schuh, S.; Ruini, C.; Perwein, M.K.E.; Daxenberger, F.; Gust, C.; Sattler, E.C.; Welzel, J. Line-Field Confocal Optical Coherence Tomography: A New Tool for the Differentiation between Nevi and Melanomas? Cancers 2022, 14, 1140. [Google Scholar] [CrossRef] [PubMed]
- Verzì, A.E.; Broggi, G.; Caltabiano, R.; Micali, G.; Lacarrubba, F. Line-field confocal optical coherence tomography of lentigo maligna with horizontal and vertical histopathologic correlations. J. Cutan. Pathol. 2023, 50, 118–122. [Google Scholar] [CrossRef]
- Wolswijk, T.; Sanak, D.; Lenoir, C.; Cinotti, E.; Tognetti, L.; Rubegni, P.; Perez-Anker, J.; Puig, S.; Malvehy, J.; Perrot, J.; et al. Line-field confocal optical coherence tomography can help differentiating melanoma from pigmented basal cell carcinoma: A case report. Skin. Res. Technol. 2023, 29, e13376. [Google Scholar] [CrossRef]
- Perez-Anker, J.; Puig, S.; Alos, L.; García, A.; Alejo, B.; Cinotti, E.; Cano, C.O.; Tognetti, L.; Lenoir, C.; Monnier, J.; et al. Morphological evaluation of melanocytic lesions with three-dimensional line-field confocal optical coherence tomography: Correlation with histopathology and reflectance confocal microscopy. A pilot study. Clin. Exp. Dermatol. 2022, 47, 2222–2233. [Google Scholar] [CrossRef]
- Soglia, S.; Pérez-Anker, J.; Albero, R.; Alós, L.; Berot, V.; Castillo, P.; Cinotti, E.; Del Marmol, V.; Fakih, A.; García, A.; et al. Understanding the anatomy of dermoscopy of melanocytic skin tumours: Correlation in vivo with line-field optical coherence tomography. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- El Zeinaty, P.; Suppa, M.; Del Marmol, V.; Tavernier, C.; Dauendorffer, J.; Lebbé, C.; Baroudjian, B. Line-field confocal optical coherence tomography (LC-OCT): A novel tool of cutaneous imaging for non-invasive diagnosis of pigmented lesions of genital area. J. Eur. Acad. Dermatol. Venereol. 2023, 37, E1081–E1084. [Google Scholar] [CrossRef]
- Ruini, C.; Schuh, S.; Gust, C.; Hartmann, D.; French, L.E.; Sattler, E.C.; Welzel, J. In-Vivo LC-OCT Evaluation of the Downward Proliferation Pattern of Keratinocytes in Actinic Keratosis in Comparison with Histology: First Impressions from a Pilot Study. Cancers 2022, 13, 2856. [Google Scholar] [CrossRef]
- Ruini, C.; Schuh, S.; Sattler, E.; Welzel, J. Line-field confocal optical coherence tomography-Practical applications in dermatology and comparison with established imaging methods. Skin. Res. Technol. 2021, 27, 340–352. [Google Scholar] [CrossRef]
- Ruini, C.; Schuh, S.; Gust, C.; Kendziora, B.; Frommherz, L.; French, L.E.; Hartmann, D.; Welzel, J.; Sattler, E.C. Line-field confocal optical coherence tomography for the in vivo real-time diagnosis of different stages of keratinocyte skin cancer: A preliminary study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2388–2397. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, C.; Cinotti, E.; Tognetti, L.; Cano, C.O.; Diet, G.; Miyamoto, M.; Rocq, L.; Trépant, A.; Perez-Anker, J.; Puig, S.; et al. Line-field confocal optical coherence tomography of actinic keratosis: A case series. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e900–e902. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, C.; Diet, G.; Cinotti, E.; Tognetti, L.; Cano, C.O.; Rocq, L.; Trépant, A.; Monnier, J.; Perez-Anker, J.; Rubegni, P.; et al. Line-field confocal optical coherence tomography of sebaceous hyperplasia: A case series. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e509–e511. [Google Scholar] [CrossRef]
- Verzì, A.E.; Micali, G.; Lacarrubba, F. Line-Field Confocal Optical Coherence Tomography May Enhance Monitoring of Superficial Basal Cell Carcinoma Treated with Imiquimod 5% Cream: A Pilot Study. Cancers 2021, 13, 4913. [Google Scholar] [CrossRef]
- Lacarrubba, F.; Verzì, A.E.; Puglisi, D.F.; Broggi, G.; Caltabiano, R.; Micali, G. Line-field confocal optical coherence tomography of xanthogranuloma: Correlation with vertical and horizontal histopathology. J. Cutan. Pathol. 2021, 48, 1208–1211. [Google Scholar] [CrossRef]
- Cinotti, E.; Tognetti, L.; Cartocci, A.; Lamberti, A.; Gherbassi, S.; Cano, C.O.; Lenoir, C.; Dejonckheere, G.; Diet, G.; Fontaine, M.; et al. Line-field confocal optical coherence tomography for actinic keratosis and squamous cell carcinoma: A descriptive study. Clin. Exp. Dermatol. 2021, 46, 1530–1541. [Google Scholar] [CrossRef]
- Cappilli, S.; Dejonckheere, G.; Hajjar, N.; Cinotti, E.; Tognetti, L.; Perez-Anker, J.; Rubegni, P.; Puig, S.; Malvehy, J.; Perrot, J.L.; et al. Line-field confocal optical coherence tomography: A case on the importance of full-lesion examination for basal cell carcinoma. Int. J. Dermatol. 2022, 61, e248–e250. [Google Scholar] [CrossRef]
- Cappilli, S.; Suppa, M.; Tognetti, L.; Cinotti, E.; Rubegni, P.; Del Marmol, V.; Di Stefani, A.; Peris, K. Line-field confocal optical coherence tomography of fibroepithelioma of Pinkus. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e698–e700. [Google Scholar] [CrossRef] [PubMed]
- Gust, C.; Schuh, S.; Welzel, J.; Daxenberger, F.; Hartmann, D.; French, L.E.; Ruini, C.; Sattler, E.C. Line-Field Confocal Optical Coherence Tomography Increases the Diagnostic Accuracy and Confidence for Basal Cell Carcinoma in Equivocal Lesions: A Prospective Study. Cancers 2022, 14, 1082. [Google Scholar] [CrossRef]
- Cappilli, S.; Cinotti, E.; Lenoir, C.; Tognetti, L.; Perez-Anker, J.; Rubegni, P.; Puig, S.; Malvehy, J.; Perrot, J.; del Marmol, V.; et al. Line-field confocal optical coherence tomography of basosquamous carcinoma: A case series with histopathological correlation. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, E.; Bertello, M.; Cartocci, A.; Fiorani, D.; Tognetti, L.; Solmi, V.; Cappilli, S.; Peris, K.; Perrot, J.L.; Suppa, M.; et al. Comparison of reflectance confocal microscopy and line-field optical coherence tomography for the identification of keratinocyte skin tumours. Skin. Res. Technol. 2022, 29, e13215. [Google Scholar] [CrossRef]
- Di Stefani, A.; Fionda, B.; Cappilli, S.; Tagliaferri, L.; Peris, K. Extramammary Paget disease imaged by LC-OCT and treated with radiotherapy. Int. J. Dermatol. 2023, 62, e503–e505. [Google Scholar] [CrossRef]
- Soglia, S.; Pérez-Anker, J.; Fraghì, A.; Ariasi, C.; La Rosa, G.; Lenoir, C.; Suppa, M.; Calzavara-Pinton, P.G.; Venturini, M. Line-field confocal optical coherence tomography and reflectance confocal microscopy of Merkel cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e1223–e1225. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, C.; Perez-Anker, J.; Tognetti, L.; Cinotti, E.; Trépant, A.L.; Rubegni, P.; Puig, S.; Perrot, J.L.; Malvehy, J.; del Marmol, V.; et al. Line-field confocal optical coherence tomography of seborrheic keratosis: A case series. J. Eur. Acad. Dermatol. Venereol. 2023, 37, E1011–E1013. [Google Scholar] [CrossRef]
- Donelli, C.; Suppa, M.; Tognetti, L.; Perrot, J.L.; Calabrese, L.; Pérez-Anker, J.; Malvehy, J.; Rubegni, P.; Cinotti, E. Line-Field Confocal Optical Coherence Tomography for the Diagnosis of Skin Carcinomas: Real-Life Data over Three Years. Curr. Oncol. 2023, 30, 8853–8864. [Google Scholar] [CrossRef] [PubMed]
- Lacarrubba, F.; Verzì, A.E.; Polita, M.; Aleo, A.; Micali, G. Line-field confocal optical coherence tomography in the treatment monitoring of actinic keratosis with tirbanibulin: A pilot study. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e1131–e1133. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, E.; Bertello, M.; Dragotto, M.; Cartocci, A.; Tognetti, L.; Cappilli, S.; Peris, K.; Perrot, J.L.; Del Marmol, V.; Rubegni, P.; et al. Comparison of reflectance confocal microscopy and line-field optical coherence tomography for the identification of basal cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e1147–e1150. [Google Scholar] [CrossRef] [PubMed]
- Aktas, D.; Palmisano, G.; Cinotti, E.; Tognetti, L.; Perrot, J.; Perez-Anker, J.; Rubegni, P.; Puig, S.; Malvehy, J.; Peris, K.; et al. The role of line-field confocal optical coherence tomography in the differential diagnosis of infiltrative basal cell carcinoma with scar-like lesions: A case series. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e1396–e1398. [Google Scholar] [CrossRef]
- Cortonesi, G.; Donelli, C.; Rubegni, P.; Miracco, C.; Cinotti, E. Non-invasive Imaging Techniques for the Diagnosis of Clear Cell Acanthomas: Dermoscopy, Reflectance Confocal Microscopy and Line-Field Confocal Optical Coherence Tomography. Dermatol. Pract. Concept. 2023, 13, e2023231. [Google Scholar] [CrossRef]
- Maione, V.; Bighetti, S.; Bettolini, L.; Zambelli, C.; Calzavara-Pinton, P. The role of line-field confocal optical coherence tomography (LC-OCT) in the diagnosis of eccrine poroma: A case report. Australas. J. Dermatol. 2023, 64, e216–e219. [Google Scholar] [CrossRef]
- Verzì, A.E.; Russo, A.; Castellino, N.; Caltabiano, R.; Fallico, M.; Cappellani, F.; Micali, G.; Lacarrubba, F. Line-field confocal optical coherence tomography of eyelid margin growths: A case series. Skin. Res. Technol. 2024, 30, e13559. [Google Scholar] [CrossRef] [PubMed]
- Soglia, S.; Pérez-Anker, J.; Ghini, I.; Lenoir, C.; Maione, V.; Sala, R.; Tonon, F.; Suppa, M.; Calzavara-Pinton, P.G.; Malvehy, J.; et al. Line-field confocal optical coherence tomography: A new in vivo assessment tool for cutaneous mycosis fungoides. J. Eur. Acad. Dermatol. Venereol. 2024, 38, e296–e298. [Google Scholar] [CrossRef]
- Jacobsen, K.; Wenande, E.; Ortner, V.K.; Schmidt, G.; Haedersdal, M. Surgical planning with line-field confocal optical coherence tomography for recurrent infiltrative basal cell carcinoma: Visualizing subclinical tumor for margin adjustment. J. Dtsch. Dermatol. Ges. 2024, 22, 462–465. [Google Scholar] [CrossRef]
- Paradisi, A.; Cornacchia, L.; Cappilli, S.; Abeni, D.; Federico, F.; Di Stefani, A.; Mannino, M.; Peris, K. Preoperative evaluation of high-risk basal cell carcinoma with line-field confocal optical coherence tomography (LC-OCT) reduces Mohs micrographic surgery stage number: A case-control study. EJC Skin. Cancer 2024, 2, 100015. [Google Scholar] [CrossRef]
- Tognetti, L.; Cinotti, E.; Suppa, M.; Guazzo, R.; Habougit, C.; Santi, F.; Diet, G.; Fontaine, M.; Berot, V.; Monnier, J.; et al. Line field confocal optical coherence tomography: An adjunctive tool in the diagnosis of autoimmune bullous diseases. J. Biophotonics. 2021, 14, e202000449. [Google Scholar] [CrossRef]
- Verzì, A.; Broggi, G.; Micali, G.; Sorci, F.; Caltabiano, R.; Lacarrubba, F. Line-field confocal optical coherence tomography of psoriasis, eczema and lichen planus: A case series with histopathological correlation. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1884–1889. [Google Scholar] [CrossRef]
- Truong, T.M.; Pathak, G.N.; Rao, B.K. Line-field confocal optical coherence tomography imaging findings of scalp psoriasis. JAAD Case Rep. 2023, 39, 106–108. [Google Scholar] [CrossRef]
- Orsini, C.; Trovato, E.; Cortonesi, G.; Pedrazzani, M.; Suppa, M.; Rubegni, P.; Tognetti, L.; Cinotti, E. Line-field confocal optical coherence tomography: New insights for psoriasis treatment monitoring. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 325–331. [Google Scholar] [CrossRef]
- Falcinelli, F.; Lazzeri, L.; Tognetti, L.; Bardelli, M.; Rubegni, P.; Russo, F. Development of a sebo-psoriasis-like dermatosis: A newly paradoxical reaction due to ixekizumab described with line-field confocal optical coherence tomography. Ital. J. Dermatol. Venerol. 2024, 159, 197–198. [Google Scholar] [CrossRef]
- Russo, F.; Lazzeri, L.; Falcinelli, F. Reading Patch Test Through Line-Field Confocal Optical Coherence Tomography Eyes. Dermatitis. 2024, 35, 118–120. [Google Scholar] [CrossRef]
- Suppa, M.; Fontaine, M.; Dejonckheere, G.; Cinotti, E.; Yélamos, O.; Diet, G.; Tognetti, L.; Miyamoto, M.; Cano, C.O.; Perez-Anker, J.; et al. Line-field confocal optical coherence tomography of basal cell carcinoma: A descriptive study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1099–1110. [Google Scholar] [CrossRef]
- Ruini, C.; Schuh, S.; Gust, C.; Kendziora, B.; Frommherz, L.; French, L.E.; Hartmann, D.; Welzel, J.; Sattler, E. Line-field optical coherence tomography: In vivo diagnosis of basal cell carcinoma subtypes compared with histopathology. Clin. Exp. Dermatol. 2021, 46, 1471–1481. [Google Scholar] [CrossRef]
- Cappilli, S.; Suppa, M.; Ricci, C.; del Marmol, V.; Peris, K.; Di Stefani, A. Line-field confocal optical coherence tomography of cutaneous vascular lesions: Morphological assessment and histopathological correlations. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1664–1668. [Google Scholar] [CrossRef]
- Kurzeja, M.; Warszawik-Hendzel, O.; Rakowska, A.; Graczyk, A.; Fedorczuk, D.; Czuwara, J.; Olszewska, M.; Rudnicka, L. Line-field confocal optical coherence tomography: A new diagnostic method of lichen planopilaris. Skin. Res. Technol. 2023, 29, e13495. [Google Scholar] [CrossRef]
- Kurzeja, M.; Warszawik-Hendzel, O.; Rakowska, A.; Graczyk, A.; Waskiel-Burnat, A.; Czuwara, J.; Olszewska, M.; Rudnicka, L. Line-field confocal optical coherence tomography-A new diagnostic method in hair loss associated with folliculitis decalvans. J. Eur. Acad. Dermatol. Venereol. 2024, 38, e267–e270. [Google Scholar] [CrossRef]
- Lacarrubba, F.; Verzì, A.E.; Dall’Oglio, F.; Villani, A.; Micali, G. Alopecia areata: Line-field confocal optical coherence tomography features and dermoscopy correlation. Skin. Res. Technol. 2024, 30, e13596. [Google Scholar] [CrossRef] [PubMed]
- Verzì, A.E.; Lacarrubba, F.; Dall’Oglio, F.; Rini, S.; Tosti, A.; Micali, G. Subclinical, early hair regrowth in alopecia areata patients under treatment with baricitinib detected by line-field confocal optical coherence tomography evaluation. J. Eur. Acad. Dermatol. Venereol. 2024, 38, e459–e461. [Google Scholar] [CrossRef]
- Falcinelli, F.; Calabrese, L.; Lamberti, A.; Tognetti, L.; Rubegni, P.; Cinotti, E. The corkscrew hairs of tinea capitis through line-field confocal optical coherence tomography. J. Eur. Acad. Dermatol. Venereol. 2024, 38, e528–e530. [Google Scholar] [CrossRef]
- Eijkenboom, Q.L.; Daxenberger, F.; Gust, C.; Hartmann, D.; Guertler, A.; Steckmeier, S.; Deussing, M.; French, L.E.; Welzel, J.; Schuh, S.; et al. Line-field confocal optical coherence tomography, a novel non-invasive tool for the diagnosis of onychomycosis. J. Dtsch. Dermatol. Ges. 2024, 22, 367–375. [Google Scholar] [CrossRef]
- Hobelsberger, S.; Steininger, J.; Bauer, A.; Beissert, S.; Gellrich, F.F. Line-field confocal optical coherence tomography for the diagnosis of onychomycosis in comparison with healthy nails: A case series. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e1234–e1236. [Google Scholar] [CrossRef]
- Eijkenboom, Q.L.; Daxenberger, F.; Guertler, A.; Steckmeier, S.; French, L.E.; Sattler, E.C. Line-field confocal optical coherence tomography (LC-OCT) for the in vivo examination of nails: Analysis of typical features for the differentiation of nail disorders. J. Eur. Acad. Dermatol. Venereol. 2024, 38, e413–e416. [Google Scholar] [CrossRef] [PubMed]
- Ruini, C.; Schuh, S.; Pellacani, G.; French, L.; Welzel, J.; Sattler, E. In vivo imaging of Sarcoptes scabiei infestation using line-field confocal optical coherence tomography. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e808–e809. [Google Scholar] [CrossRef]
- Ruini, C.; Schuh, S.; Hartmann, D.; French, L.; Welzel, J.; Sattler, E. Noninvasive real-time imaging of mite skin infestations with line-field confocal optical coherence tomography. Br. J. Dermatol. 2021, 184, e3. [Google Scholar] [CrossRef] [PubMed]
- Lacarrubba, F.; Verzì, A.E.; Puglisi, D.F.; Micali, G. Line-field confocal optical coherence tomography: A novel, non-invasive imaging technique for a rapid, in-vivo diagnosis of herpes infection of the skin. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e404–e406. [Google Scholar] [CrossRef]
- Verzì, A.E.; Micali, G.; Lacarrubba, F. Line-field confocal optical coherence tomography in molluscum contagiosum: A case series. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e934–e936. [Google Scholar] [CrossRef] [PubMed]
- Cortonesi, G.; Rubegni, P.; Tognetti, L.; Habougit, C.; Planello, J.; Perrot, J.; Cinotti, E. Line-field confocal optical coherence tomography imaging of human cowpox virus skin infection. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e1066–e1067. [Google Scholar] [CrossRef]
- Cappilli, S.; Bocchino, E.; Cornacchia, L.; Peris, K.; Di Stefani, A. Sharpen your sight: Trombiculosis under the dermatoscope with LC-OCT new insights. Dermatol. Ther. 2022, 35, e15951. [Google Scholar] [CrossRef]
- Orsini, C.; Taddeucci, P.; Bertello, M.; Cinotti, E.; Cortonesi, G.; Rubegni, P.; Russo, F. Line-field confocal optical coherence tomography for the diagnosis of nodular scabies mimicking breast cancer skin metastasis. Int. J. Dermatol. 2023, 62, e195–e196. [Google Scholar] [CrossRef]
- Pathak, G.N.; Truong, T.M.; Rao, B.K. Line-field confocal optical coherence tomography assessment of pityriasis rosea. JAAD Case Rep. 2023, 39, 135–138. [Google Scholar] [CrossRef]
- Idoudi, S.; Battistella, M.; El Zeinaty, P.; Tavernier, C.; Lebbe, C.; Baroudijian, B. Line-field confocal optical coherence tomography in vivo description of Sarcoptes scabiei and histological correlation. J. Eur. Acad. Dermatol. Venereol. 2024. [Google Scholar] [CrossRef]
- Cinotti, E.; Barbarossa, L.; Cortonesi, G.; Lamberti, A.; La Marca, F.; Tognetti, L.; Rubegni, P.; Perrot, J.L. Non-Invasive Imaging for the Diagnosis of Genital Warts and Their Imitators. J. Clin. Med. 2024, 13, 1345. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, L.; Carraro, A.; Cinotti, E.; Suppa, M.; del Marmol, V.; Perrot, J.L.; Rubegni, P. Line-field confocal optical coherence tomography for non-invasive diagnosis of lichenoid dermatoses of the childhood: A case series. Skin. Res. Technol. 2021, 27, 1178–1181. [Google Scholar] [CrossRef]
- Gallay, C.; Ventéjou, S.; Christen-Zaech, S. Line-field confocal optical coherence tomography of pyogenic granulomas in children: Report of two cases. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e37–e39. [Google Scholar] [CrossRef]
- Del Río-Sancho, S.; Gallay, C.; Ventéjou, S.; Christen-Zaech, S. In vivo evaluation of skin of children with LC-OCT: An objective assessment. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1897–1905. [Google Scholar] [CrossRef]
- Del Río-Sancho, S.; Gallay, C.; Ventéjou, S.; Christen-Zaech, S. Non-invasive imaging of agminated Spitz nevi with line-field confocal optical coherence tomography. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e658–e659. [Google Scholar] [CrossRef] [PubMed]
- Cappilli, S.; Guerriero, C.; Iacoangeli, A.; Verzì, A.E.; Cinotti, E.; Suppa, M.; Peris, K.; DI Stefani, A. Utility of line-field confocal optical coherence tomography in the pediatric population. Ital. J. Dermatol. Venerol. 2023, 158, 197–204. [Google Scholar] [CrossRef]
- Tognetti, L.; Fiorani, D.; Suppa, M.; Cinotti, E.; Fontaine, M.; Marmol, V.; Rubegni, P.; Perrot, J. Examination of circumscribed palmar hypokeratosis with line-field confocal optical coherence tomography: Dermoscopic, ultrasonographic and histopathologic correlates. Indian. J. Dermatol. Venereol. Leprol. 2020, 86, 206–208. [Google Scholar] [CrossRef] [PubMed]
- Bruzziches, F.; Tognetti, L.; Habougit, C.; Suppa, M.; del Marmol, V.; Cinotti, E.; Rubegni, P.; Perrot, J.L. Non-invasive imaging of a rare presentation of infantile generalized eruptive histiocytosis with xanthogranuloma-like appearance: Dermoscopy, reflectance confocal microscopy, and line-field optical coherence tomography. Int. J. Dermatol. 2022, 61, e288–e290. [Google Scholar] [CrossRef]
- Tognetti, L.; Cinotti, E.; Coriolani, G.; Suppa, M.; Perrot, J.L.; Vascotto, M.; Grosso, S.; Rubegni, P. Cutaneous lesions of Anderson-Fabry disease examined with a novel technique: Line-field confocal optical coherence tomography. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e371–e373. [Google Scholar] [CrossRef]
- Verzì, A.E.; Broggi, G.; Cinotti, E.; Tognetti, L.; Caltabiano, R.; Rubegni, P.; Micali, G.; Lacarrubba, F. Line-field confocal optical coherence tomography of Darier’s disease: A case series with histopathological correlation. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e885–e887. [Google Scholar] [CrossRef]
- Di Stefani, A.; Cappilli, S.; Ricci, C.; Costantini, A.; Paradisi, A.; Peris, K. Line-field confocal optical coherence tomography (LC-OCT) in Hailey-Hailey disease: Another brick in the wall. Int. J. Dermatol. 2023, 62, e178–e179. [Google Scholar] [CrossRef]
- Maione, V.; Tonon, F.; Soglia, S.; Venturuzzo, A.; Venturini, M.; Calzavara-Pinton, P. Line-Field Confocal Optical Coherence Tomography of a Suspected Case of Galli-Galli Disease. Dermatol. Pract. Concept. 2024, 14, e2024007. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, L.; Carraro, A.; Lamberti, A.; Cinotti, E.; Suppa, M.; Perrot, J.L.; Rubegni, P. Kaposi sarcoma of the glans: New findings by line field confocal optical coherence tomography examination. Skin. Res. Technol. 2021, 27, 285–287. [Google Scholar] [CrossRef]
- Chauvel-Picard, J.; Bérot, V.; Tognetti, L.; Cano, C.O.; Fontaine, M.; Lenoir, C.; Pérez-Anker, J.; Puig, S.; Dubois, A.; Forestier, S.; et al. Line-field confocal optical coherence tomography as a tool for three-dimensional in vivo quantification of healthy epidermis: A pilot study. J. Biophotonics. 2022, 15, e202100236. [Google Scholar] [CrossRef]
- Pedrazzani, M.; Breugnot, J.; Rouaud-Tinguely, P.; Cazalas, M.; Davis, A.; Bordes, S.; Dubois, A.; Closs, B. Comparison of line-field confocal optical coherence tomography images with histological sections: Validation of a new method for in vivo and non-invasive quantification of superficial dermis thickness. Skin. Res. Technol. 2020, 26, 398–404. [Google Scholar] [CrossRef]
- Breugnot, J.; Rouaud-Tinguely, P.; Gilardeau, S.; Rondeau, D.; Bordes, S.; Aymard, E.; Closs, B. Utilizing deep learning for dermal matrix quality assessment on in vivo line-field confocal optical coherence tomography images. Skin. Res. Technol. 2023, 29, e13221. [Google Scholar] [CrossRef] [PubMed]
- Bonnier, F.; Pedrazzani, M.; Fischman, S.; Viel, T.; Lavoix, A.; Pegoud, D.; Nili, M.; Jimenez, Y.; Ralambondrainy, S.; Cauchard, J.-H.; et al. Line-field confocal optical coherence tomography coupled with artificial intelligence algorithms to identify quantitative biomarkers of facial skin ageing. Sci. Rep. 2023, 13, 13881. [Google Scholar] [CrossRef]
- Razi, S.; Raquepo, T.M.; Truong, T.M.; Rao, B. Analyzing the effects of a chemical peel on post-inflammatory hyperpigmentation using line-field confocal optical coherence tomography. Skin. Res. Technol. 2023, 29, e13496. [Google Scholar] [CrossRef]
- Razi, S.; Truong, T.M.; Khan, S.; Sanabria, B.; Rao, B. In vivo assessment of early effects of diamond-tipped microdermabrasion through the lens of line-field confocal optical coherence tomography. J. Cosmet. Dermatol. 2024. [Google Scholar] [CrossRef]
- Razi, S.; Raquepo, T.M.; Rao, B. Analyzing the in vivo cutaneous effects of diode laser treatment using line-field confocal optical coherence tomography. J. Cosmet. Dermatol. 2024, 23, 717–719. [Google Scholar] [CrossRef]
- Jdid, R.; Pedrazzani, M.; Lejeune, F.; Fischman, S.; Cazorla, G.; Forestier, S.; Ben Khalifa, Y. Skin dark spot mapping and evaluation of brightening product efficacy using Line-field Confocal Optical Coherence Tomography (LC-OCT). Skin. Res. Technol. 2024, 30, e13623. [Google Scholar] [CrossRef]
- Razi, S.; Truong, T.M.; Khan, S.; Sanabria, B.; Rao, B. Hydradermabrasion through the lens of Line-Field Confocal Optical Coherence Tomography. Skin. Res. Technol. 2024, 30, e13684. [Google Scholar] [CrossRef]
- Tognetti, L.; Rizzo, A.; Fiorani, D.; Cinotti, E.; Perrot, J.L.; Rubegni, P. New findings in non-invasive imaging of aquagenic keratoderma: Line-field optical coherence tomography, dermoscopy and reflectance confocal microscopy. Skin. Res. Technol. 2020, 26, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, L.; Ekinde, S.; Habougit, C.; Cinotti, E.; Rubegni, P.; Perrot, J.L. Delayed Tattoo Reaction From Red Dye With Overlapping Clinicopathological Features: Examination With High-Frequency Ultrasound and Line-Field Optical Coherence Tomography. Dermatol. Pract. Concept. 2020, 10, e2020053. [Google Scholar] [CrossRef]
- Tognetti, L.; Fiorani, D.; Cinotti, E.; Rubegni, P. Tridimensional skin imaging in aquagenic keratoderma: Virtual histology by line-field confocal optical coherence tomography. Int. J. Dermatol. 2021, 60, e52–e54. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, L.; Bertello, M.; Cinotti, E.; Rubegni, P. Acquired digital fibrokeratoma: First observation by high-resolution skin ultrasound and line-field confocal optical coherence tomography. Indian. J. Dermatol. Venereol. Leprol. 2022, 88, 275. [Google Scholar] [CrossRef]
- Tognetti, L.; Cinotti, E.; Falcinelli, F.; Miracco, C.; Suppa, M.; Perrot, J.; Rubegni, P. Line-field confocal optical coherence tomography: A new tool for non-invasive differential diagnosis of pustular skin disorders. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1873–1883. [Google Scholar] [CrossRef]
- Thamm, J.R.; Welzel, J.; Schuh, S. Line-field confocal optical coherence tomography, optical coherence tomography and reflectance confocal microscopy in a case of cutaneous sarcoidosis. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e739–e741. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, G.; Cappilli, S.; Lugli, A.P.; Di Stefani, A.; Peris, K. Solitary cutaneous focal mucinosis: In-vivo line-field confocal optical coherence tomography (LC-OCT) features correlate to histopathology. Int. J. Dermatol. 2024, 63, 970–971. [Google Scholar] [CrossRef]
- Verzì, A.E.; Micali, G.; Lacarrubba, F. Line-field confocal optical coherence tomography of miliaria crystallina: An in vivo, three-dimensional imaging. JAAD Case Rep. 2024, 48, 83–84. [Google Scholar] [CrossRef]
- Lamberti, A.; Falcinelli, F.; Cinotti, E.; Bruzziches, F.; Rubegni, P.; Cota, C. Unilateral lichen planus pigmentosus inversus: Line-field confocal optical coherence tomography features and histopathological correlation. J. Eur. Acad. Dermatol. Venereol. 2024. [Google Scholar] [CrossRef]
- Falcinelli, F.; Lazzeri, L.; Rubegni, P.; Russo, F. Line-field confocal optical coherence tomography (LC-OCT) in bullous striae distensae. Ital. J. Dermatol. Venerol. 2024, 159, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Castellano, R.; D’Onghia, M.; Tognetti, L.; Suppa, M.; Giorgino, G.; Rubegni, P.; Cinotti, E. Line-field confocal optical coherence tomography to help the diagnosis of graphite tattoo. J. Eur. Acad. Dermatol. Venereol. 2024, 38, e729–e730. [Google Scholar] [CrossRef]
- Ariasi, C.; Licata, G.; Perazzolli, G.; Tonon, F.; Venturini, M.; Calzavara-Pinton, P.G.; Soglia, S. Features of tattoo-associated cutaneous lymphoid hyperplasia on reflectance confocal microscopy and line-field confocal optical coherence tomography. Australas. J. Dermatol. 2024, 65, e50–e55. [Google Scholar] [CrossRef] [PubMed]
- Pellacani, G.; Witkowski, A.; Cesinaro, A.M.; Losi, A.; Colombo, G.; Campagna, A.; Longo, C.; Piana, S.; De Carvalho, N.; Giusti, F.; et al. Cost-benefit of reflectance confocal microscopy in the diagnostic performance of melanoma. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 413–419. [Google Scholar] [CrossRef]
- Muzumdar, S.; Wu, R.; Rothe, M.J.; Grant-Kels, J.M. Reflectance confocal microscopy decreases the cost of skin lesion diagnosis: A single institution retrospective chart review. J. Am. Acad. Dermatol. 2022, 86, 209–211. [Google Scholar] [CrossRef]
| Authors and Year of Publication | Disease | Number of Patients | |
|---|---|---|---|
| melanocytic lesions | Lenoir et al., 2021 [21] | dermal nevi | 7 |
| Schuh et al., 2022 [23] | nevi and melanoma | 75 | |
| Perez-Anker et al., 2022 [26] | morphological evaluation of melanocytic lesions | 12 | |
| Verzi’ et al., 2023 [24] | lentigo maligna | 1 | |
| Zeinaty et al., 2023 [28] | pigmented lesions of genital area | 9 | |
| Wolswijk et al., 2023 [25] | nodular melanoma | 1 | |
| Soglia et al., 2024 [27] | melanocytic skin tumours | ||
| non- melanocytic lesions | Dubois et al., 2018 [16] | BCC | 86 |
| Ruini et al., 2021 [29] | AK | 50 | |
| Ruini et al., 2021 [30] | BCC | 52 | |
| Ruini et al., 2021 [31] | keratinocyte skin cancers | 73 | |
| Lenoir et al., 2021 [32] | AK | 16 | |
| Lenoir et al., 2021 [33] | sebaceous hyperplasia | 12 | |
| Verzì et al., 2021 [34] | monitoring of superficial BCC treated with imiquimod 5% cream | 12 | |
| Lacarrubba et al., 2021 [35] | Xanthogranuloma | 1 | |
| Cinotti et al., 2021 [36] | AK and SCC | 158 | |
| Cappilli et al., 2021 [37] | BCC | 1 | |
| Cappilli et al., 2022 [38] | fibroepithelioma of Pinkus | 5 | |
| Gust et al., 2022 [39] | BCC | 154 | |
| Cappilli et al., 2022 [40] | basosquamous carcinoma | 8 | |
| Cinotti et al., 2022 [41] | keratinocyte skin cancers | 52 | |
| Di Stefani et al., 2023 [42] | extramammary Paget disease | 1 | |
| Di Stefani et al., 2023 [22] | eyelid skin lesions | 51 | |
| Soglia et al., 2023 [43] | merkel cell carcinoma | 1 | |
| Lenoir et al., 2023 [44] | seborrheic keratosis | 29 | |
| Donelli et al., 2023 [45] | keratinocyte skin cancers | 360 | |
| Lacarrubba et al., 2023 [46] | treatment monitoring of AK with tirbanibulin | 10 | |
| Cinotti et al., 2023 [47] | BCC | 62 | |
| Aktas et al., 2023 [48] | differential diagnosis of infiltrative BCC with scar-like lesion | 4 | |
| Cortonesi et al., 2023 [49] | clear cell acanthoma | 7 | |
| Maione et al., 2023 [50] | eccrine poroma | 1 | |
| Verzì et al., 2023 [51] | BCC, SCC, nevi, seborrheic keratoses, pyogenic granuloma, trichilemmal cysts, hidrocystoma | 28 | |
| Soglia et al., 2023 [52] | cutaneous mycosis fungoides | 10 | |
| Jacobsen et al., 2024 [53] | surgical planning for recurrent infiltrative BCC | ||
| Paradisi et al., 2024 [54] | preoperative evaluation of high-risk BCC | 60 | |
| inflammatory and autoimmune skin diseases | Tognetti et al., 2021 [55] | autoimmune bullous diseases | 30 |
| Verzì et al., 2022 [56] | psoriasis, eczema and lichen planus | 15 | |
| Truong et al. 2023 [57] | scalp psoriasis | 1 | |
| Orsini et al., 2024 [58] | psoriasis treatment monitoring | 17 | |
| Falcinelli et al., 2024 [59] | sebo-psoriasis-like dermatosis reaction to ixekizumab | 1 | |
| Russo et al., 2024 [60] | evaluation of Patch Test |
| Authors and Year of Publication | Disease | Number of Patients | |
|---|---|---|---|
| hair and nails | Hobelsberger S et al., 2023 [70] | healthy nails and onychomycosis | 13 |
| Kurzeja et al., 2023 [64] | lichen planopilaris | 6 | |
| Eijkenboom et al., 2024 [71] | leukonychia, subungual haemorrhage, psoriasis, lichen planus, longitudinal melanonychia, subungual melanoma and onychomycosis | 16 | |
| Eijkenboom et al., 2024 [69] | onychomycosis | 100 | |
| Lacarrubba et al., 2024 [66] | Alopecia areata | 65 | |
| Verzì et al., 2024 [67] | Alopecia areata | 10 | |
| Kurzeja et al., 2024 [65] | folliculitis decalvans | 5 | |
| Falcinelli et al., 2024 [68] | tinea capitis | 1 | |
| skin infectious disorders | Ruini et al., 2020 [72] | scabies | 1 |
| Ruini et al., 2021 [73] | scabies | 1 | |
| Lacarrubba et al., 2021 [74] | herpes infection | 5 | |
| Verzì et al., 2021 [75] | molluscum contagiosum | 6 | |
| Cortonesi et al., 2022 [76] | cowpox virus skin infection | 1 | |
| Cappilli et al., 2022 [77] | trombiculosis | 1 | |
| Orsini et al., 2023 [78] | nodular scabies | 1 | |
| Pathak et al., 2023 [79] | pityriasis rosea | 1 | |
| Idoudi et al., 2024 [80] | scabies | 1 | |
| Cinotti et al., 2024 [81] | genital warts, molluscum contagiosus, Fordyce’s spot, acquired lymphangiomas | 14 | |
| pediatric conditions | Tognetti et al., 2021 [82] | lichenoid dermatoses | 10 |
| C Gallay et al., 2022 [83] | pyogenic granuloma | 2 | |
| Del Río-Sancho, 2023 [84] | healthy skin | 67 | |
| Del Río-Sancho et al., 2023 [85] | agminated spitz nevus | 1 | |
| Cappilli S. et al., 2023 [86] | melanocytic lesions, vascular lesions, scabies, viral warts, pyogenic granulomas, tinea corporis | 73 |
| Authors and Year of Publication | Disease | Number of Patients | |
|---|---|---|---|
| skin vascular lesions | Tognetti et al., 2021 [93] | kaposi sarcoma | 1 |
| Cappilli et al., 2023 [63] | cherry haemangiomas, angiokeratomas, pyogenic granulomas, venous lakes, targetoid haemosiderotic haemangiomas, kaposi sarcomas, extraungual glomus tumours | 50 | |
| genetic diseases | Tognetti et al., 2020 [87] | circumscribed palmar hypokeratosis | 3 |
| Bruzziches et al., 2022 [88] | generalized eruptive histiocytosis | 1 | |
| Tognetti et al., 2022 [89] | Anderson–Fabry disease | 1 | |
| Di Stefani et al., 2023 [91] | Hailey–Haiey disease | 1 | |
| Verzì et al., 2023 [90] | Darier’s disease | 8 | |
| Maione et al., 2024 [92] | Galli–Galli disease | 1 | |
| cosmetic applications | Chauvel-Picard et al., 2022 [94] | in vivo quantification of healthy epidermis | 8 |
| Pedrazzani et al., 2020 [95] | quantification of superficial dermis thickness | 36 | |
| Breugnot et al., 2023 [96] | dermal matrix quality assessment | 57 | |
| Bonnier et al., 2023 [97] | quantitative biomarkers of facial skin aging | 100 | |
| Razi et al., 2022 [98] | effects of a chemical peel on post-inflammatory hyperpigmentation | 1 | |
| Razi et al., 2024 [99] | early effects of diamond-tipped microdermabrasion | 8 | |
| Razi et al., 2024 [100] | cutaneous effects of diode laser | 3 | |
| Jdid et al., 2024 [101] | skin dark spot mapping and evaluation of brightening product efficacy | 26 | |
| Razi et al., 2024 [102] | hydradermabrasion | 8 | |
| Others | Tognetti et al., 2020 [103] | aquagenic keratoderma | 3 |
| Tognetti et al., 2020 [104] | delayed Tattoo Reaction | 1 | |
| Tognetti et al., 2021 [105] | aquagenic keratoderma | 1 | |
| Tognetti et al., 2022 [106] | acquired digital fibrokeratoma | 1 | |
| Tognetti et al., 2022 [107] | pustular skin disorders | 19 | |
| Thamm et al., 2023 [108] | cutaneous sarcoidosis | 1 | |
| Palmisano et al., 2024 [109] | solitary cutaneous focal mucinosis | 1 | |
| Verzì et al., 2024 [110] | miliaria crystallina | 1 | |
| Lamberti et al., 2024 [111] | lichen planus pigmentosus inversus | 1 | |
| Falcinelli et al., 2024 [112] | bullous striae distensae | 1 | |
| Castellano et al., 2024 [113] | graphite tattoo | 1 | |
| Ariasi et al., 2024 [114] | tattoo-associated cutaneous lymphoid hyperplasia | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cappilli, S.; Paradisi, A.; Di Stefani, A.; Palmisano, G.; Pellegrino, L.; D’Onghia, M.; Ricci, C.; Tognetti, L.; Verzì, A.E.; Rubegni, P.; et al. Line-Field Confocal Optical Coherence Tomography: A New Skin Imaging Technique Reproducing a “Virtual Biopsy” with Evolving Clinical Applications in Dermatology. Diagnostics 2024, 14, 1821. https://doi.org/10.3390/diagnostics14161821
Cappilli S, Paradisi A, Di Stefani A, Palmisano G, Pellegrino L, D’Onghia M, Ricci C, Tognetti L, Verzì AE, Rubegni P, et al. Line-Field Confocal Optical Coherence Tomography: A New Skin Imaging Technique Reproducing a “Virtual Biopsy” with Evolving Clinical Applications in Dermatology. Diagnostics. 2024; 14(16):1821. https://doi.org/10.3390/diagnostics14161821
Chicago/Turabian StyleCappilli, Simone, Andrea Paradisi, Alessandro Di Stefani, Gerardo Palmisano, Luca Pellegrino, Martina D’Onghia, Costantino Ricci, Linda Tognetti, Anna Elisa Verzì, Pietro Rubegni, and et al. 2024. "Line-Field Confocal Optical Coherence Tomography: A New Skin Imaging Technique Reproducing a “Virtual Biopsy” with Evolving Clinical Applications in Dermatology" Diagnostics 14, no. 16: 1821. https://doi.org/10.3390/diagnostics14161821






