Ultrafast Brain MRI at 3 T for MS: Evaluation of a 51-Second Deep Learning-Enhanced T2-EPI-FLAIR Sequence
Abstract
:1. Introduction
2. Materials and Methods
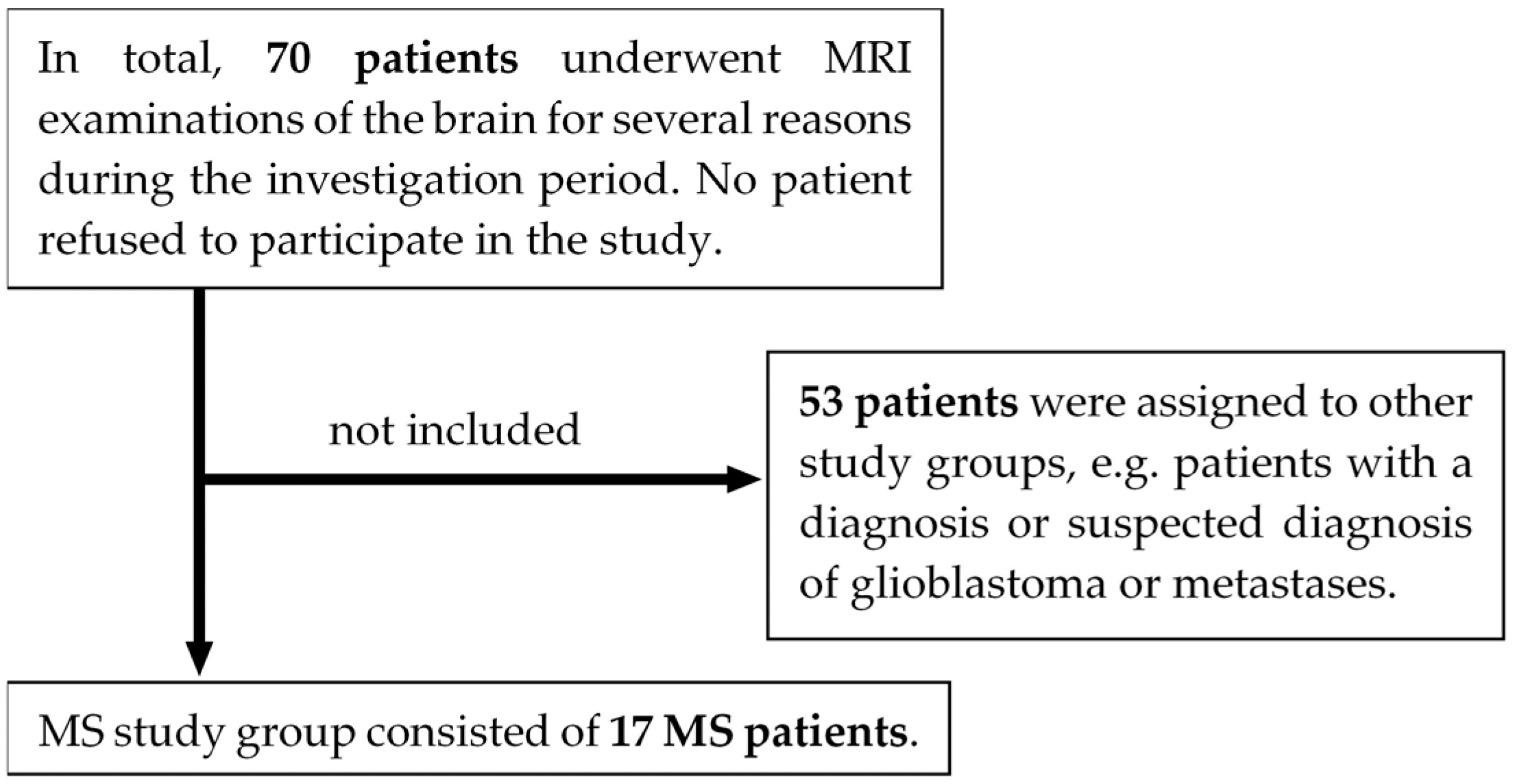
2.1. Study Design
2.2. Imaging Protocol and Image Acquisition
2.3. FLAIRUF Sequence
2.4. Image Evaluation
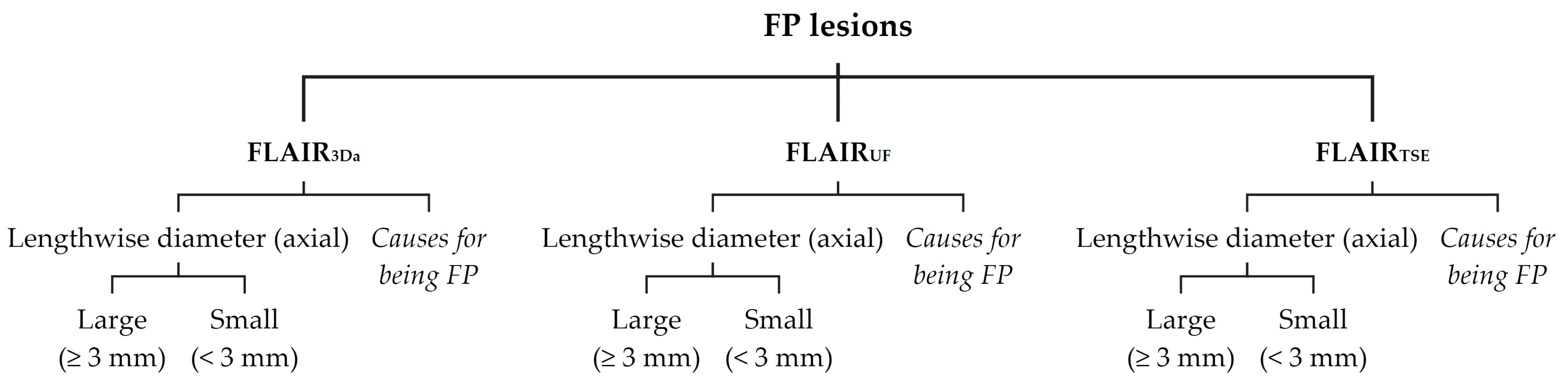
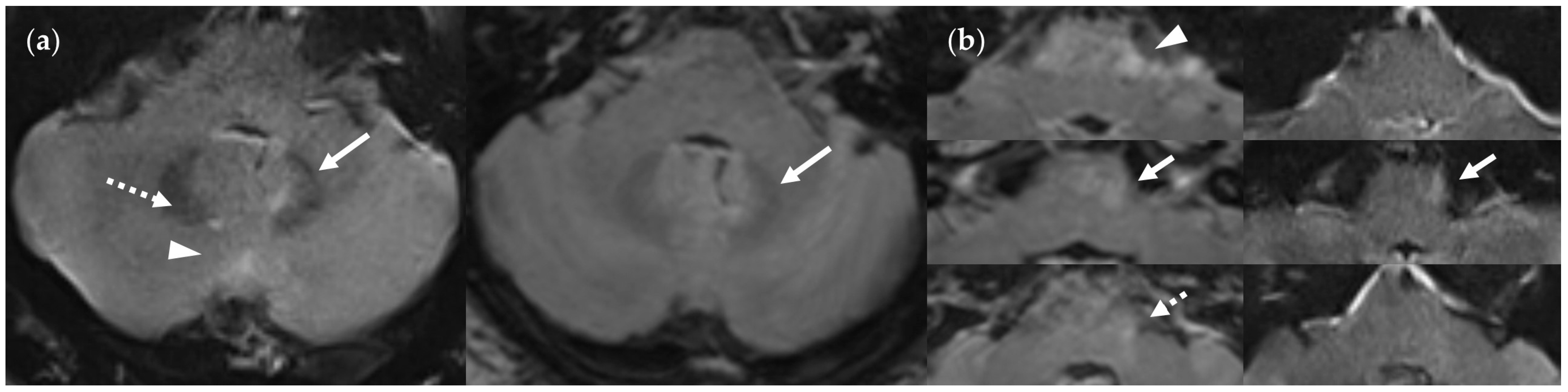
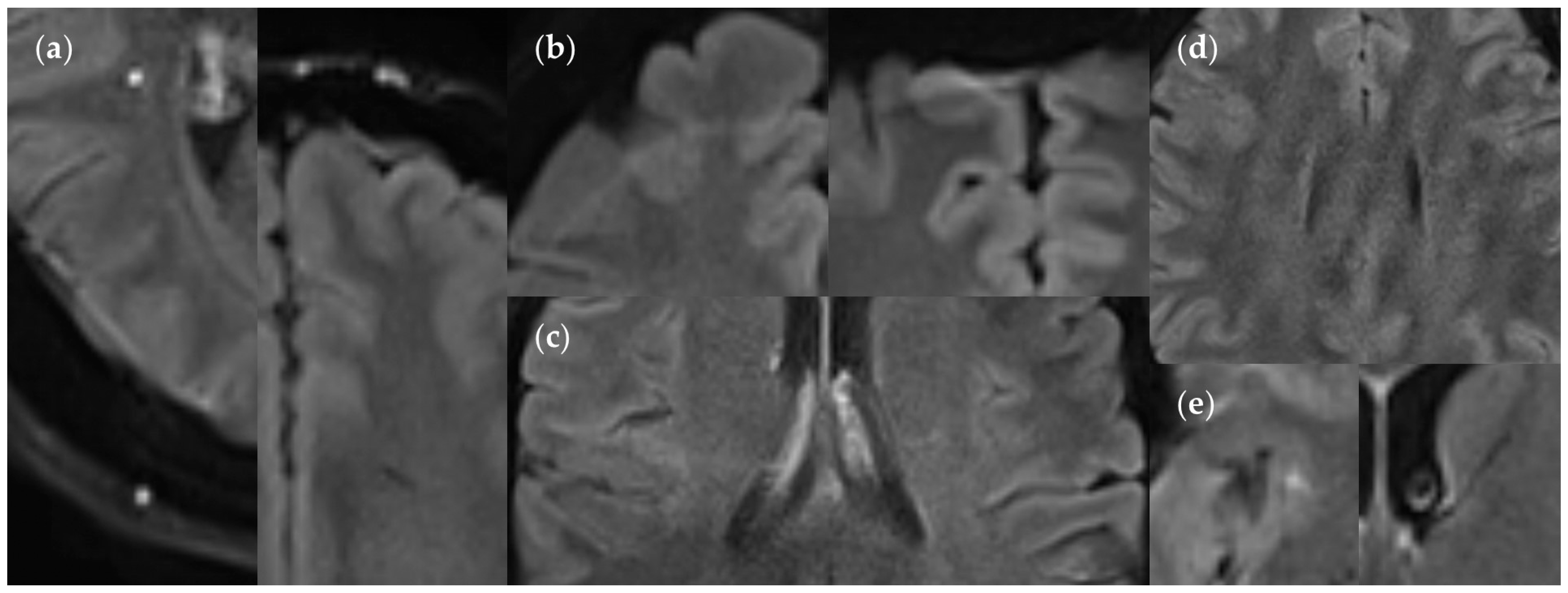
2.4.1. Lesion Assessment
2.4.2. Image Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Image Acquisitions and Lesions
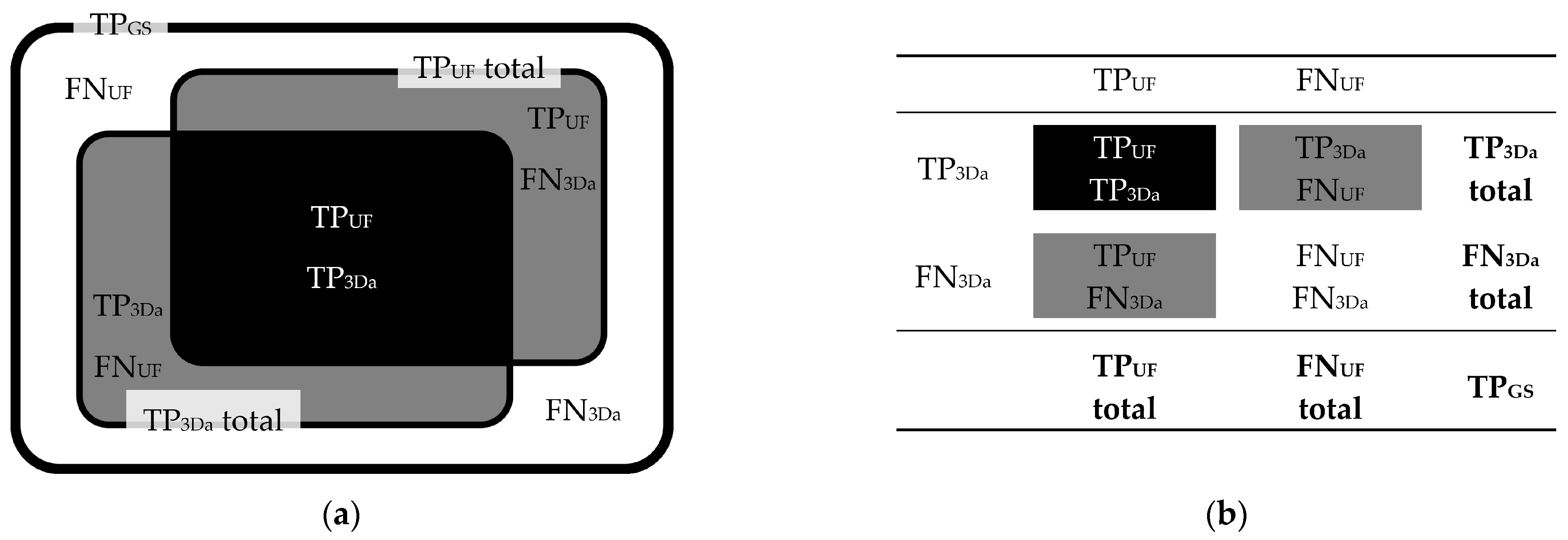
3.2. Lesion Detection
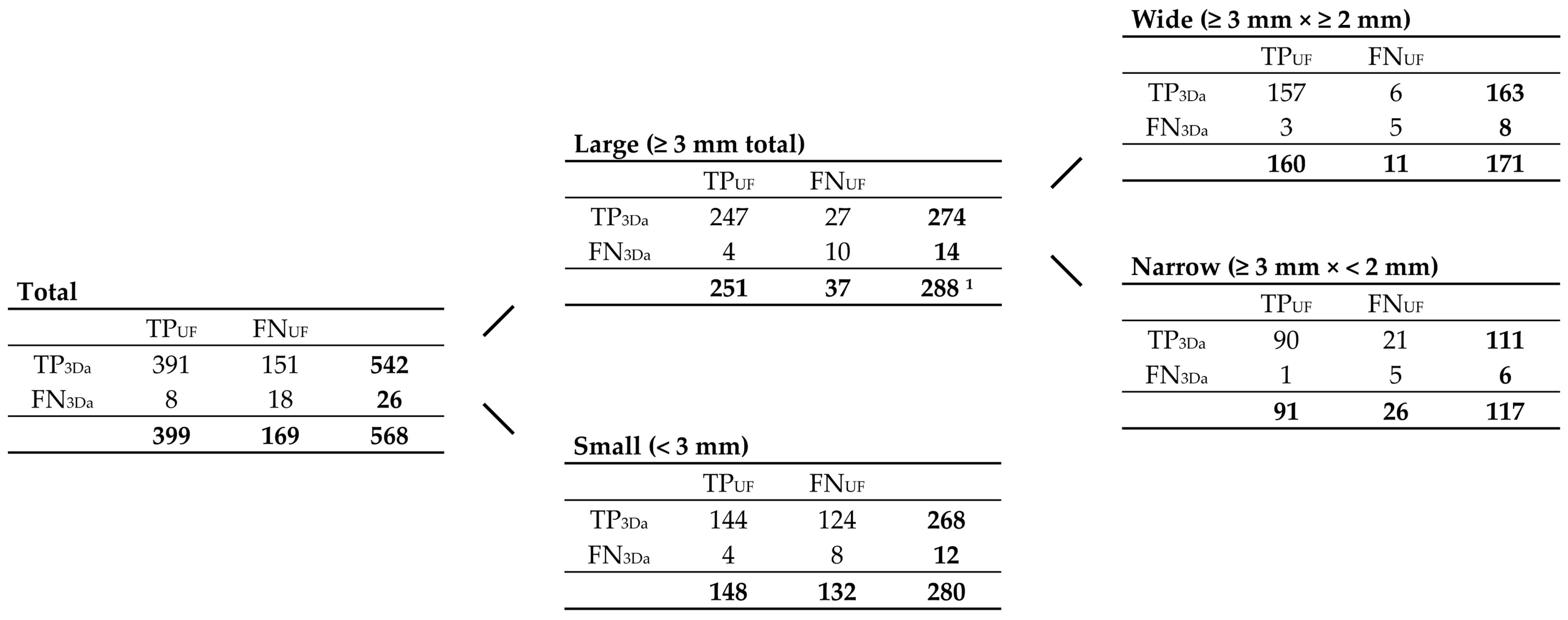
3.2.1. FLAIRUF Compared with FLAIR3Da
3.2.2. FLAIRUF Compared with FLAIRTSE
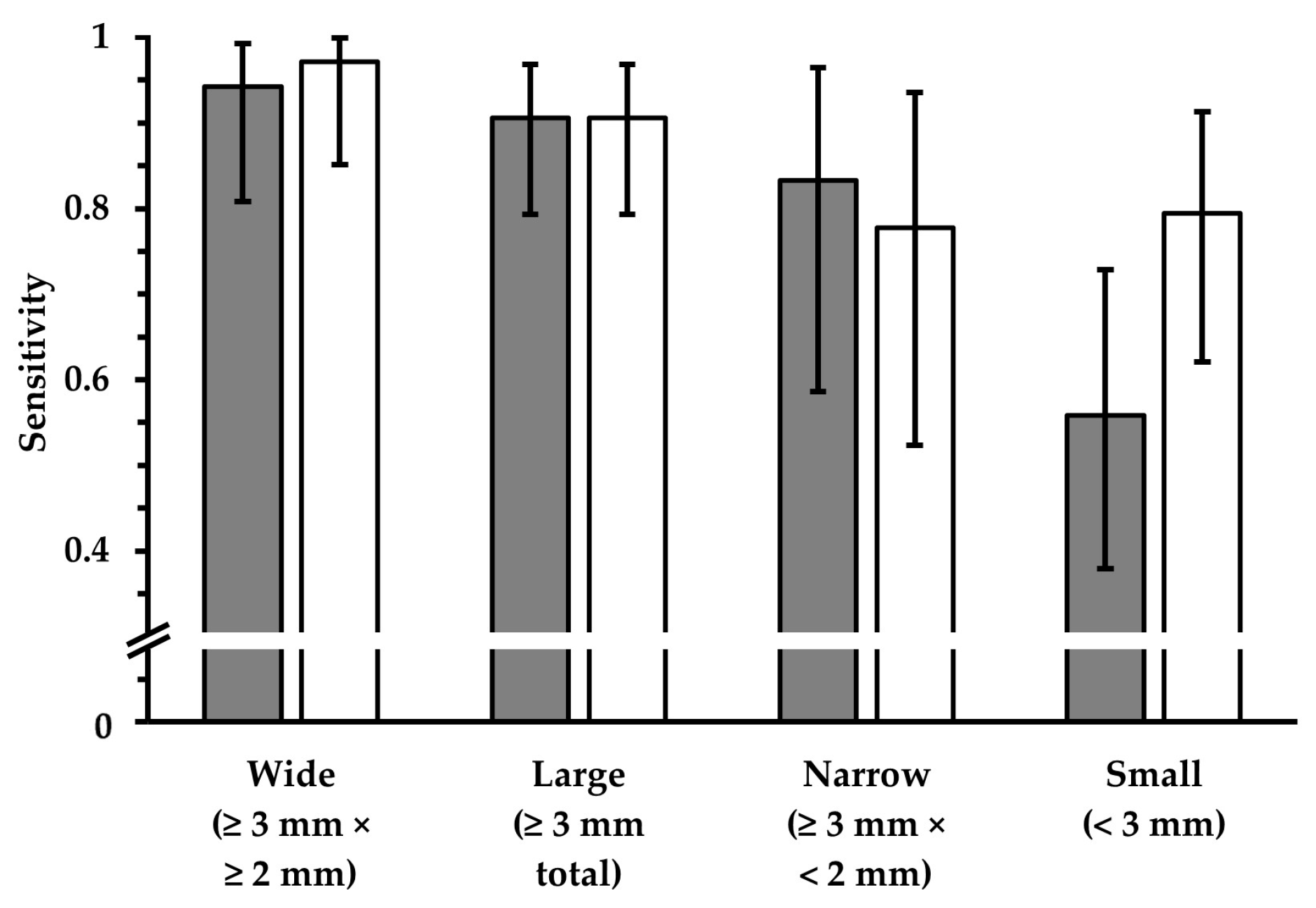
3.2.3. Dependence on Size and Location within FLAIRUF
3.3. Image Quality
3.3.1. FLAIRUF Compared with FLAIR3Da
3.3.2. FLAIRUF Compared with FLAIRTSE
3.3.3. Positional Dependence of SNR and CNR in FLAIRUF
4. Discussion
4.1. Main Findings of This Study
4.2. Significance of MRI and Ultrafast MRI
4.3. Limitations of the FLAIRUF Images
4.4. Considerations on Ratings for Lesion Conspicuity in FLAIRUF
4.5. Outcomes Correlated with Technical Features
4.6. Limitations of the Study
4.7. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mansfield, P. Multi-planar image formation using NMR spin echoes. J. Phys. C Solid State Phys. 1977, 10, L55–L58. [Google Scholar] [CrossRef]
- Poustchi-Amin, M.; Mirowitz, S.A.; Brown, J.J.; McKinstry, R.C.; Li, T. Principles and applications of echo-planar imaging: A review for the general radiologist. Radiographics 2001, 21, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Okudera, T.; Tamura, H.; Ogawa, T.; Hatazawa, J. Assessment of Lacunar Hemorrhage Associated With Hypertensive Stroke by Echo-Planar Gradient-Echo T2*-Weighted MRI. Stroke 2000, 31, 1646–1650. [Google Scholar] [CrossRef] [PubMed]
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Leray, E.; Moreau, T.; Fromont, A.; Edan, G. Epidemiology of multiple sclerosis. Rev. Neurol. 2016, 172, 3–13. [Google Scholar] [CrossRef]
- McDonald, W.I.; Compston, A.; Edan, G.; Goodkin, D.; Hartung, H.P.; Lublin, F.D.; McFarland, H.F.; Paty, D.W.; Polman, C.H.; Reingold, S.C.; et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann. Neurol. 2001, 50, 121–127. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Edan, G.; Filippi, M.; Hartung, H.-P.; Kappos, L.; Lublin, F.D.; Metz, L.M.; McFarland, H.F.; O′Connor, P.W.; et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann. Neurol. 2005, 58, 840–846. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Kaunzner, U.W.; Gauthier, S.A. MRI in the assessment and monitoring of multiple sclerosis: An update on best practice. Ther. Adv. Neurol. Disord. 2017, 10, 247–261. [Google Scholar] [CrossRef]
- Filippi, M.; Rocca, M.A.; Wiessmann, M.; Mennea, S.; Cercignani, M.; Yousry, T.A.; Sormani, M.P.; Comi, G. A Comparison of MR Imaging with Fast-FLAIR, HASTE-FLAIR, and EPI-FLAIR Sequences in the Assessment of Patients with Multiple Sclerosis. AJNR Am. J. Neuroradiol. 1999, 20, 1931–1938. [Google Scholar] [PubMed]
- Gebarski, S.S.; Gabrielsen, T.O.; Gilman, S.; Knake, J.E.; Latack, J.T.; Aisen, A.M. The initial diagnosis of multiple sclerosis: Clinical impact of magnetic resonance imaging. Ann. Neurol. 1985, 17, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Barkhof, F.; Filippi, M.; Miller, D.H.; Scheltens, P.; Campi, A.; Polman, C.H.; Comi, G.; Adèr, H.J.; Losseff, N.; Valk, J. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 1997, 120, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Grahl, S.; Pongratz, V.; Schmidt, P.; Engl, C.; Bussas, M.; Radetz, A.; Gonzalez-Escamilla, G.; Groppa, S.; Zipp, F.; Lukas, C.; et al. Evidence for a white matter lesion size threshold to support the diagnosis of relapsing remitting multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 29, 124–129. [Google Scholar] [CrossRef]
- Selvikvåg Lundervold, A.; Lundervold, A. An overview of deep learning in medical imaging focusing on MRI. Z. Med. Phys. 2019, 29, 102–127. [Google Scholar] [CrossRef] [PubMed]
- Radmanesh, A.; Muckley, M.J.; Murrell, T.; Lindsey, E.; Sriram, A.; Knoll, F.; Sodickson, D.K.; Lui, Y.W. Exploring the Acceleration Limits of Deep Learning Variational Network–based Two-dimensional Brain MRI. Radiol. Artif. Intell. 2022, 4, e210313. [Google Scholar] [CrossRef]
- Mani, A.; Santini, T.; Puppala, R.; Dahl, M.; Venkatesh, S.; Walker, E.; DeHaven, M.; Isitan, C.; Ibrahim, T.S.; Wang, L.; et al. Applying Deep Learning to Accelerated Clinical Brain Magnetic Resonance Imaging for Multiple Sclerosis. Front. Neurol. 2021, 12, 685276. [Google Scholar] [CrossRef]
- Estler, A.; Hauser, T.-K.; Mengel, A.; Brunnée, M.; Zerweck, L.; Richter, V.; Zuena, M.; Schuhholz, M.; Ernemann, U.; Gohla, G. Deep Learning Accelerated Image Reconstruction of Fluid-Attenuated Inversion Recovery Sequence in Brain Imaging: Reduction of Acquisition Time and Improvement of Image Quality. Acad. Radiol. 2024, 31, 180–186. [Google Scholar] [CrossRef]
- Iwamura, M.; Ide, S.; Sato, K.; Kakuta, A.; Tatsuo, S.; Nozaki, A.; Wakayama, T.; Ueno, T.; Haga, R.; Kakizaki, M.; et al. Thin-slice Two-dimensional T2-weighted Imaging with Deep Learning-based Reconstruction: Improved Lesion Detection in the Brain of Patients with Multiple Sclerosis. Magn. Reson. Med. Sci. 2024, 23, 184–192. [Google Scholar] [CrossRef]
- Toledano-Massiah, S.; Sayadi, A.; de Boer, R.; Gelderblom, J.; Mahdjoub, R.; Gerber, S.; Zuber, M.; Zins, M.; Hodel, J. Accuracy of the Compressed Sensing Accelerated 3D-FLAIR Sequence for the Detection of MS Plaques at 3T. AJNR Am. J. Neuroradiol. 2018, 39, 454–458. [Google Scholar] [CrossRef]
- Liebig, P.A.; Heidemann, R.M.; Hensel, B.; Porter, D.A. A new approach to accelerate readout segmented EPI with compressed sensing. Magn. Reson. Med. 2020, 84, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xi, B.; Sun, H.; Wang, J.; Chen, C.; Wen, X.; Zhang, Y.; Zeng, M. The clinical feasibility of artificial intelligence-assisted compressed sensing single-shot fluid-attenuated inversion recovery (ACS-SS-FLAIR) for evaluation of uncooperative patients with brain diseases: Comparison with the conventional T2-FLAIR with parallel imaging. Acta Radiol. 2022, 64, 1943–1949. [Google Scholar] [CrossRef]
- Cristobal-Huerta, A.; Poot, D.H.J.; Vogel, M.W.; Krestin, G.P.; Hernandez-Tamames, J.A. Compressed Sensing 3D-GRASE for faster High-Resolution MRI. Magn. Reson. Med. 2019, 82, 984–999. [Google Scholar] [CrossRef]
- Runge, V.M.; Richter, J.K.; Heverhagen, J.T. Simultaneous Multi-Slice—A Concise Review Covering Major Applications in Clinical Practice. MAGNETOM Flash 2017, 68, 96–101. [Google Scholar]
- Setsompop, K.; Cauley, S.F.; Wald, L.L. Advancing Diffusion MRI Using Simultaneous Multi-Slice Echo Planar Imaging. MAGNETOM Flash 2015, 63, 16–22. [Google Scholar]
- Runge, V.M.; Richter, J.K.; Heverhagen, J.T. Speed in Clinical Magnetic Resonance. Investig. Radiol. 2017, 52, 1–17. [Google Scholar] [CrossRef]
- Schulz, J.; Marques, J.P.; ter Telgte, A.; Dorst, A.; de Leeuw, F.-E.; Meijer, F.J.; Norris, D.G. Clinical application of Half Fourier Acquisition Single Shot Turbo Spin Echo (HASTE) imaging accelerated by simultaneous multi-slice acquisition. Eur. J. Radiol. 2018, 98, 200–206. [Google Scholar] [CrossRef]
- Cho, J.; Liao, C.; Tian, Q.; Zhang, Z.; Xu, J.; Lo, W.-C.; Poser, B.A.; Stenger, V.A.; Stockmann, J.; Setsompop, K.; et al. Highly accelerated EPI with wave encoding and multi-shot simultaneous multislice imaging. Magn. Reson. Med. 2022, 88, 1180–1197. [Google Scholar] [CrossRef]
- Eliezer, M.; Vaussy, A.; Toupin, S.; Barbe, R.; Kannengiesser, S.; Stemmer, A.; Houdart, E. Iterative denoising accelerated 3D SPACE FLAIR sequence for brain MR imaging at 3T. Diagn. Interv. Imaging 2022, 103, 13–20. [Google Scholar] [CrossRef]
- Quint, R.; Vaussy, A.; Stemmer, A.; Hautefort, C.; Houdart, E.; Eliezer, M. Iterative Denoising Accelerated 3D FLAIR Sequence for Hydrops MR Imaging at 3T. AJNR Am. J. Neuroradiol. 2023, 44, 1064–1069. [Google Scholar] [CrossRef]
- Granberg, T.; Uppman, M.; Hashim, F.; Cananau, C.; Nordin, L.E.; Shams, S.; Berglund, J.; Forslin, Y.; Aspelin, P.; Fredrikson, S.; et al. Clinical Feasibility of Synthetic MRI in Multiple Sclerosis: A Diagnostic and Volumetric Validation Study. AJNR Am. J. Neuroradiol. 2016, 37, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, A.; Hori, M.; Yokoyama, K.; Takemura, M.; Andica, C.; Tabata, T.; Kamagata, K.; Suzuki, M.; Kumamaru, K.; Nakazawa, M.; et al. Synthetic MRI in the Detection of Multiple Sclerosis Plaques. AJNR Am. J. Neuroradiol. 2017, 38, 257–263. [Google Scholar] [CrossRef]
- Hagiwara, A.; Otsuka, Y.; Hori, M.; Tachibana, Y.; Yokoyama, K.; Fujita, S.; Andica, C.; Kamagata, K.; Irie, R.; Koshino, S.; et al. Improving the Quality of Synthetic FLAIR Images with Deep Learning Using a Conditional Generative Adversarial Network for Pixel-by-Pixel Image Translation. AJNR Am. J. Neuroradiol. 2019, 40, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.; Baek, H.; Cho, S.; Ha, J.; Kim, T.; Hwang, M. Clinical feasibility of 1-min ultrafast brain MRI compared with routine brain MRI using synthetic MRI: A single center pilot study. J. Neurol. 2019, 266, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Dong, Z.; Reese, T.G.; Bilgic, B.; Manhard, M.K.; Chen, J.; Polimeni, J.R.; Wald, L.L.; Setsompop, K. Echo planar time-resolved imaging (EPTI). Magn. Reson. Med. 2019, 81, 3599–3615. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Dong, Z.; Reese, T.G.; Rosen, B.; Wald, L.L.; Setsompop, K. 3D Echo Planar Time-resolved Imaging (3D-EPTI) for ultrafast multi-parametric quantitative MRI. Neuroimage 2022, 250, 118963. [Google Scholar] [CrossRef]
- Kerleroux, B.; Kober, T.; Hilbert, T.; Serru, M.; Philippe, J.; Sirinelli, D.; Morel, B. Clinical equivalence assessment of T2 synthesized pediatric brain magnetic resonance imaging. J. Neuroradiol. 2019, 46, 130–135. [Google Scholar] [CrossRef]
- Gruenebach, N.; Abello Mercado, M.A.; Grauhan, N.F.; Sanner, A.; Kronfeld, A.; Groppa, S.; Schoeffling, V.I.; Hilbert, T.; Brockmann, M.A.; Othman, A.E. Clinical feasibility and validation of the accelerated T2 mapping sequence GRAPPATINI in brain imaging. Heliyon 2023, 9, e15064. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.; Clifford, B.; Lo, W.-C.; Applewhite, B.P.; Tabari, A.; Goncalves Filho, A.L.M.; Hosseini, Z.; Longo, M.G.F.; Cauley, S.F.; Setsompop, K.; et al. Clinical Evaluation of a 2-Minute Ultrafast Brain MR Protocol for Evaluation of Acute Pathology in the Emergency and Inpatient Settings. AJNR Am. J. Neuroradiol. 2024, 45, 379–385. [Google Scholar] [CrossRef]
- Altmann, S.; Grauhan, N.F.; Brockstedt, L.; Kondova, M.; Schmidtmann, I.; Paul, R.; Clifford, B.; Feiweier, T.; Hosseini, Z.; Uphaus, T.; et al. Ultrafast Brain MRI with Deep Learning Reconstruction for Suspected Acute Ischemic Stroke. Radiology 2024, 310, e231938. [Google Scholar] [CrossRef]
- Verclytte, S.; Gnanih, R.; Verdun, S.; Feiweier, T.; Clifford, B.; Ambarki, K.; Pasquini, M.; Ding, J. Ultrafast MRI using deep learning echoplanar imaging for a comprehensive assessment of acute ischemic stroke. Eur. Radiol. 2023, 33, 3715–3725. [Google Scholar] [CrossRef]
- Altmann, S.; Abello Mercado, M.A.; Brockstedt, L.; Kronfeld, A.; Clifford, B.; Feiweier, T.; Uphaus, T.; Groppa, S.; Brockmann, M.A.; Othman, A.E. Ultrafast Brain MRI Protocol at 1.5 T Using Deep Learning and Multi-shot EPI. Acad. Radiol. 2023, 30, 2988–2998. [Google Scholar] [CrossRef] [PubMed]
- Clifford, B.; Conklin, J.; Huang, S.Y.; Feiweier, T.; Hosseini, Z.; Gonçalves Filho, A.L.M.; Tabari, A.; Demir, S.; Lo, W.-C.; Longo, M.G.F.; et al. An artificial intelligence-accelerated 2-minute multi-shot echo planar imaging protocol for comprehensive high-quality clinical brain imaging. Magn. Reson. Med. 2022, 87, 2453–2463. [Google Scholar] [CrossRef] [PubMed]
- Clifford, B.; Conklin, J.; Huang, S.; Feiweier, T.; Hosseini, Z.; Gonçalves Filho, A.L.M.; Tabari, A.; Demir, S.; Lo, W.-C.; Figueiro Longo, M.G.; et al. Clinical evaluation of an AI-accelerated two-minute multi-shot EPI protocol for comprehensive high-quality brain imaging. In Proceedings of the ISMRM & SMRT Annual Meeting & Exhibition, Virtual Meeting, 15–20 May 2021. [Google Scholar]
- Conklin, J.; Clifford, B.; Bollmann, S.; Lo, W.-C.; Bilgic, B.; Cauley, S.; Setsompop, K.; Feiweier, T.; Kirsch, J.; Gonzalez, R.; et al. A comprehensive multi-shot EPI protocol for high-quality clinical brain imaging in 3 minutes. In Proceedings of theISMRM & SMRT Annual Meeting & Exhibition, Virtual Meeting, 8–14 August 2020. [Google Scholar]
- Tabari, A.; Clifford, B.; Gonçalves Filho, A.L.M.; Hosseini, Z.; Feiweier, T.; Lo, W.-C.; Figueiro Longo, M.G.; Setsompop, K.; Bilgic, B.; Rapalino, O.; et al. Ultrafast Brain Imaging with Deep Learning Multi-Shot EPI: Preliminary Clinical Evaluation. MAGNETOM Flash 2021, 79, 66–70. [Google Scholar]
- Skare, S.; Sprenger, T.; Norbeck, O.; Rydén, H.; Blomberg, L.; Avventi, E.; Engström, M. A 1-minute full brain MR exam using a multicontrast EPI sequence. Magn. Reson. Med. 2018, 79, 3045–3054. [Google Scholar] [CrossRef]
- Sprenger, T.; Kits, A.; Norbeck, O.; van Niekerk, A.; Berglund, J.; Rydén, H.; Avventi, E.; Skare, S. NeuroMix—A single-scan brain exam. Magn. Reson. Med. 2021, 87, 2178–2193. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.F.; Kits, A.; Bystam, J.; Kaijser, M.; Skorpil, M.; Sprenger, T.; Skare, S. Diagnostic performance of a new multicontrast one-minute full brain exam (EPIMix) in neuroradiology: A prospective study. J. Magn. Reson. Imaging 2019, 50, 1824–1833. [Google Scholar] [CrossRef]
- Ryu, K.H.; Baek, H.; Skare, S.; Moon, J.I.; Choi, B.H.; Park, S.E.; Ha, J.Y.; Kim, T.B.; Hwang, M.; Sprenger, T. Clinical Experience of 1-Minute Brain MRI Using a Multicontrast EPI Sequence in a Different Scan Environment. AJNR Am. J. Neuroradiol. 2020, 41, 424–429. [Google Scholar] [CrossRef]
- Kits, A.; Luca, F.; Kolloch, J.; Müller, S.; Mazya, M.; Skare, S.; Delgado, A.F. One-Minute Multi-contrast Echo Planar Brain MRI in Ischemic Stroke: A Retrospective Observational Study of Diagnostic Performance. J. Magn. Reson. Imaging 2021, 54, 1088–1095. [Google Scholar] [CrossRef]
- Burén, S.; Kits, A.; Lönn, L.; Luca, F.; Sprenger, T.; Skare, S.; Delgado, A.F. A 78 Seconds Complete Brain MRI Examination in Ischemic Stroke: A Prospective Cohort Study. J. Magn. Reson. Imaging 2022, 56, 884–892. [Google Scholar] [CrossRef]
- Chung, M.S.; Lee, J.Y.; Jung, S.C.; Baek, S.; Shim, W.H.; Park, J.E.; Kim, H.S.; Choi, C.G.; Kim, S.J.; Lee, D.H.; et al. Reliability of fast magnetic resonance imaging for acute ischemic stroke patients using a 1.5-T scanner. Eur. Radiol. 2019, 29, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.Y.; Baek, H.J.; Ryu, K.H.; Choi, B.H.; Moon, J.I.; Park, S.E.; Kim, T.B. One-Minute Ultrafast Brain MRI with Full Basic Sequences: Can It Be a Promising Way Forward for Pediatric Neuroimaging? AJR Am. J. Roentgenol. 2020, 215, 198–205. [Google Scholar] [CrossRef]
- Meshksar, A.; Villablanca, J.P.; Khan, R.; Carmody, R.; Coull, B.; Nael, K. Role of EPI-FLAIR in patients with acute stroke: A comparative analysis with FLAIR. AJNR Am. J. Neuroradiol. 2014, 35, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Nael, K.; Khan, R.; Choudhary, G.; Meshksar, A.; Villablanca, P.; Tay, J.; Drake, K.; Coull, B.M.; Kidwell, C.S. Six-minute magnetic resonance imaging protocol for evaluation of acute ischemic stroke: Pushing the boundaries. Stroke 2014, 45, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Traboulsee, A.; Simon, J.H.; Stone, L.; Fisher, E.; Jones, D.E.; Malhotra, A.; Newsome, S.D.; Oh, J.; Reich, D.S.; Richert, N.; et al. Revised Recommendations of the Consortium of MS Centers Task Force for a Standardized MRI Protocol and Clinical Guidelines for the Diagnosis and Follow-Up of Multiple Sclerosis. AJNR Am. J. Neuroradiol. 2016, 37, 394–401. [Google Scholar] [CrossRef]
- Tawfik, A.I.; Kamr, W.H. Diagnostic value of 3D-FLAIR magnetic resonance sequence in detection of white matter brain lesions in multiple sclerosis. Egypt. J. Radiol. Nucl. Med. 2020, 51, 127. [Google Scholar] [CrossRef]
- Hammernik, K.; Schlemper, J.; Qin, C.; Duan, J.; Summers, R.M.; Rueckert, D. Σ-net: Systematic Evaluation of Iterative Deep Neural Networks for Fast Parallel MR Image Reconstruction. arXiv 2019, arXiv:1912.09278v1. [Google Scholar] [CrossRef]
- Uecker, M.; Lai, P.; Murphy, M.J.; Virtue, P.; Elad, M.; Pauly, J.M.; Vasanawala, S.S.; Lustig, M. ESPIRiT—An Eigenvalue Approach to Autocalibrating Parallel MRI: Where SENSE meets GRAPPA. Magn. Reson. Med. 2014, 71, 990–1001. [Google Scholar] [CrossRef]
- Zhang, T.; Pauly, J.M.; Vasanawala, S.S.; Lustig, M. Coil compression for accelerated imaging with Cartesian sampling. Magn. Reson. Med. 2013, 69, 571–582. [Google Scholar] [CrossRef]
- Demir, S.; Clifford, B.; Lo, W.-C.; Tabari, A.; Gonçalves Filho, A.L.M.; Lang, M.; Cauley, S.F.; Setsompop, K.; Bilgic, B.; Lev, M.H.; et al. Optimization of magnetization transfer contrast for EPI FLAIR brain imaging. Magn. Reson. Med. 2022, 87, 2380–2387. [Google Scholar] [CrossRef]
- Jezzard, P.; Balaban, R.S. Correction for geometric distortion in echo planar images from B0 field variations. Magn. Reson. Med. 1995, 34, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Grauhan, N.F.; Grünebach, N.; Brockstedt, L.; Sanner, A.; Feiweier, T.; Schöffling, V.; Brockmann, M.A.; Othman, A.E. Reduction of Distortion Artifacts in Brain MRI Using a Field Map-based Correction Technique in Diffusion-weighted Imaging: A Prospective Study. Clin. Neuroradiol. 2024, 34, 85–91. [Google Scholar] [CrossRef]
- Tintoré, M.; Rovira, A.; Martínez, M.J.; Rio, J.; Díaz-Villoslada, P.; Brieva, L.; Borrás, C.; Grivé, E.; Capellades, J.; Montalban, X. Isolated demyelinating syndromes: Comparison of different MR imaging criteria to predict conversion to clinically definite multiple sclerosis. AJNR Am. J. Neuroradiol. 2000, 21, 702–706. [Google Scholar] [PubMed]
- Bender, R.; Lange, S.; Ziegler, A. Multiples Testen. Dtsch. Med. Wochenschr. 2007, 132, e26–e29. [Google Scholar] [CrossRef]
- Noyes, K.; Weinstock-Guttman, B. Impact of diagnosis and early treatment on the course of multiple sclerosis. Am. J. Manag. Care 2013, 19, s321–s331. [Google Scholar]
- Cohan, S.; Chen, C.; Baraban, E.; Stuchiner, T.; Grote, L. MRI utility in the detection of disease activity in clinically stable patients with multiple sclerosis: A retrospective analysis of a community based cohort. BMC Neurol. 2016, 16, 184. [Google Scholar] [CrossRef]
- Eran, A.; García, M.; Malouf, R.; Bosak, N.; Wagner, R.; Ganelin-Cohen, E.; Artsy, E.; Shifrin, A.; Rozenberg, A. MRI in predicting conversion to multiple sclerosis within 1 year. Brain Behav. 2018, 8, e01042. [Google Scholar] [CrossRef]
- Sutherland, G.; Russell, N.; Gibbard, R.; Dobrescu, A. The Value of Radiology, Part II; The Conference Board of Canada: Ottawa, ON, Canada, 2019. [Google Scholar]
- van Nynatten, L.; Gershon, A. Radiology wait times: Impact on Patient Care and Potential Solutions. Univ. West. Ont. Med. J. 2017, 86, 65–66. [Google Scholar] [CrossRef]
- van Sambeek, J.R.; Joustra, P.E.; Das, S.F.; Bakker, P.J.; Maas, M. Reducing MRI access times by tackling the appointment-scheduling strategy. BMJ Qual. Saf. 2011, 20, 1075–1080. [Google Scholar] [CrossRef]
- Nuti, S.; Vainieri, M. Managing waiting times in diagnostic medical imaging. BMJ Open 2012, 2, e001255. [Google Scholar] [CrossRef]
- Biloglav, Z.; Medaković, P.; Buljević, J.; Žuvela, F.; Padjen, I.; Vrkić, D.; Ćurić, J. The analysis of waiting time and utilization of computed tomography and magnetic resonance imaging in Croatia: A nationwide survey. Croat. Med. J. 2020, 61, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Boldor, N.; Vaknin, S.; Myers, V.; Hakak, N.; Somekh, M.; Wilf-Miron, R.; Luxenburg, O. Reforming the MRI system: The Israeli National Program to shorten waiting times and increase efficiency. Isr. J. Health Policy Res. 2021, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Henkelman, R.M.; Stanisz, G.J.; Graham, S.J. Magnetization transfer in MRI: A review. NMR Biomed. 2001, 14, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, A.; Gulani, V.; Griswold, M.A.; Seiberlich, N. Parallel MR imaging. J. Magn. Reson. Imaging 2012, 36, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Aja-Fernández, S.; Vegas-Sánchez-Ferrero, G.; Tristán-Vega, A. Noise estimation in parallel MRI: GRAPPA and SENSE. Magn. Reson. Imaging 2014, 32, 281–290. [Google Scholar] [CrossRef]
- Breuer, F.A.; Kannengiesser, S.A.; Blaimer, M.; Seiberlich, N.; Jakob, P.M.; Griswold, M.A. General formulation for quantitative G-factor calculation in GRAPPA reconstructions. Magn. Reson. Med. 2009, 62, 739–746. [Google Scholar] [CrossRef]
- Bernstein, M.A.; King, K.F.; Zhou, X.J. Handbook of MRI Pulse Sequences; Elsevier Academic Press: Amsterdam, The Netherlands, 2004; pp. 732–734. ISBN 978-0-12-092861-3. [Google Scholar]
- Schenck, J.F. The role of magnetic susceptibility in magnetic resonance imaging: MRI magnetic compatibility of the first and second kinds. Med. Phys. 1996, 23, 815–850. [Google Scholar] [CrossRef]
- Phalke, V.V.; Gujar, S.; Quint, D.J. Comparison of 3.0 T versus 1.5 T MR: Imaging of the spine. Neuroimaging Clin. N. Am. 2006, 16, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Linera, J. 3T MRI: Advances in brain imaging. Eur. J. Radiol. 2008, 67, 415–426. [Google Scholar] [CrossRef]
- Stadler, A.; Schima, W.; Ba-Ssalamah, A.; Kettenbach, J.; Eisenhuber, E. Artifacts in body MR imaging: Their appearance and how to eliminate them. Eur. Radiol. 2007, 17, 1242–1255. [Google Scholar] [CrossRef]
- Arena, L.; Morehouse, H.T.; Safir, J. MR imaging artifacts that simulate disease: How to recognize and eliminate them. Radiographics 1995, 15, 1373–1394. [Google Scholar] [CrossRef] [PubMed]


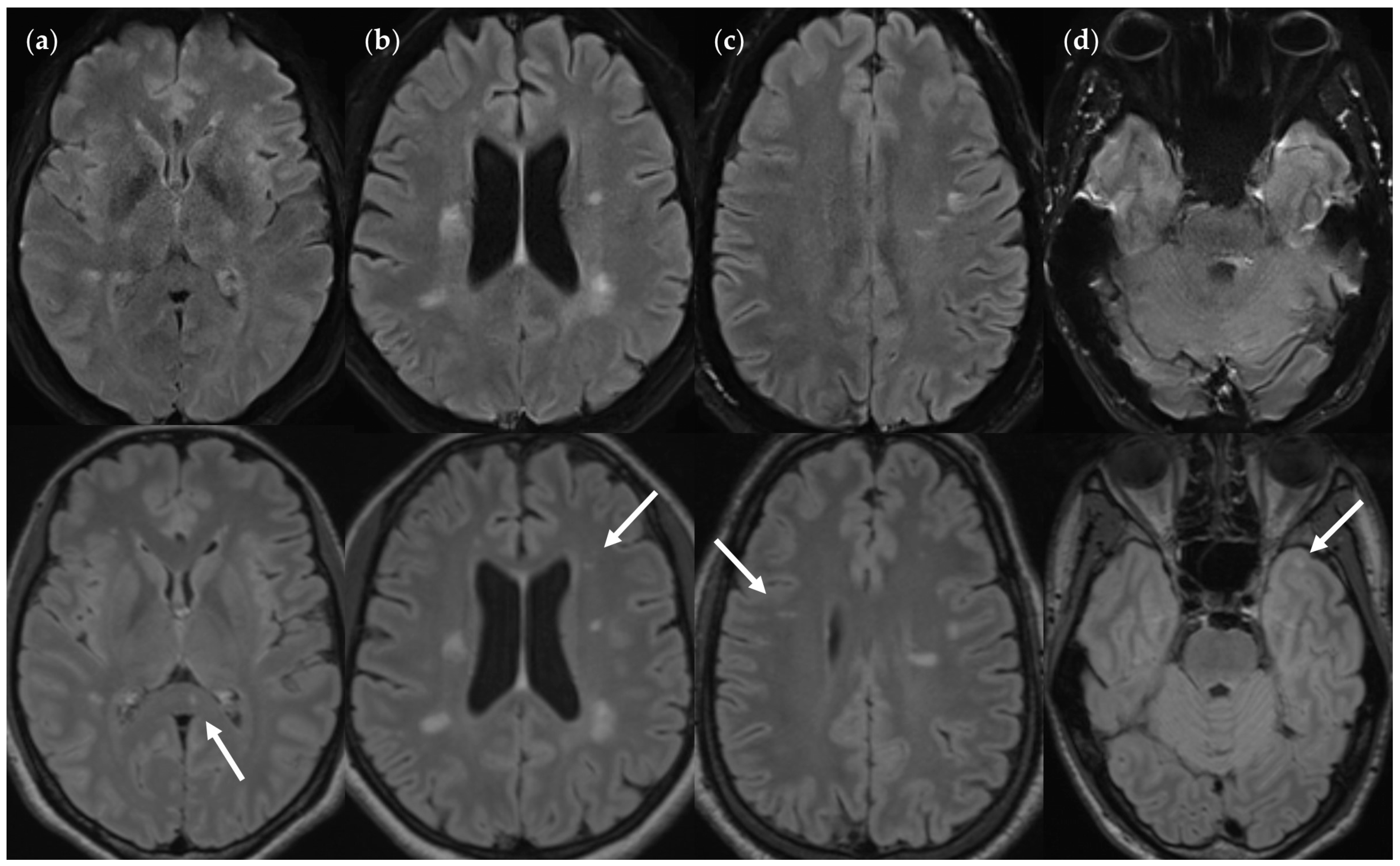

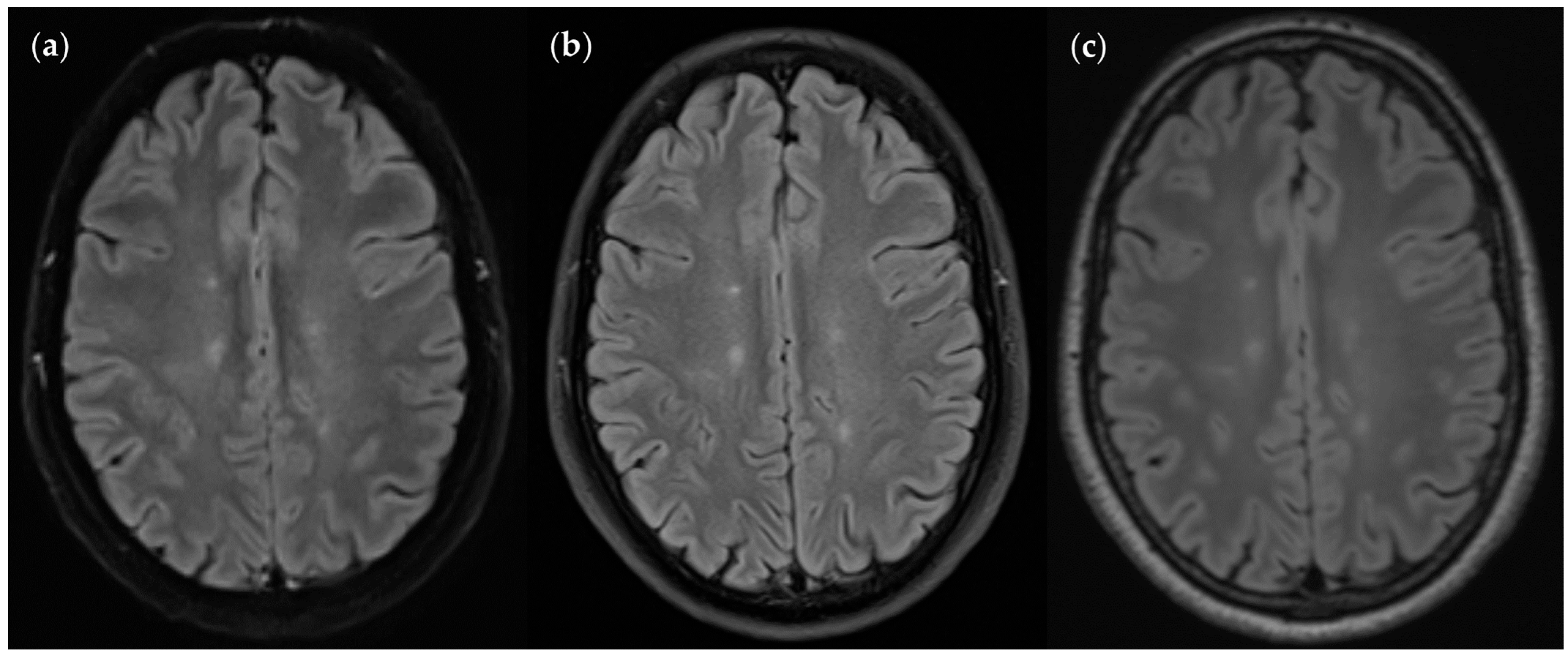





| Characteristics | Values |
|---|---|
| Number of patients | 17 |
| Mean age ± standard deviation | 33 ± 10 years |
| Median age (range) | 29 (21–60) years |
| Distribution between sexes | 71% male (n = 12), 29% female (n = 5) |
| Parameter | FLAIRUF | FLAIRTSE | FLAIR3D |
|---|---|---|---|
| Orientation | Axial | Axial | - |
| Sequence type | Multi-shot EPI | TSE | SPACE |
| TR (ms) | 8000 | 8800 | 5000 |
| TE (ms) | 88 | 87 | 386 |
| TI (ms) | 2372 | 2480 | 1800 |
| Flip angle (°) | 180 | 150 | 120 (VFA) |
| Voxel size (mm) | 0.9 × 0.9 × 4 | 0.7 × 0.7 × 4 | 0.5 × 0.5 × 0.9 1 |
| Gap between slices (mm) | 0.8 | 0 | - |
| Phase encoding direction | P → A | R → L | A → P |
| Acceleration mode | DL-based | GRAPPA | GRAPPA |
| Acceleration factor | 2 | 2 | 2 |
| In-plane FOV (read × phase; mm) | 230 × 230 | 230 × 187 | 256 × 256 1 |
| Number of slices | 32 | 36 | 192 |
| Time of acquisition (min:s) | 0:51 | 2:22 | 4:57 |
| Total | Sensitivity | PPV | ||||||
|---|---|---|---|---|---|---|---|---|
| Lesion Size | S | TP | FN | TPGS | FP | 95% CI [LL, UL] | p | 95% CI [LL, UL] |
| n | n | n | n | % | % | |||
| Large (≥3 mm) | UF | 251 | 37 | 288 | 11 | 87.2 [82.7, 90.8] | <0.001 | 95.8 [92.6, 97.9] |
| 3Da | 274 | 14 | 11 | 95.1 [92.0, 97.3] | 96.1 [93.2, 98.1] | |||
| Wide (×≥2 mm) | UF | 160 | 11 | 171 | 6 | 93.6 [88.8, 96.7] | 0.50 | 96.4 [92.3, 98.7] |
| 3Da | 163 | 8 | 8 | 95.3 [91.0, 98.0] | 95.3 [91.0, 98.0] | |||
| Narrow (×<2 mm) | UF | 91 | 26 | 117 | 5 | 77.8 [69.2, 84.9] | <0.001 | 94.8 [88.3, 98.3] |
| 3Da | 111 | 6 | 3 | 94.9 [89.2, 98.1] | 97.4 [92.5, 99.5] | |||
| Small (<3 mm) | UF | 148 | 132 | 280 | 33 | 52.9 [46.8, 58.8] | <0.001 | 81.8 [75.4, 87.1] |
| 3Da | 268 | 12 | 15 | 95.7 [92.6, 97.8] | 94.7 [91.4, 97.0] | |||
| FLAIRUF | FLAIR3Da | ||||||
|---|---|---|---|---|---|---|---|
| Presumed Type of Cause | FNUF Total | FNUF and TP3Da | FNUF and FN3Da | FN3Da and TPUF | FN3Da Total | ||
| n | n | n | n | n | n | ||
| Not detectable | SR/CNR/SNR 2 | 28 | 22 | 6 | 0 | 1 | 1 |
| Mistaken for natural structure 1 | 7 | 4 | 3 | 3 | 0 | 3 | |
| Mistaken for/masked by pulsation artifact | 0 | 0 | 0 | 7 3 | 3 3 | 10 3 | |
| Masked by distortion artifact | 2 | 1 | 1 | 0 | 0 | 0 | |
| Total | 37 | 27 | 10 | 4 | 14 | ||
| FLAIRUF | FLAIR3Da | ||||||
|---|---|---|---|---|---|---|---|
| Presumed Type of Cause | FNUF Total | FNUF and TP3Da | FNUF and FN3Da | FN3Da and TPUF | FN3Da Total | ||
| n | n | n | n | n | n | ||
| Not detectable | SR/CNR/SNR 2 | 110 | 105 | 5 | 0 | 3 | 3 |
| Mistaken for natural structure 1 | 21 | 19 | 2 | 3 | 1 | 4 | |
| Mistaken for/masked by pulsation artifact | 0 | 0 | 0 | 5 3 | 0 | 5 3 | |
| Masked by distortion artifact | 1 | 0 | 1 | 0 | 0 | 0 | |
| Total | 132 | 124 | 8 | 4 | 12 | ||
| FLAIRUF | FLAIR3Da | ||||||
|---|---|---|---|---|---|---|---|
| Presumed Causal Phenomenon | FPUF Large (≥3 mm) | FPUF Small (<3 mm) | FPUF Total | FP3Da Large (≥3 mm) | FP3Da Small (<3 mm) | FP3Da Total | |
| n | n | n | n | n | n | ||
| Partially imaged natural structure 1 | SR 2 | 9 | 24 | 33 | 6 | 7 | 13 |
| Partially imaged nearby large lesion | 0 | 2 | 2 | 0 | 1 | 1 | |
| Pulsation artifact | 2 3 | 7 3 | 9 3 | 5 4 | 7 5 | 12 5 | |
| Total | 11 | 33 | 44 | 11 | 15 | 26 | |
| Total | Sensitivity | |||||
|---|---|---|---|---|---|---|
| Lesion Size | S | tseTP | tseFN | tseTPGS | 95% CI [LL, UL] | p |
| n | n | n | % | |||
| Large (≥3 mm) | UF | 48 | 5 | 53 | 90.6 [79.3, 96.9] | 0.68 |
| TSE | 48 | 5 | 90.6 [79.3, 96.9] | |||
| Wide (×≥2 mm) | UF | 33 | 2 | 35 | 94.3 [80.8, 99.3] | >0.99 |
| TSE | 34 | 1 | 97.1 [85.1, 99.9] | |||
| Narrow (×<2 mm) | UF | 15 | 3 | 18 | 83.3 [58.6, 96.4] | >0.99 |
| TSE | 14 | 4 | 77.8 [52.4, 93.6] | |||
| Small (<3 mm) | UF | 19 | 15 | 34 | 55.9 [37.9, 72.8] | 0.08 |
| TSE | 27 | 7 | 79.4 [62.1, 91.3] | |||
| FLAIRUF | FLAIRTSE | ||||||
|---|---|---|---|---|---|---|---|
| Presumed Type of Cause | tseFNUF Total | tseFNUF and TPTSE | tseFNUF and FNTSE | FNTSE and tseTPUF | FNTSE Total | ||
| n | n | n | n | n | n | ||
| Not detectable | SR/CNR/SNR 2 | 3 | 3 | 0 | 0 | 3 | 3 |
| Mistaken for natural structure 1 | 2 | 0 | 2 | 2 | 0 | 2 | |
| Total | 5 | 3 | 2 | 3 | 5 | ||
| FLAIRUF | FLAIRTSE | ||||||
|---|---|---|---|---|---|---|---|
| Presumed Type of Cause | tseFNUF Total | tseFNUF and TPTSE | tseFNUF and FNTSE | FNTSE and tseTPUF | FNTSE Total | ||
| n | n | n | n | n | n | ||
| Not detectable | SR/CNR/SNR 2 | 13 | 10 | 3 | 3 | 3 | 6 |
| Mistaken for natural structure 1 | 2 | 2 | 0 | 0 | 1 | 1 | |
| Total | 15 | 12 | 3 | 4 | 7 | ||
| FLAIRUF | FLAIRTSE | ||||||
|---|---|---|---|---|---|---|---|
| Presumed Causal Phenomenon | tseFPUF Large (≥3 mm) | tseFPUF Small (<3 mm) | tseFPUF Total | FPTSE Large (≥3 mm) | FPTSE Small (<3 mm) | FPTSE Total | |
| n | n | n | n | n | n | ||
| Partially imaged natural structure 1 | SR 2 | 1 | 1 | 2 | 1 | 3 | 4 |
| Partially imaged nearby large lesion | 0 | 1 | 1 | 0 | 1 | 1 | |
| Total | 1 | 2 | 3 | 1 | 4 | 5 | |
| Conspicuity | ||||||||
|---|---|---|---|---|---|---|---|---|
| Size | Location | 1 | 2 | 3 | 4 | 5 | TP3Da | p |
| n | n | n | n | n | n | |||
| Large (≥3 mm total) | All | 37 | 180 | 30 | 13 | 14 | 274 | <0.001 3 |
| Small (<3 mm) | All | 12 | 94 | 38 | 44 | 80 | 268 | |
| Large (≥3 mm total) | Frontal | 7 | 55 | 4 | 4 | 3 | 73 | 0.002 4 |
| Parietal | 5 | 40 | 6 | 4 | 3 | 58 | ||
| Temporal | 12 | 48 | 7 | 2 | 3 | 72 | ||
| Occipital | 6 | 9 | 0 | 0 | 0 | 15 | ||
| Central 1 | 3 | 7 | 8 | 3 | 1 | 22 | ||
| Infratentorial 2 | 4 | 21 | 5 | 0 | 4 | 34 | ||
| Small (<3 mm) | Frontal | 7 | 46 | 16 | 16 | 25 | 110 | 0.15 4 |
| Parietal | 2 | 20 | 10 | 6 | 22 | 60 | ||
| Temporal | 3 | 16 | 7 | 14 | 20 | 60 | ||
| Occipital | 0 | 0 | 1 | 1 | 1 | 3 | ||
| Central 1 | 0 | 11 | 4 | 5 | 11 | 31 | ||
| Infratentorial 2 | 0 | 1 | 0 | 2 | 1 | 4 | ||
| Large (≥3 mm total) | Frontal | 7 | 55 | 4 | 4 | 3 | 73 | 0.42 4 |
| Parietal | 5 | 40 | 6 | 4 | 3 | 58 | ||
| Temporal | 12 | 48 | 7 | 2 | 3 | 72 | ||
| Infratentorial 2 | 4 | 21 | 5 | 0 | 4 | 34 | ||
| Small (≥3 mm total) | Occipital | 6 | 9 | 0 | 0 | 0 | 15 | |
| 0.002 3 | ||||||||
| Frontal and Parietal and Temporal and Infratentorial 2 | 28 | 164 | 22 | 10 | 13 | 237 | ||
| 0.01 3 | ||||||||
| Central 1 | 3 | 7 | 8 | 3 | 1 | 22 | ||
| Location | Large (≥3 mm total) | Small (<3 mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TP3Da | M and 95% CI [LL, UL] | SD | Mdn | p | TP3Da | M and 95% CI [LL, UL] | SD | Mdn | p | |
| n | mm | mm | mm | n | mm | mm | mm | |||
| Frontal | 73 | 5.1 [4.6, 5.6] | 1.9 | 4.6 | 0.26 | 110 | 2.1 [2.0, 2.2] | 0.5 | 2.3 | 0.06 |
| Parietal | 58 | 5.8 [5.0, 6.7] | 3.2 | 4.6 | 60 | 2.1 [1.9, 2.2] | 0.6 | 2.1 | ||
| Temporal | 72 | 5.3 [4.7, 5.9] | 2.5 | 4.6 | 60 | 2.0 [1.8, 2.1] | 0.6 | 2.1 | ||
| Occipital | 15 | 5.6 [4.6, 6.6] | 1.8 | 5.4 | 3 | 2.8 [2.6, 2.9] | 0.1 | 2.8 | ||
| Central | 22 | 5.5 [3.8, 7.2] | 3.8 | 4.5 | 31 | 2.2 [2.1, 2.4] | 0.5 | 2.4 | ||
| Infratentorial | 34 | 6.5 [5.4, 7.5] | 3.0 | 5.2 | 4 | 2.3 [1.9, 2.8] | 0.3 | 2.4 | ||
| Total | 274 | 5.5 [5.2, 5.9] | 2.7 | 4.7 | 268 | 2.1 [2.0, 2.2] | 0.6 | 2.2 | ||
| Location | Wide (≥3 mm × ≥2 mm) | Narrow (≥3 mm × <2 mm) | Large (≥3 mm Total) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conspicuity | Total | Conspicuity | Total | TP3Da | |||||||||
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | ||||
| n | n | n | n | n | n | n | n | n | n | n | n | n | |
| Frontal | 7 | 35 | 0 | 0 | 1 | 43 | 0 | 20 | 4 | 4 | 2 | 30 | 73 |
| Parietal | 2 | 28 | 2 | 1 | 0 | 33 | 3 | 12 | 4 | 3 | 3 | 25 | 58 |
| Temporal | 5 | 30 | 4 | 1 | 0 | 40 | 7 | 18 | 3 | 1 | 3 | 32 | 72 |
| Occipital | 4 | 8 | 0 | 0 | 0 | 12 | 2 | 1 | 0 | 0 | 0 | 3 | 15 |
| Central | 0 | 4 | 5 | 1 | 0 | 10 | 3 | 3 | 3 | 2 | 1 | 12 | 22 |
| Infratentorial | 3 | 17 | 3 | 0 | 2 | 25 | 1 | 4 | 2 | 0 | 2 | 9 | 34 |
| Total | 21 | 122 | 14 | 3 | 3 | 163 | 16 | 58 | 16 | 10 | 11 | 111 | 274 |
| Parameter | FLAIRUF | FLAIR3Da | p | ||||
|---|---|---|---|---|---|---|---|
| Mdn (IQR) | M ± SD | L/H | Mdn (IQR) | M ± SD | L/H | ||
| SNR | 3 (3–3) | 3.00 ± 0.00 | 3/3 | 1 (1–1) | 1.18 ± 0.39 | 1/2 | <0.001 |
| CNR | 3 (2–3) | 2.53 ± 0.51 | 2/3 | 1 (1–1) | 1.18 ± 0.39 | 1/2 | <0.001 |
| Artifacts | FLAIRUF | FLAIR3Da | p | ||||
|---|---|---|---|---|---|---|---|
| Mdn (IQR) | M ± SD | L/H | Mdn (IQR) | M ± SD | L/H | ||
| Distortions | |||||||
| Frontal | 3 (2–3) | 2.6 ± 0.6 | 1/3 | 0 (0–0) | 0.0 ± 0.0 | 0/0 | <0.001 |
| Frontobasal | 3 (2–3) | 2.7 ± 0.5 | 2/3 | 0 (0–0) | 0.0 ± 0.0 | 0/0 | <0.001 |
| Temporopolar | 3 (2.5–3) | 2.8 ± 0.4 | 2/3 | 0 (0–0) | 0.0 ± 0.0 | 0/0 | <0.001 |
| Infratentorial | 1 (1–1) | 1.1 ± 0.3 | 1/2 | 0 (0–0) | 0.0 ± 0.0 | 0/0 | <0.001 |
| Pulsatile flow | |||||||
| infratentorial | 1 (1–4) | 2.2 ± 1.6 | 0/4 | 3 (3–4) | 3.4 ± 0.7 | 2/4 | 0.02 |
| supratentorial | 1 (0–1) | 0.7 ± 0.8 | 0/3 | 0 (0–0) | 0.0 ± 0.0 | 0/0 | 0.004 |
| Chemical shift | |||||||
| Frontal | 0 (0–0) | 0.1 ± 0.3 | 0/1 | 0 (0–0) | 0.0 ± 0.0 | 0/0 | 0.16 |
| Residual Aliasing | |||||||
| Central | 0 (0–0) | 0.2 ± 0.6 | 0/2 | 0 (0–0) | 0.0 ± 0.0 | 0/0 | 0.10 |
| Spikes 1 | 0 (0–1) | 0.4 ± 0.6 | 0/2 | 0 (0–0) | 0.0 ± 0.0 | 0/0 | 0.03 |
| Subject motion | 0 (0–0) | 0.0 ± 0.0 | 0/0 | 0 (0–0) | 0.1 ± 0.2 | 0/1 | 0.32 |
| Parameter | FLAIRUF | FLAIRTSE | ||||
|---|---|---|---|---|---|---|
| Mdn (IQR) | M ± SD | L/H | Mdn (IQR) | M ± SD | L/H | |
| SNR | 3 (3–3) | 3.00 ± 0.00 | 3/3 | 3 (3–3) | 3.00 ± 0.00 | 3/3 |
| CNR | 3 (2.5–3) | 2.67 ± 0.58 | 2/3 | 2 (2–2) | 2.00 ± 0.00 | 2/2 |
| Artifacts | FLAIRUF | FLAIRTSE | ||||
|---|---|---|---|---|---|---|
| Mdn (IQR) | M ± SD | L/H | Mdn (IQR) | M ± SD | L/H | |
| Distortions | ||||||
| Frontal | 3 (3–3) | 3.0 ± 0.0 | 3/3 | 0 (0–0) | 0.0 ± 0.0 | 0/0 |
| Frontobasal | 3 (3–3) | 3.0 ± 0.0 | 3/3 | 0 (0–0) | 0.0 ± 0.0 | 0/0 |
| Temporopolar | 3 (3–3) | 3.0 ± 0.0 | 3/3 | 0 (0–0) | 0.0 ± 0.0 | 0/0 |
| Infratentorial | 1 (1–1) | 1.0 ± 0.0 | 1/1 | 0 (0–0) | 0.0 ± 0.0 | 0/0 |
| Pulsatile flow | ||||||
| infratentorial | 1 (1–1) | 1.0 ± 0.0 | 1/1 | 2 (1.5–3) | 2.3 ± 1.5 | 1/4 |
| supratentorial | 0 (0–0) | 0.0 ± 0.0 | 0/0 | 0 (0–0.5) | 0.3 ± 0.6 | 0/1 |
| Chemical shift | ||||||
| Frontal | 0 (0–0) | 0.0 ± 0.0 | 0/0 | 0 (0–0) | 0.0 ± 0.0 | 0/0 |
| Residual Aliasing | ||||||
| Central | 0 (0–1) | 0.7 ± 1.2 | 0/2 | 0 (0–0) | 0.0 ± 0.0 | 0/0 |
| Spikes 1 | 0 (0–0.5) | 0.3 ± 0.6 | 0/1 | 0 (0–0) | 0.0 ± 0.0 | 0/0 |
| Subject motion | 0 (0–0) | 0.0 ± 0.0 | 0/0 | 0 (0–0.5) | 0.3 ± 0.6 | 0/1 |
| SNR Substandard | Yes Percentage | |||||||
|---|---|---|---|---|---|---|---|---|
| Location | Yes | No | TPGS | 95% CI [LL, UL] | p | |||
| n | n | n | % | |||||
| All | 147 | 421 | 568 | 25.9 [22.3, 29.7] | ||||
| Frontal | 27 | 163 | 190 | 14.2 [9.6, 20.0] |  | <0.001 |  | 0.07 |
| Parietal | 29 | 91 | 120 | 24.2 [16.8, 32.8] | ||||
| Temporal | 24 | 111 | 135 | 17.8 [11.7, 25.3] | ||||
| Occipital | 1 | 17 | 18 | 5.6 [0.1, 27.3] | ||||
| Central | 35 | 19 | 54 | 64.8 [50.6, 77.3] |  | 0.67 | ||
| Infratentorial | 31 | 20 | 51 | 60.8 [46.1, 74.2] | ||||
| Frontal and Parietal and Temporal and Occipital | 81 | 382 | 463 | 17.5 [14.1, 21.3] |  | <0.001 | ||
| Central and Infratentorial | 66 | 39 | 105 | 62.9 [52.9, 72.1] | ||||
| CNR Substandard | Yes Percentage | |||||||
|---|---|---|---|---|---|---|---|---|
| Location | Yes | No | TPGS | 95% CI [LL, UL] | p | |||
| n | n | n | % | |||||
| All | 33 | 535 | 568 | 5.8 [4.0, 8.1] | ||||
| Frontal | 6 | 184 | 190 | 3.2 [1.2, 6.8] |  | <0.001 |  | 0.15 |
| Parietal | 7 | 113 | 120 | 5.8 [2.4, 11.6] | ||||
| Temporal | 2 | 133 | 135 | 1.5 [0.2, 5.2] | ||||
| Occipital | 0 | 18 | 18 | 0.0 [0.0, 18.5] | ||||
| Central | 0 | 54 | 54 | 0.0 [0.0, 6.6] | ||||
| Infratentorial | 18 | 33 | 51 | 35.3 [22.4, 49.9] | ||||
| Frontal and Parietal and Temporal and Occipital and Central | 15 | 502 | 517 | 2.9 [1.6, 4.7] |  | <0.001 | ||
| Infratentorial | 18 | 33 | 51 | 35.3 [22.4, 49.9] | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuhholz, M.; Ruff, C.; Bürkle, E.; Feiweier, T.; Clifford, B.; Kowarik, M.; Bender, B. Ultrafast Brain MRI at 3 T for MS: Evaluation of a 51-Second Deep Learning-Enhanced T2-EPI-FLAIR Sequence. Diagnostics 2024, 14, 1841. https://doi.org/10.3390/diagnostics14171841
Schuhholz M, Ruff C, Bürkle E, Feiweier T, Clifford B, Kowarik M, Bender B. Ultrafast Brain MRI at 3 T for MS: Evaluation of a 51-Second Deep Learning-Enhanced T2-EPI-FLAIR Sequence. Diagnostics. 2024; 14(17):1841. https://doi.org/10.3390/diagnostics14171841
Chicago/Turabian StyleSchuhholz, Martin, Christer Ruff, Eva Bürkle, Thorsten Feiweier, Bryan Clifford, Markus Kowarik, and Benjamin Bender. 2024. "Ultrafast Brain MRI at 3 T for MS: Evaluation of a 51-Second Deep Learning-Enhanced T2-EPI-FLAIR Sequence" Diagnostics 14, no. 17: 1841. https://doi.org/10.3390/diagnostics14171841





