Inflammatory Protein Panel: Exploring Diagnostic Insights for Peripheral Artery Disease Diagnosis in a Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Design
2.3. Patient Recruitment
2.4. Baseline Characteristics
2.5. Measurement of Plasma Protein Levels
2.6. Development and Evaluation of the Diagnostic Model
2.7. Statistical Analysis
3. Results
3.1. Patients
3.2. Plasma Protein Concentrations
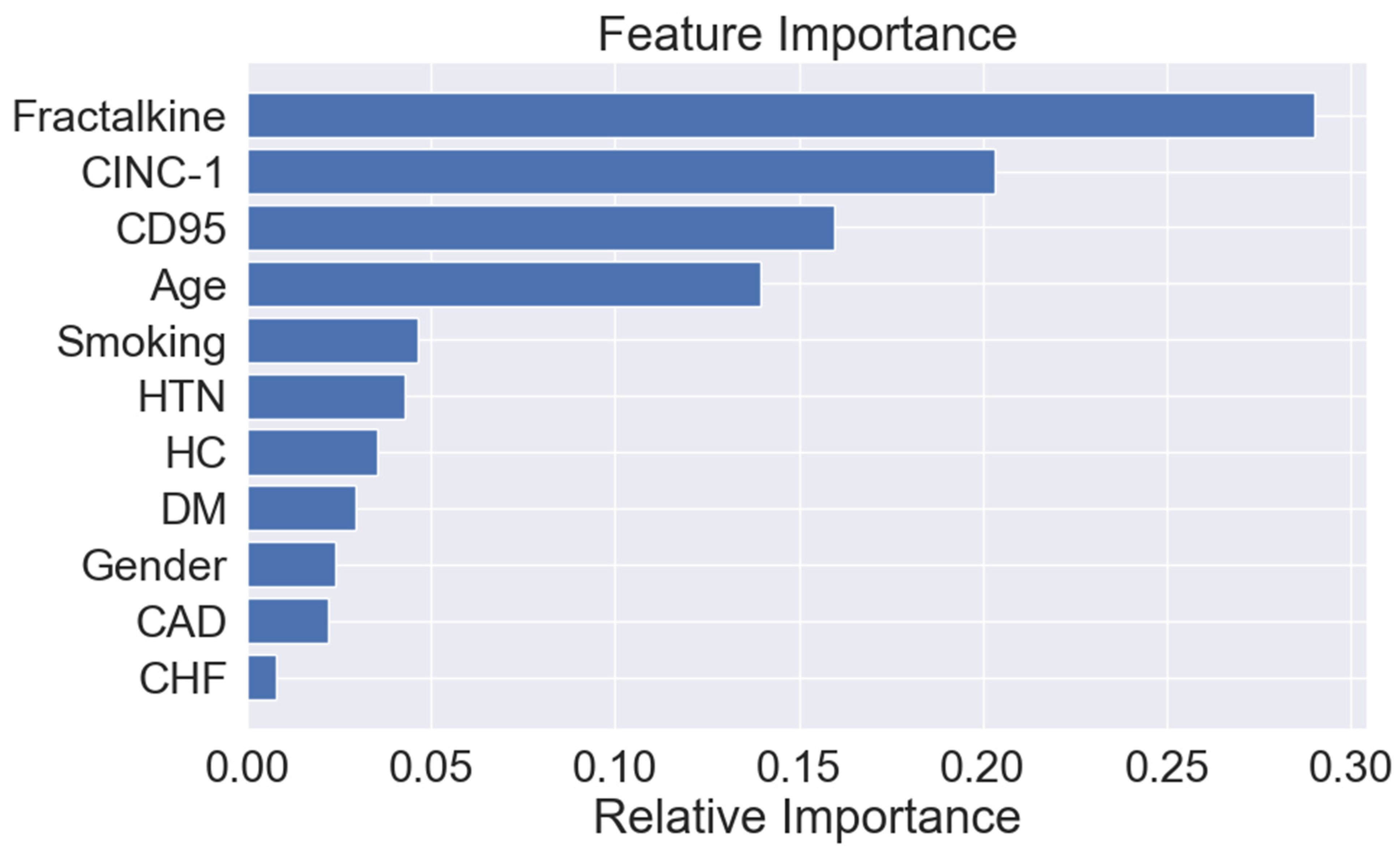
3.3. Associations between Proteins and PAD Diagnosis
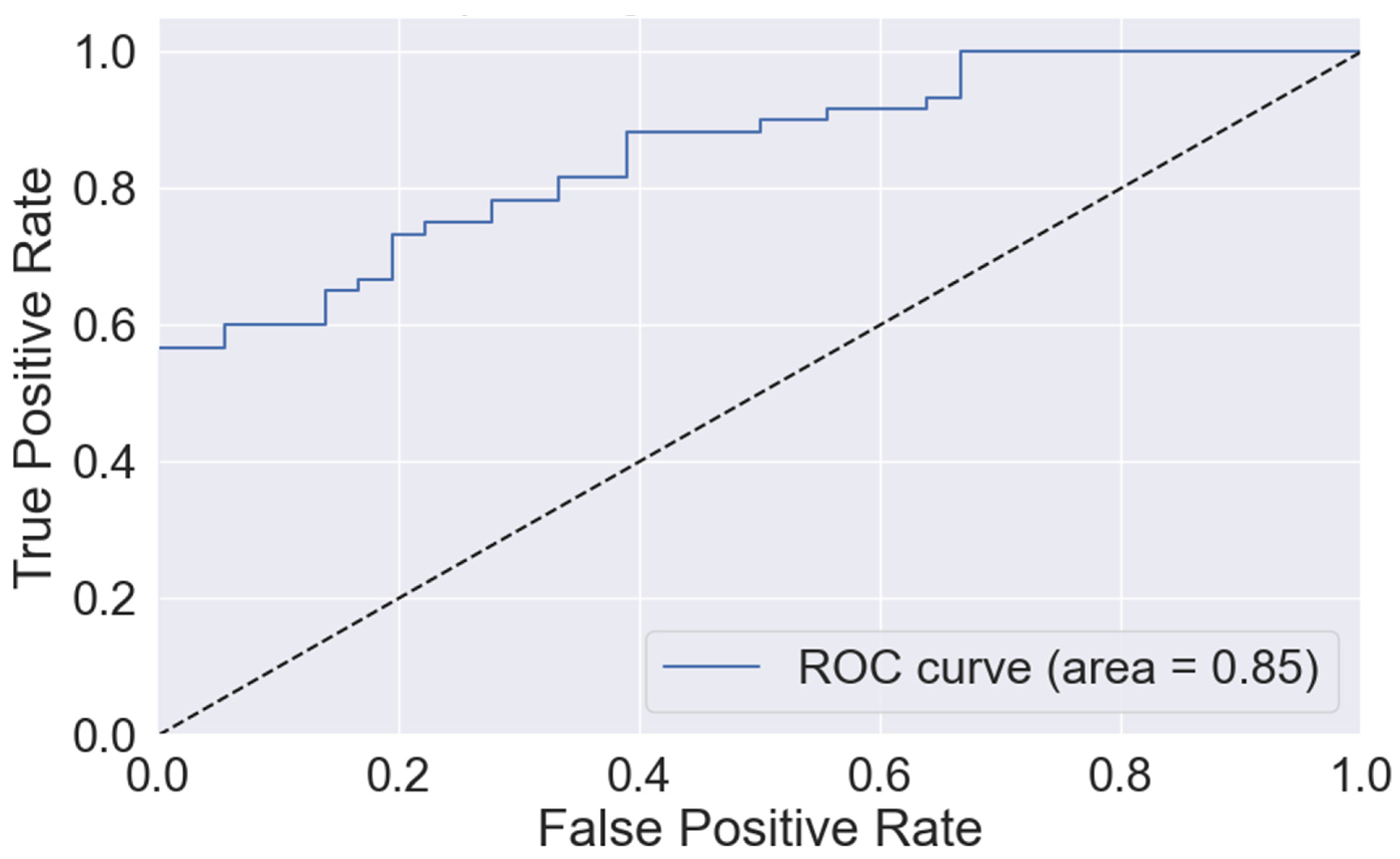
3.4. Model Performance
4. Discussion
4.1. Key Findings
4.2. Comparison to Existing Literature
4.3. Explanation of Findings
4.4. Implications
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zemaitis, M.R.; Boll, J.M.; Dreyer, M.A. Peripheral Arterial Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Olin, J.W.; Sealove, B.A. Peripheral Artery Disease: Current Insight Into the Disease and Its Diagnosis and Management. Mayo Clin. Proc. 2010, 85, 678–692. [Google Scholar] [CrossRef]
- Mehta, A.; Dhindsa, D.S.; Hooda, A.; Nayak, A.; Massad, C.S.; Rao, B.; Makue, L.F.; Rajani, R.R.; Alabi, O.; Quyyumi, A.A.; et al. Premature Atherosclerotic Peripheral Artery Disease: An Underrecognized and Undertreated Disorder with a Rising Global Prevalence. Trends Cardiovasc. Med. 2021, 31, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Lorenzetti, B.B.; Veiga, F.H.; Canetti, C.A.; Poole, S.; Cunha, F.Q.; Ferreira, S.H. Cytokine-Induced Neutrophil Chemoattractant 1 (CINC-1) Mediates the Sympathetic Component of Inflammatory Mechanical Hypersensitivitiy in Rats. Eur. Cytokine Netw. 2002, 13, 456–461. [Google Scholar] [PubMed]
- Padgett, L.E.; Dinh, H.Q.; Wu, R.; Gaddis, D.E.; Araujo, D.J.; Winkels, H.; Nguyen, A.; McNamara, C.A.; Hedrick, C.C. Naive CD8+ T Cells Expressing CD95 Increase Human Cardiovascular Disease Severity. Arter. Thromb. Vasc. Biol. 2020, 40, 2845–2859. [Google Scholar] [CrossRef]
- Damås, J.K.; Boullier, A.; Waehre, T.; Smith, C.; Sandberg, W.J.; Green, S.; Aukrust, P.; Quehenberger, O. Expression of Fractalkine (CX3CL1) and Its Receptor, CX3CR1, Is Elevated in Coronary Artery Disease and Is Reduced during Statin Therapy. Arter. Thromb. Vasc. Biol. 2005, 25, 2567–2572. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Sun, J.; Söderholm, M.; Melander, O.; Orho-Melander, M.; Nilsson, J.; Borné, Y.; Engström, G. Association of TIM-1 (T-Cell Immunoglobulin and Mucin Domain 1) with Incidence of Stroke. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1777–1786. [Google Scholar] [CrossRef]
- Aday, A.W.; Matsushita, K. Epidemiology of Peripheral Artery Disease and Polyvascular Disease. Circ. Res. 2021, 128, 1818–1832. [Google Scholar] [CrossRef] [PubMed]
- Parvar, S.L.; Fitridge, R.; Dawson, J.; Nicholls, S.J. Medical and Lifestyle Management of Peripheral Arterial Disease. J. Vasc. Surg. 2018, 68, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Cornejo Del Río, V.; Mostaza, J.; Lahoz, C.; Sánchez-Arroyo, V.; Sabín, C.; López, S.; Patrón, P.; Fernández-García, P.; Fernández-Puntero, B.; Vicent, D.; et al. Prevalence of Peripheral Artery Disease (PAD) and Factors Associated: An Epidemiological Analysis from the Population-Based Screening PRE-Diabetes and Type 2 DIAbetes (SPREDIA-2) Study. PLoS ONE 2017, 12, e0186220. [Google Scholar] [CrossRef]
- Duval, S.; Massaro, J.M.; Jaff, M.R.; Boden, W.E.; Alberts, M.J.; Califf, R.M.; Eagle, K.A.; D’Agostino, R.B.; Pedley, A.; Fonarow, G.C.; et al. An Evidence-Based Score to Detect Prevalent Peripheral Artery Disease (PAD). Vasc. Med. 2012, 17, 342–351. [Google Scholar] [CrossRef]
- Gouda, P.; Ramasundarahettige, C.; Anand, S.; Muhlhoffer, E.; Berkowitz, S.; Fox, K.A.; Eikelboom, J.; Welsh, R. Clinical Factors Associated with Peripheral Artery Disease in Patients with Documented Coronary Artery Disease: A Post Hoc Analysis of the COMPASS Trial. Atherosclerosis 2021, 331, 38–44. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Moons, K.G.M.; Dhiman, P.; Riley, R.D.; Beam, A.L.; Calster, B.V.; Ghassemi, M.; Liu, X.; Reitsma, J.B.; van Smeden, M.; et al. TRIPOD+AI Statement: Updated Guidance for Reporting Clinical Prediction Models That Use Regression or Machine Learning Methods. BMJ 2024, 385, e078378. [Google Scholar] [CrossRef] [PubMed]
- Gul, F.; Janzer, S.F. Peripheral Vascular Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. J. Am. Coll. Cardiol. 2019, 73, e285–e350. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison, H.C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef]
- Luminex Assays, Multiplex Immunoassays. Available online: https://www.bio-techne.com/ (accessed on 6 May 2023).
- Luminex Assays—CA. Available online: https://www.thermofisher.com/ca/en/home/life-science/antibodies/immunoassays/procartaplex-assays-luminex.html (accessed on 18 December 2021).
- MAGPIX® System|xMAP Instrument|Luminex Corporation. Available online: https://www.luminexcorp.com/magpix-system/ (accessed on 18 December 2021).
- xPONENT® Software for xMAP® Instruments; Luminex Corporation: Austin, TX, USA, 2024.
- Rigatti, S.J. Random Forest. J. Insur. Med. 2017, 47, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-Y.; Lu, Y. Decision Tree Methods: Applications for Classification and Prediction. Shanghai Arch. Psychiatry 2015, 27, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Elfanagely, O.; Toyoda, Y.; Othman, S.; Mellia, J.A.; Basta, M.; Liu, T.; Kording, K.; Ungar, L.; Fischer, J.P. Machine Learning and Surgical Outcomes Prediction: A Systematic Review. J. Surg. Res. 2021, 264, 346–361. [Google Scholar] [CrossRef]
- Bektaş, M.; Tuynman, J.B.; Costa Pereira, J.; Burchell, G.L.; van der Peet, D.L. Machine Learning Algorithms for Predicting Surgical Outcomes after Colorectal Surgery: A Systematic Review. World J. Surg. 2022, 46, 3100–3110. [Google Scholar] [CrossRef]
- Senders, J.T.; Staples, P.C.; Karhade, A.V.; Zaki, M.M.; Gormley, W.B.; Broekman, M.L.D.; Smith, T.R.; Arnaout, O. Machine Learning and Neurosurgical Outcome Prediction: A Systematic Review. World Neurosurg. 2018, 109, 476–486.e1. [Google Scholar] [CrossRef]
- Hajian-Tilaki, K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Casp. J. Intern. Med. 2013, 4, 627–635. [Google Scholar]
- Loh, W.-Y.; Zhou, P. Variable Importance Scores. J. Data Sci. 2021, 19, 569–592. [Google Scholar] [CrossRef]
- SPSS Software. Available online: https://www.ibm.com/analytics/spss-statistics-software (accessed on 18 December 2021).
- Zagorski, J.; Gellar, M.A.; Obraztsova, M.; Kline, J.A.; Watts, J.A. Inhibition of CINC-1 Decreases Right Ventricular Damage Caused by Experimental Pulmonary Embolism in Rats. J. Immunol. 2007, 179, 7820–7826. [Google Scholar] [CrossRef]
- Janin, A.; Deschaumes, C.; Daneshpouy, M.; Estaquier, J.; Micic-Polianski, J.; Rajagopalan-Levasseur, P.; Akarid, K.; Mounier, N.; Gluckman, E.; Socié, G.; et al. CD95 Engagement Induces Disseminated Endothelial Cell Apoptosis in Vivo: Immunopathologic Implications. Blood 2002, 99, 2940–2947. [Google Scholar] [CrossRef]
- Loh, S.X.; Ekinci, Y.; Spray, L.; Jeyalan, V.; Olin, T.; Richardson, G.; Austin, D.; Alkhalil, M.; Spyridopoulos, I. Fractalkine Signalling (CX3CL1/CX3CR1 Axis) as an Emerging Target in Coronary Artery Disease. J. Clin. Med. 2023, 12, 4821. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.G.; Jung, K.; Dudley, J.T.; Li, L.; Leeper, N.J.; Shah, N.H. Predicting Future Cardiovascular Events in Patients with Peripheral Artery Disease Using Electronic Health Record Data. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e004741. [Google Scholar] [CrossRef] [PubMed]
- Miceli, G.; Basso, M.G.; Rizzo, G.; Pintus, C.; Tuttolomondo, A. The Role of the Coagulation System in Peripheral Arterial Disease: Interactions with the Arterial Wall and Its Vascular Microenvironment and Implications for Rational Therapies. Int. J. Mol. Sci. 2022, 23, 14914. [Google Scholar] [CrossRef] [PubMed]
- Stoltzfus, J.C. Logistic Regression: A Brief Primer. Acad. Emerg. Med. 2011, 18, 1099–1104. [Google Scholar] [CrossRef]
- Kia, B.; Mendes, A.; Parnami, A.; George, R.; Mobley, K.; Ditto, W.L. Nonlinear Dynamics Based Machine Learning: Utilizing Dynamics-Based Flexibility of Nonlinear Circuits to Implement Different Functions. PLoS ONE 2020, 15, e0228534. [Google Scholar] [CrossRef]
- Chatterjee, P.; Cymberknop, L.J.; Armentano, R.L. Nonlinear Systems in Healthcare towards Intelligent Disease Prediction. In Nonlinear Systems—Theoretical Aspects and Recent Applications; IntechOpen: Rijeka, Croatia, 2019; Volume 1, p. e88163. [Google Scholar] [CrossRef]
- Smith, B.J.; Silva-Costa, L.C.; Martins-de-Souza, D. Human Disease Biomarker Panels through Systems Biology. Biophys. Rev. 2021, 13, 1179–1190. [Google Scholar] [CrossRef]
- Couronné, R.; Probst, P.; Boulesteix, A.-L. Random Forest versus Logistic Regression: A Large-Scale Benchmark Experiment. BMC Bioinform. 2018, 19, 270. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.M.; Demsas, F.; Leeper, N.J.; Ross, E.G. Leveraging Machine Learning and Artificial Intelligence to Improve Peripheral Artery Disease Detection, Treatment, and Outcomes. Circ. Res. 2021, 128, 1833–1850. [Google Scholar] [CrossRef]
- Nakar, S.; Vinker, S.; Neuman, S.; Kitai, E.; Yaphe, J. Baseline Tests or Screening: What Tests Do Family Physicians Order Routinely on Their Healthy Patients? J. Med. Screen. 2002, 9, 133–134. [Google Scholar] [CrossRef]
- Akbari, C.M.; Stone, L. Accreditation and Credentialing in the Vascular Laboratory. Semin. Vasc. Surg. 2002, 15, 178–181. [Google Scholar] [CrossRef]
- Burns, P.; Gough, S.; Bradbury, A.W. Management of Peripheral Arterial Disease in Primary Care. BMJ 2003, 326, 584–588. [Google Scholar] [CrossRef]
- Koh, C.E.; Walker, S.R. Vascular Surgery Consults: A Significant Workload. ANZ J. Surg. 2007, 77, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Nishimiya, K.; Matsumoto, Y.; Shimokawa, H. Recent Advances in Vascular Imaging. Arter. Thromb. Vasc. Biol. 2020, 40, e313–e321. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Farber, A.; Menard, M.T.; Conte, M.S.; Kaufman, J.A.; Powell, R.J.; Choudhry, N.K.; Hamza, T.H.; Assmann, S.F.; Creager, M.A.; Cziraky, M.J.; et al. Surgery or Endovascular Therapy for Chronic Limb-Threatening Ischemia. N. Engl. J. Med. 2022, 387, 2305–2316. [Google Scholar] [CrossRef] [PubMed]
- Caetano, A.P.; Conde Vasco, I.; Veloso Gomes, F.; Costa, N.V.; Luz, J.H.; Spaepen, E.; Formiga, A.; Coimbra, É.; Neves, J.; Bilhim, T. Successful Revascularization Has a Significant Impact on Limb Salvage Rate and Wound Healing for Patients with Diabetic Foot Ulcers: Single-Centre Retrospective Analysis with a Multidisciplinary Approach. Cardiovasc. Interv. Radiol. 2020, 43, 1449–1459. [Google Scholar] [CrossRef]
- Margolis, J.; Barron, J.J.; Grochulski, W.D. Health Care Resources and Costs for Treating Peripheral Artery Disease in a Managed Care Population: Results from Analysis of Administrative Claims Data. J. Manag. Care Pharm. 2005, 11, 727–734. [Google Scholar] [CrossRef] [PubMed]
| PAD (n = 312) | Non-PAD (n = 164) | p | |
|---|---|---|---|
| Age, mean (SD) | 71 (10) | 65 (12) | <0.001 |
| Female sex | 109 (35) | 67 (41) | 0.204 |
| Dyslipidemia | 263 (84) | 100 (61) | <0.001 |
| Hypertension | 257 (82) | 96 (59) | <0.001 |
| Diabetes | 131 (42) | 34 (21) | <0.001 |
| Current smoking | 78 (25) | 35 (21) | 0.002 |
| Past smoking | 171 (55) | 71 (43) | 0.001 |
| Congestive heart failure | 11 (4) | 4 (2) | 0.519 |
| Coronary artery disease | 118 (38) | 34 (21) | <0.001 |
| Previous stroke | 51 (16) | 13 (8) | 0.011 |
| Statin | 229 (73) | 93 (57) | <0.001 |
| Acetylsalicylic acid | 251 (80) | 99 (60) | <0.001 |
| Beta-blocker | 134 (41) | 50 (30) | 0.001 |
| ACE-I/ARB | 216 (66) | 74 (45) | 0.001 |
| Hydrochlorothiazide or furosemide | 41 (13) | 17 (10) | 0.190 |
| Calcium channel blocker | 82 (25) | 34 (21) | 0.079 |
| Insulin | 22 (7) | 6 (4) | 0.255 |
| Oral antihyperglycemic agent | 24 (8) | 8 (5) | 0.201 |
| Non-PAD (n = 164) | PAD (n = 312) | ||||
|---|---|---|---|---|---|
| Mean | Standard Deviation | Mean | Standard Deviation | p | |
| CINC-1 | 63.08 | 41.99 | 79.42 | 69.51 | <0.001 |
| CD95 | 4.56 | 1.94 | 5.48 | 3.12 | 0.001 |
| Fractalkine | 904.21 | 423.76 | 1093.42 | 1063.36 | 0.020 |
| TIM-1 | 22.48 | 8.58 | 27.04 | 19.51 | 0.125 |
| Unadjusted Odds Ratio [95% CI] | p-Value | Adjusted Odds Ratio [95% CI] * | p-Value | |
|---|---|---|---|---|
| CINC-1 | 1.36 [0.05–3.33] | 0.503 | 3.10 [0.33–6.39] | 0.322 |
| CD95 | 2.47 [2.03–3.25] | 0.001 | 2.63 [1.73–3.99] | 0.001 |
| Fractalkine | 2.73 [1.99–4.14] | 0.001 | 2.58 [1.63–3.90] | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Nassereldine, R.; Shaikh, F.; Younes, H.; AbuHalimeh, B.; Zamzam, A.; Abdin, R.; Qadura, M. Inflammatory Protein Panel: Exploring Diagnostic Insights for Peripheral Artery Disease Diagnosis in a Cross-Sectional Study. Diagnostics 2024, 14, 1847. https://doi.org/10.3390/diagnostics14171847
Li B, Nassereldine R, Shaikh F, Younes H, AbuHalimeh B, Zamzam A, Abdin R, Qadura M. Inflammatory Protein Panel: Exploring Diagnostic Insights for Peripheral Artery Disease Diagnosis in a Cross-Sectional Study. Diagnostics. 2024; 14(17):1847. https://doi.org/10.3390/diagnostics14171847
Chicago/Turabian StyleLi, Ben, Rakan Nassereldine, Farah Shaikh, Houssam Younes, Batool AbuHalimeh, Abdelrahman Zamzam, Rawand Abdin, and Mohammad Qadura. 2024. "Inflammatory Protein Panel: Exploring Diagnostic Insights for Peripheral Artery Disease Diagnosis in a Cross-Sectional Study" Diagnostics 14, no. 17: 1847. https://doi.org/10.3390/diagnostics14171847
APA StyleLi, B., Nassereldine, R., Shaikh, F., Younes, H., AbuHalimeh, B., Zamzam, A., Abdin, R., & Qadura, M. (2024). Inflammatory Protein Panel: Exploring Diagnostic Insights for Peripheral Artery Disease Diagnosis in a Cross-Sectional Study. Diagnostics, 14(17), 1847. https://doi.org/10.3390/diagnostics14171847






