Influence of Bridging Stent Graft Implantation into the Renal Artery during Complex Endovascular Aortic Procedures on the Renal Resistance Index
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population and Selection Criteria
2.2. Clinical Data and Surgical Aspects
2.3. Perioperative Renal Protection
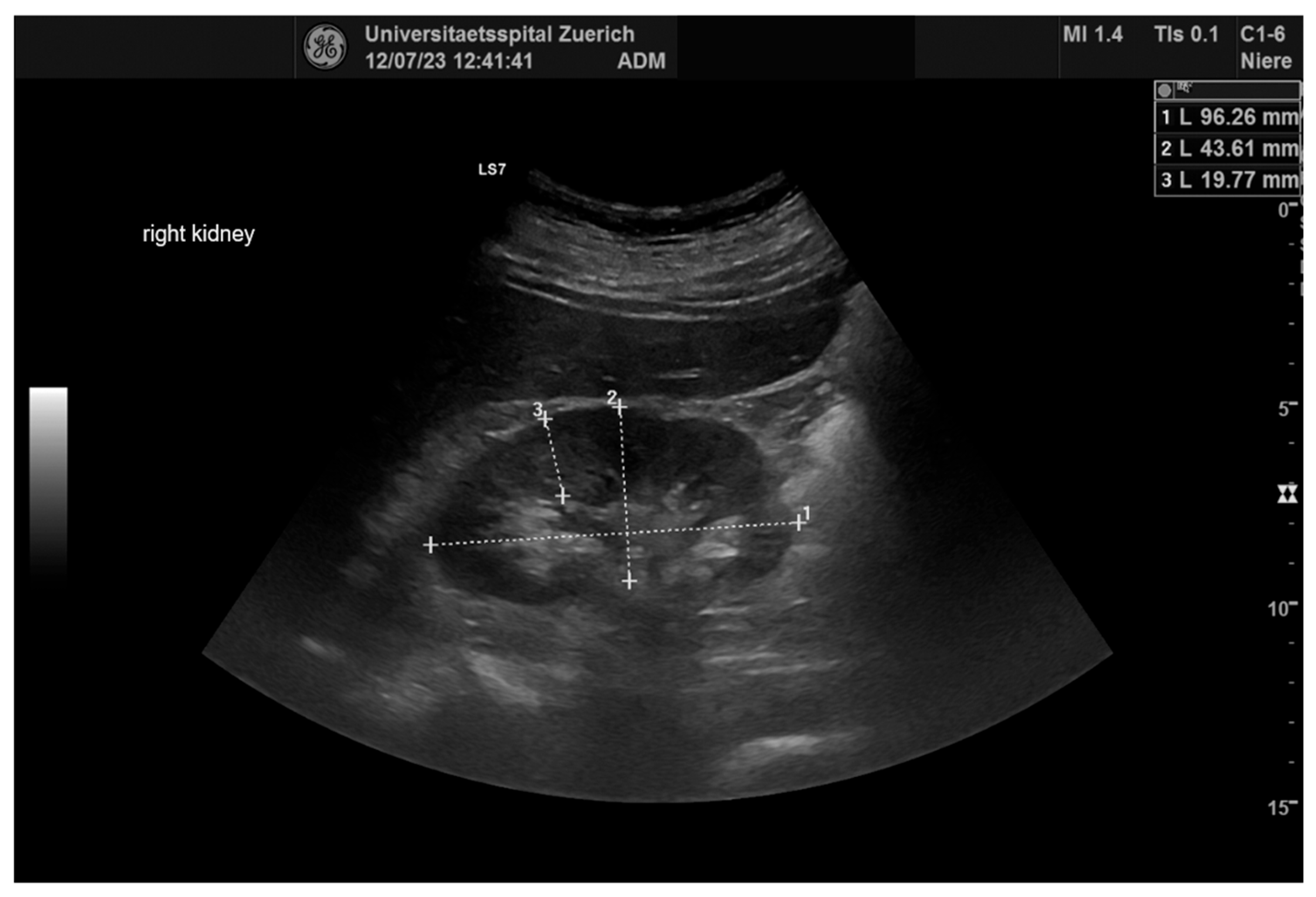
2.4. Ultrasonography
2.5. Statistical Analysis
3. Results
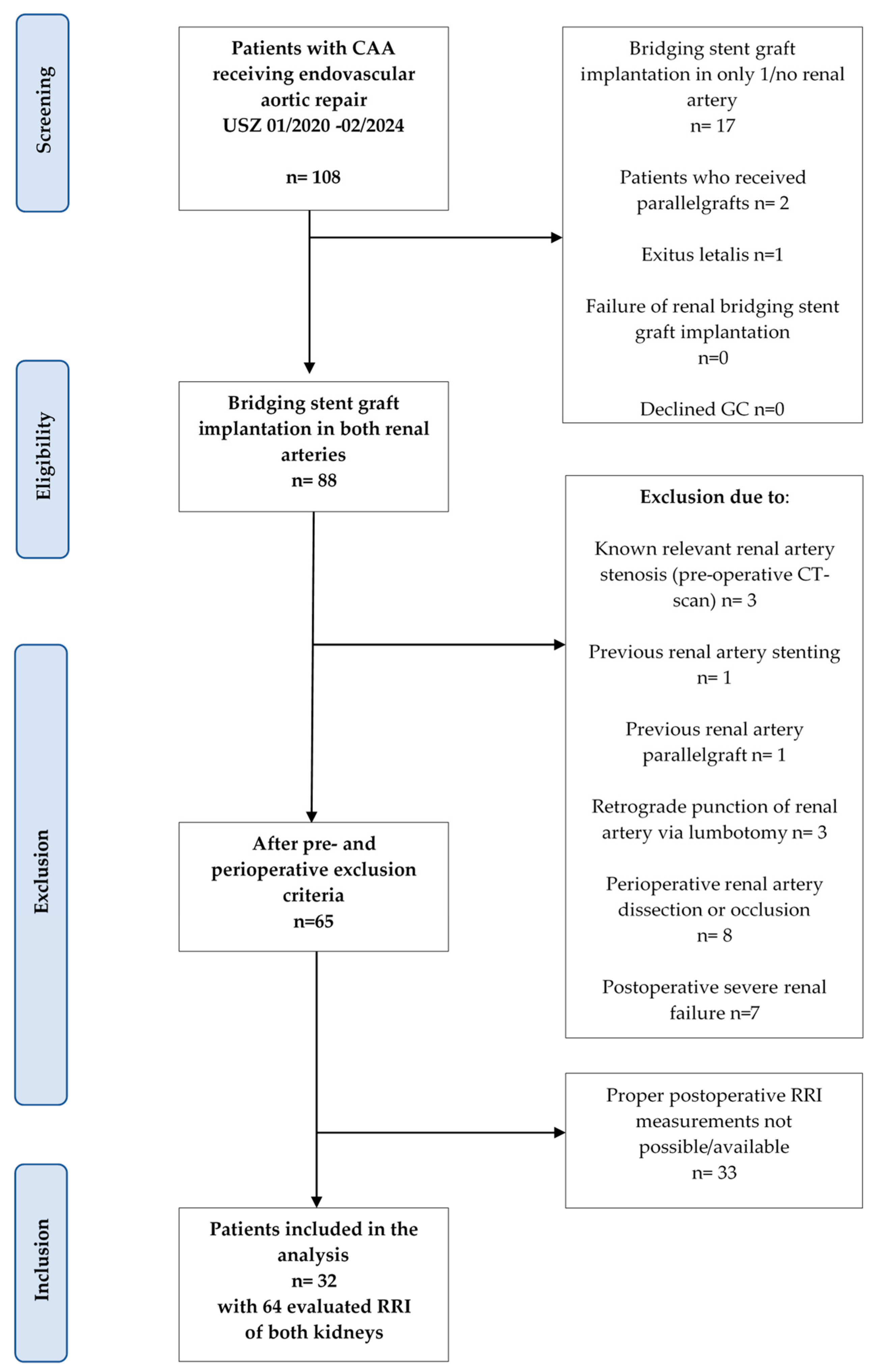
3.1. Screening Algorithm
3.2. Patient’s Characteristics
3.3. Anatomic Characterization of the Underlying Pathology
3.4. Endovascular Treatment Modalities
3.5. Bridging Stent Graft Characteristics
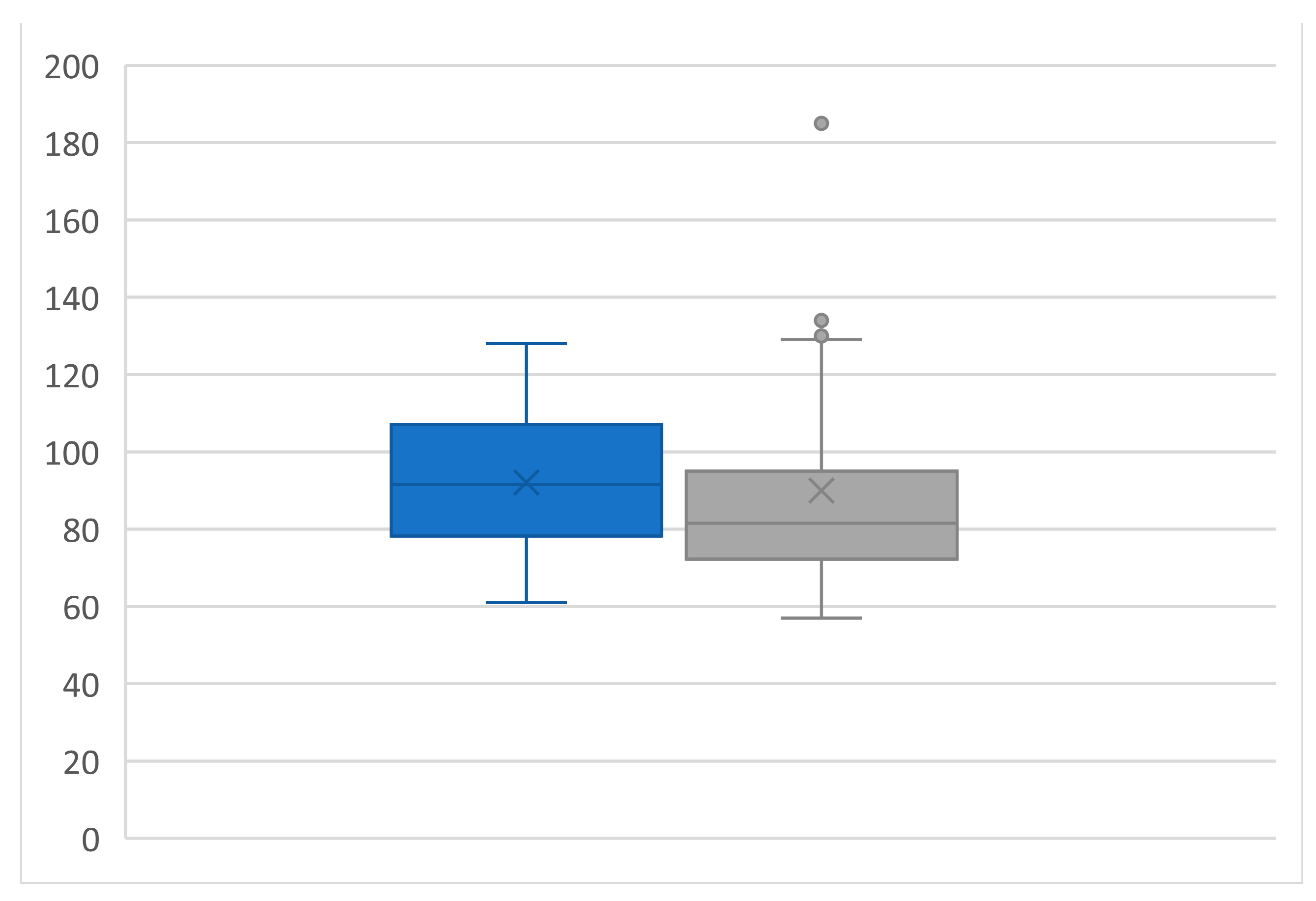
3.6. Ultrasound Evaluation
3.7. Renal Functional Outcomes
3.8. Subgroup Analysis
4. Discussion
4.1. Limitations
4.2. Impact on Clinical Practice
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nordon, I.M.; Hinchliffe, R.J.; Loftus, I.M.; Thompson, M.M. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat. Rev. Cardiol. 2011, 8, 92–102. [Google Scholar] [CrossRef]
- Chaikof, E.L.; Dalman, R.L.; Eskandari, M.K.; Jackson, B.M.; Lee, W.A.; Mansour, M.A.; Mastracci, T.M.; Mell, M.; Murad, M.H.; Nguyen, L.L.; et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 2018, 67, 2–77.e72. [Google Scholar] [CrossRef] [PubMed]
- Wanhainen, A.; Van Herzeele, I.; Bastos Goncalves, F.; Bellmunt Montoya, S.; Berard, X.; Boyle, J.R.; D’Oria, M.; Prendes, C.F.; Karkos, C.D.; Kazimierczak, A.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 192–331. [Google Scholar] [CrossRef] [PubMed]
- Riambau, V.; Böckler, D.; Brunkwall, J.; Cao, P.; Chiesa, R.; Coppi, G.; Czerny, M.; Fraedrich, G.; Haulon, S.; Jacobs, M.J.; et al. Editor’s Choice—Management of Descending Thoracic Aorta Diseases: Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2017, 53, 4–52. [Google Scholar] [CrossRef] [PubMed]
- Waton, S.; Johal, A.; Heikkilä, K.; Cromwell, D.; Boyle, J.; Miller, F. National Vascular Registry (NVR) 2019 Annual Report; The Royal College of Surgeons of England: London, UK, 2019. [Google Scholar]
- Končar, I.B.; Jovanović, A.L.; Dučič, S.M. The role of fEVAR, chEVAR and open repair in treatment of juxtarenal aneurysms: A systematic review. J. Cardiovasc. Surg. 2020, 61, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, G.A.; Juszczak, M.T.; Antoniou, S.A.; Katsargyris, A.; Haulon, S. Editor’s Choice—Fenestrated or Branched Endovascular versus Open Repair for Complex Aortic Aneurysms: Meta-Analysis of Time to Event Propensity Score Matched Data. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 228–237. [Google Scholar] [CrossRef]
- Greenberg, R.K.; Lu, Q.; Roselli, E.E.; Svensson, L.G.; Moon, M.C.; Hernandez, A.V.; Dowdall, J.; Cury, M.; Francis, C.; Pfaff, K.; et al. Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair: A comparison of endovascular and open techniques. Circulation 2008, 118, 808–817. [Google Scholar] [CrossRef]
- Kasprzak, P.M.; Gallis, K.; Cucuruz, B.; Pfister, K.; Janotta, M.; Kopp, R. Editor’s choice—Temporary aneurysm sac perfusion as an adjunct for prevention of spinal cord ischemia after branched endovascular repair of thoracoabdominal aneurysms. Eur. J. Vasc. Endovasc. Surg. 2014, 48, 258–265. [Google Scholar] [CrossRef]
- Verhoeven, E.L.; Katsargyris, A.; Bekkema, F.; Oikonomou, K.; Zeebregts, C.J.; Ritter, W.; Tielliu, I.F. Editor’s Choice—Ten-year Experience with Endovascular Repair of Thoracoabdominal Aortic Aneurysms: Results from 166 Consecutive Patients. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 524–531. [Google Scholar] [CrossRef]
- Schanzer, A.; Simons, J.P.; Flahive, J.; Durgin, J.; Aiello, F.A.; Doucet, D.; Steppacher, R.; Messina, L.M. Outcomes of fenestrated and branched endovascular repair of complex abdominal and thoracoabdominal aortic aneurysms. J. Vasc. Surg. 2017, 66, 687–694. [Google Scholar] [CrossRef]
- Geisbüsch, S.; Kuehnl, A.; Salvermoser, M.; Reutersberg, B.; Trenner, M.; Eckstein, H.H. Editor’s Choice—Hospital Incidence, Treatment, and In Hospital Mortality Following Open and Endovascular Surgery for Thoraco-abdominal Aortic Aneurysms in Germany from 2005 to 2014: Secondary Data Analysis of the Nationwide German DRG Microdata. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Budtz-Lilly, J.; D’Oria, M.; Gallitto, E.; Bertoglio, L.; Kölbel, T.; Lindström, D.; Dias, N.; Lundberg, G.; Böckler, D.; Parlani, G.; et al. European Multicentric Experience With Fenestrated-branched ENDOvascular Stent Grafting After Previous FAILed Infrarenal Aortic Repair: The EU-FBENDO-FAIL Registry. Ann. Surg. 2023, 278, e389–e395. [Google Scholar] [CrossRef] [PubMed]
- Cochennec, F.; Kobeiter, H.; Gohel, M.S.; Majewski, M.; Marzelle, J.; Desgranges, P.; Allaire, E.; Becquemin, J.P. Impact of intraoperative adverse events during branched and fenestrated aortic stent grafting on postoperative outcome. J. Vasc. Surg. 2014, 60, 571–578. [Google Scholar] [CrossRef]
- Tenorio, E.R.; Balachandran, P.W.; Marcondes, G.B.; Lima, G.B.B.; Boba, L.M.; Mendes, B.C.; Macedo, T.A.; Oderich, G.S. Incidence, predictive factors, and outcomes of intraprocedure adverse events during fenestrated-branched endovascular aortic repair of complex abdominal and thoracoabdominal aortic aneurysms. J. Vasc. Surg. 2022, 75, 783–793.e784. [Google Scholar] [CrossRef]
- Mezzetto, L.; Scorsone, L.; Silingardi, R.; Gennai, S.; Piffaretti, G.; Mantovani, A.; Bush, R.L.; Haulon, S.; Veraldi, G.F. Bridging Stents in Fenestrated and Branched Endovascular Aneurysm Repair: A Systematic REVIEW. Ann. Vasc. Surg. 2021, 73, 454–462. [Google Scholar] [CrossRef]
- Sulzer, T.; Tenorio, E.R.; Mesnard, T.; Vacirca, A.; Baghbani-Oskouei, A.; de Bruin, J.L.; Verhagen, H.J.M.; Oderich, G.S. Intraoperative complications during standard and complex endovascular aortic repair. Semin. Vasc. Surg. 2023, 36, 189–201. [Google Scholar] [CrossRef]
- Tenorio, E.R.; Oderich, G.S.; Sandri, G.A.; Ozbek, P.; Kärkkäinen, J.M.; Vrtiska, T.; Macedo, T.A.; Gloviczki, P. Prospective nonrandomized study to evaluate cone beam computed tomography for technical assessment of standard and complex endovascular aortic repair. J. Vasc. Surg. 2020, 71, 1982–1993.e1985. [Google Scholar] [CrossRef] [PubMed]
- Boddi, M.; Natucci, F.; Ciani, E. The internist and the renal resistive index: Truths and doubts. Intern. Emerg. Med. 2015, 10, 893–905. [Google Scholar] [CrossRef]
- Bruno, R.M.; Daghini, E.; Versari, D.; Sgrò, M.; Sanna, M.; Venturini, L.; Romanini, C.; Di Paco, I.; Sudano, I.; Cioni, R.; et al. Predictive role of renal resistive index for clinical outcome after revascularization in hypertensive patients with atherosclerotic renal artery stenosis: A monocentric observational study. Cardiovasc. Ultrasound 2014, 12, 9. [Google Scholar] [CrossRef]
- Viazzi, F.; Leoncini, G.; Derchi, L.E.; Pontremoli, R. Ultrasound Doppler renal resistive index: A useful tool for the management of the hypertensive patient. J. Hypertens. 2014, 32, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Radermacher, J.; Chavan, A.; Bleck, J.; Vitzthum, A.; Stoess, B.; Gebel, M.J.; Galanski, M.; Koch, K.M.; Haller, H. Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N. Engl. J. Med. 2001, 344, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes: Developed by the task force on the management of cardiovascular disease in patients with diabetes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2019, 41, 111–188. [Google Scholar] [CrossRef]
- Oderich, G.S.; Forbes, T.L.; Chaer, R.; Davies, M.G.; Lindsay, T.F.; Mastracci, T.; Singh, M.J.; Timaran, C.; Woo, E.Y. Reporting standards for endovascular aortic repair of aneurysms involving the renal-mesenteric arteries. J. Vasc. Surg. 2021, 73, 4s–52s. [Google Scholar] [CrossRef]
- Gurm, H.S. A Practical Approach to Preventing Contrast-Associated Renal Complications in the Catheterization Laboratory. Interv. Cardiol. Clin. 2023, 12, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.W.; White, C.J.; Thornton, S.; Milani, R.V. Ultrasound velocity criteria for renal in-stent restenosis. J. Vasc. Surg. 2009, 50, 119–123. [Google Scholar] [CrossRef]
- Setacci, C.; Chisci, E.; Setacci, F.; Iacoponi, F.; de Donato, G. Grading carotid intrastent restenosis: A 6-year follow-up study. Stroke 2008, 39, 1189–1196. [Google Scholar] [CrossRef]
- Heneghan, R.E.; Starnes, B.W.; Nathan, D.P.; Zierler, R.E. Renal duplex ultrasound findings in fenestrated endovascular aortic repair for juxtarenal aortic aneurysms. J. Vasc. Surg. 2016, 63, 915–921. [Google Scholar] [CrossRef]
- Szegedi, I.; Potvorszki, F.; Mészáros, Z.R.; Daniel, C.; Csiba, L.; Oláh, L. Role of carotid duplex in the assessment of carotid artery restenosis after endarterectomy or stenting. Front. Neurol. 2023, 14, 1226220. [Google Scholar] [CrossRef]
- Bissacco, D.; Conti, M.; Domanin, M.; Bianchi, D.; Scudeller, L.; Mandigers, T.J.; Allievi, S.; Auricchio, F.; Trimarchi, S. Modifications in Aortic Stiffness After Endovascular or Open Aortic Repair: A Systematic Review and Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2022, 63, 567–577. [Google Scholar] [CrossRef]
- Andrikou, I.; Tsioufis, C.; Konstantinidis, D.; Kasiakogias, A.; Dimitriadis, K.; Leontsinis, I.; Andrikou, E.; Sanidas, E.; Kallikazaros, I.; Tousoulis, D. Renal resistive index in hypertensive patients. J. Clin. Hypertens. 2018, 20, 1739–1744. [Google Scholar] [CrossRef]
- Eagleton, M.J.; Follansbee, M.; Wolski, K.; Mastracci, T.; Kuramochi, Y. Fenestrated and branched endovascular aneurysm repair outcomes for type II and III thoracoabdominal aortic aneurysms. J. Vasc. Surg. 2016, 63, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Oderich, G.S.; Ribeiro, M.; Reis de Souza, L.; Hofer, J.; Wigham, J.; Cha, S. Endovascular repair of thoracoabdominal aortic aneurysms using fenestrated and branched endografts. J. Thorac. Cardiovasc. Surg. 2017, 153, S32–S41.e37. [Google Scholar] [CrossRef] [PubMed]
- Premprabha, D.; Sobel, J.; Pua, C.; Chong, K.; Reilly, L.M.; Chuter, T.A.; Hiramoto, J.S. Visceral branch occlusion following aneurysm repair using multibranched thoracoabdominal stent-grafts. J. Endovasc. Ther. 2014, 21, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Mastracci, T.M.; Carrell, T.; Constantinou, J.; Dias, N.; Martin-Gonzalez, T.; Katsargyris, A.; Modarai, B.; Resch, T.; Verhoeven, E.L.; Burnell, M.; et al. Editor’s Choice—Effect of Branch Stent Choice on Branch-related Outcomes in Complex Aortic Repair. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 536–542. [Google Scholar] [CrossRef]
- Walsh, S.R.; Tang, T.Y.; Boyle, J.R. Renal consequences of endovascular abdominal aortic aneurysm repair. J. Endovasc. Ther. 2008, 15, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Perini, P.; Sediri, I.; Midulla, M.; Delsart, P.; Gautier, C.; Haulon, S. Contrast-enhanced ultrasound vs. CT angiography in fenestrated EVAR surveillance: A single-center comparison. J. Endovasc. Ther. 2012, 19, 648–655. [Google Scholar] [CrossRef]




| Demographics | n = 32 | % |
|---|---|---|
| Age (years, mean ± SD) | 73.9 ± 8.2 | - |
| Male | 27 | 84% |
| Female | 5 | 16% |
| Comorbidities | ||
| Hypertension | 30 | 94% |
| Smoking | 25 | 78% |
| Hypercholesterolemia | 31 | 97% |
| Diabetes | 4 | 12% |
| Aneurysm characteristics | ||
| Juxtarenal | 22 | 69% |
| Pararenal | 2 | 6% |
| Suprarenal | 0 | 0% |
| TAAA | 8 | 25% |
| Diameter (mm, mean, ± SD, range) | 62.8 ± 11.2 (44–100) | - |
| n | % | |
|---|---|---|
| Hospitalization (days, mean ±SD) | 9.5 ± 7.8 | - |
| Indication (n = 32) | ||
| Elective, primary | 19 | 59% |
| Elective, secondary | 11 | 34% |
| Urgent (symptomatic) | 2 | 6% |
| Type (n = 32) | ||
| FEVAR | 26 | 81% |
| BEVAR | 6 | 19% |
| Endoprosthesis (n = 32) | ||
| Off-the-shelf | 5 | 16% |
| CMD | 23 | 72% |
| PMD | 4 | 12% |
| Bridging stent grafts (n = 64) | ||
| Balloon-expendable | 63 | 98% |
| Self-expendable | 1 | 2% |
| Treated visceral arteries (n = 32) | ||
| CT | 19 | 59% |
| SMA | 27 | 84% |
| BEVAR | FEVAR | Total | ||
|---|---|---|---|---|
| Product | Diameter/Length (mm) | n | n | n |
| Advanta® | ||||
| 6/22 | 0 | 12 | 12 | |
| 6/38 | 0 | 1 | 1 | |
| 8/32 | 0 | 2 | 2 | |
| Bentley® | ||||
| 7/23 | 0 | 1 | 1 | |
| E-ventus® | ||||
| 6/22 | 0 | 5 | 5 | |
| 6/58 | 1 | 0 | 1 | |
| 8/37 | 1 | 0 | 1 | |
| 8/57 | 1 | 0 | 1 | |
| iCover® | ||||
| 6/17 | 0 | 1 | 1 | |
| 6/27 | 0 | 25 | 25 | |
| 6/37 | 0 | 2 | 2 | |
| 6/57 | 8 | 0 | 8 | |
| 8/27 | 0 | 2 | 2 | |
| 8/37 | 1 | 2 | 3 | |
| Viabahn® | ||||
| 6/50 | 1 | 0 | 1 | |
| Total (n) | 13 | 53 | 66 | |
| Median diameter (mm) | 6 | 6 | 6 | |
| Median length (mm) | 57 | 27 | 27 | |
| Renal Parameters (n = 64) | Pre-Operative | Post-Operative | p |
|---|---|---|---|
| RRI | 0.66 ± 0.06 | 0.67 ± 0.07 | ns |
| Length (mm) | 107.1± 13.8 | 107.9 ± 14.5 | ns |
| Wide (mm) | 52.4± 7.9 | 52.9 ± 7.8 | ns |
| Parenchymal margin (mm) | 18.3 ± 3.2 | 18.6 ± 2.8 | ns |
| Laboratory Results (n = 32) | |||
| Creatinine (µmol/L) | 92.1 ± 18.5 | 90.0 ± 28.2 | ns |
| eGFR (ml/min) | 68.8 ± 15.8 | 72.3 ± 18.0 | ns |
| Bridging Stent Graft | RRI Pre-Operative | RRI Post-Operative | p |
|---|---|---|---|
| Diameter ≤ Median (6 mm), n =55 | 0.66 ± 0.05 | 0.67 ± 0.06 | ns |
| Diameter > Median (6 mm), n = 9 | 0.64 ± 0.07 | 0.68 ± 0.08 | 0.0014 * |
| Length ≤ Median (27 mm), n = 44 | 0.67 ± 0.05 | 0.68 ± 0.07 | ns |
| Length > Median (27 mm), n = 20 | 0.64 ± 0.07 | 0.65 ± 0.06 | ns |
| Procedure | |||
| BEVAR, n = 12 | 0.64 ± 0.06 | 0.66 ± 0.07 | 0.03 * |
| FEVAR, n= 52 | 0.66 ± 0.05 | 0.67 ± 0.06 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reitnauer, D.; Stoklasa, K.; Dueppers, P.; Reutersberg, B.; Zimmermann, A.; Stadlbauer, T.H.W. Influence of Bridging Stent Graft Implantation into the Renal Artery during Complex Endovascular Aortic Procedures on the Renal Resistance Index. Diagnostics 2024, 14, 1860. https://doi.org/10.3390/diagnostics14171860
Reitnauer D, Stoklasa K, Dueppers P, Reutersberg B, Zimmermann A, Stadlbauer THW. Influence of Bridging Stent Graft Implantation into the Renal Artery during Complex Endovascular Aortic Procedures on the Renal Resistance Index. Diagnostics. 2024; 14(17):1860. https://doi.org/10.3390/diagnostics14171860
Chicago/Turabian StyleReitnauer, Daniela, Kerstin Stoklasa, Philip Dueppers, Benedikt Reutersberg, Alexander Zimmermann, and Thomas H. W. Stadlbauer. 2024. "Influence of Bridging Stent Graft Implantation into the Renal Artery during Complex Endovascular Aortic Procedures on the Renal Resistance Index" Diagnostics 14, no. 17: 1860. https://doi.org/10.3390/diagnostics14171860
APA StyleReitnauer, D., Stoklasa, K., Dueppers, P., Reutersberg, B., Zimmermann, A., & Stadlbauer, T. H. W. (2024). Influence of Bridging Stent Graft Implantation into the Renal Artery during Complex Endovascular Aortic Procedures on the Renal Resistance Index. Diagnostics, 14(17), 1860. https://doi.org/10.3390/diagnostics14171860







