Evaluation of a Ten-Antigen Immunodot Test in Autoimmune Hepatitis and Primary Biliary Cholangitis: Lessons Learned for a Tertiary Care Academic Hospital
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Laboratory Assays
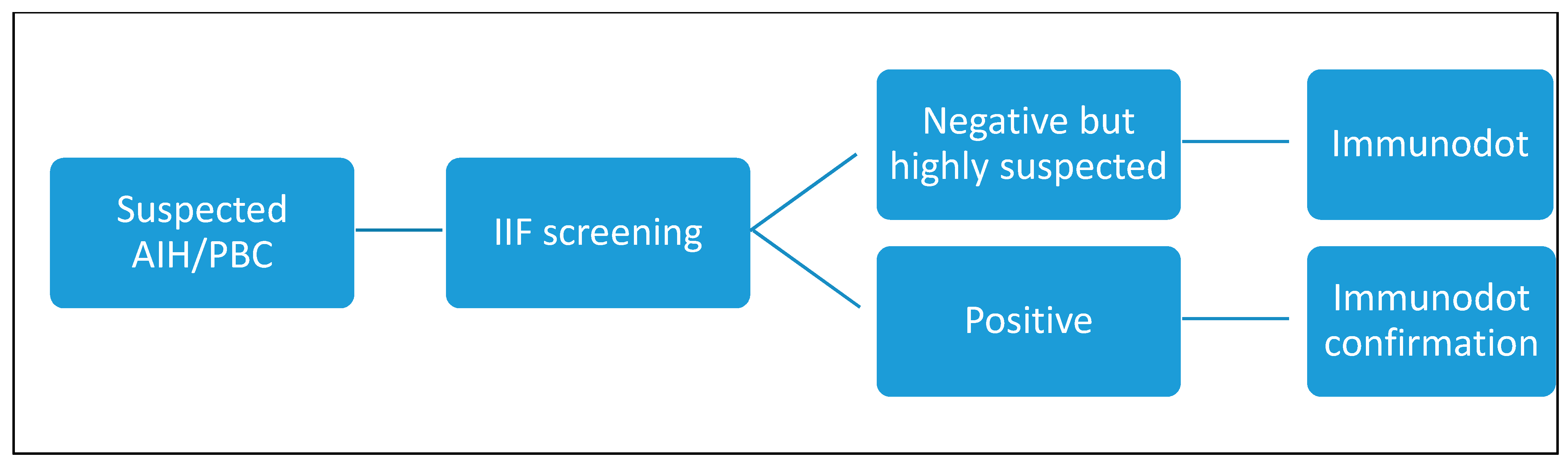
2.2.1. Indirect Immunofluorescence Screening Test
2.2.2. Confirmation Tests
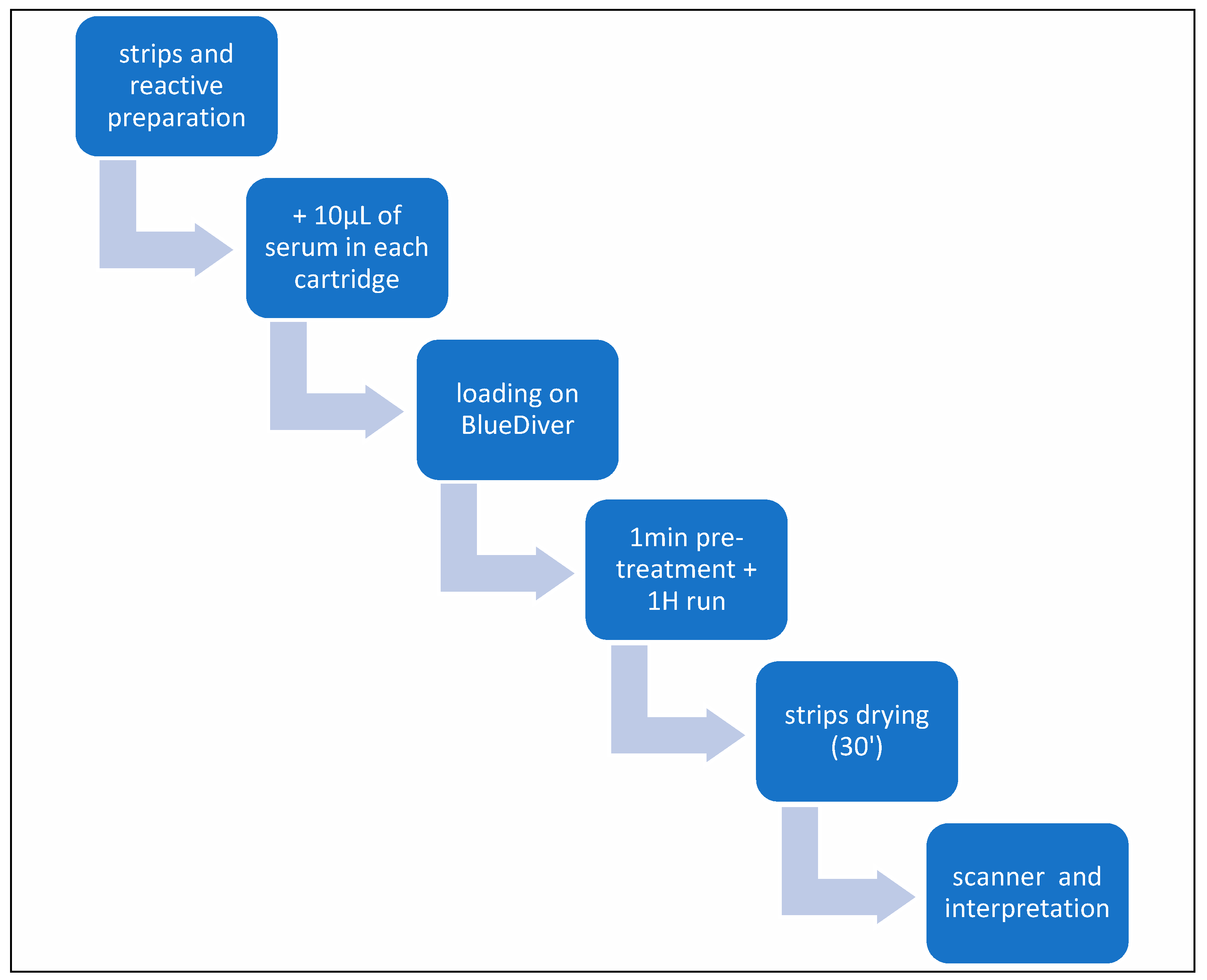
2.2.3. Liver Profile 10 Ag Dot for BDI
2.2.4. Statistical Analysis and Curve Fittings
3. Results
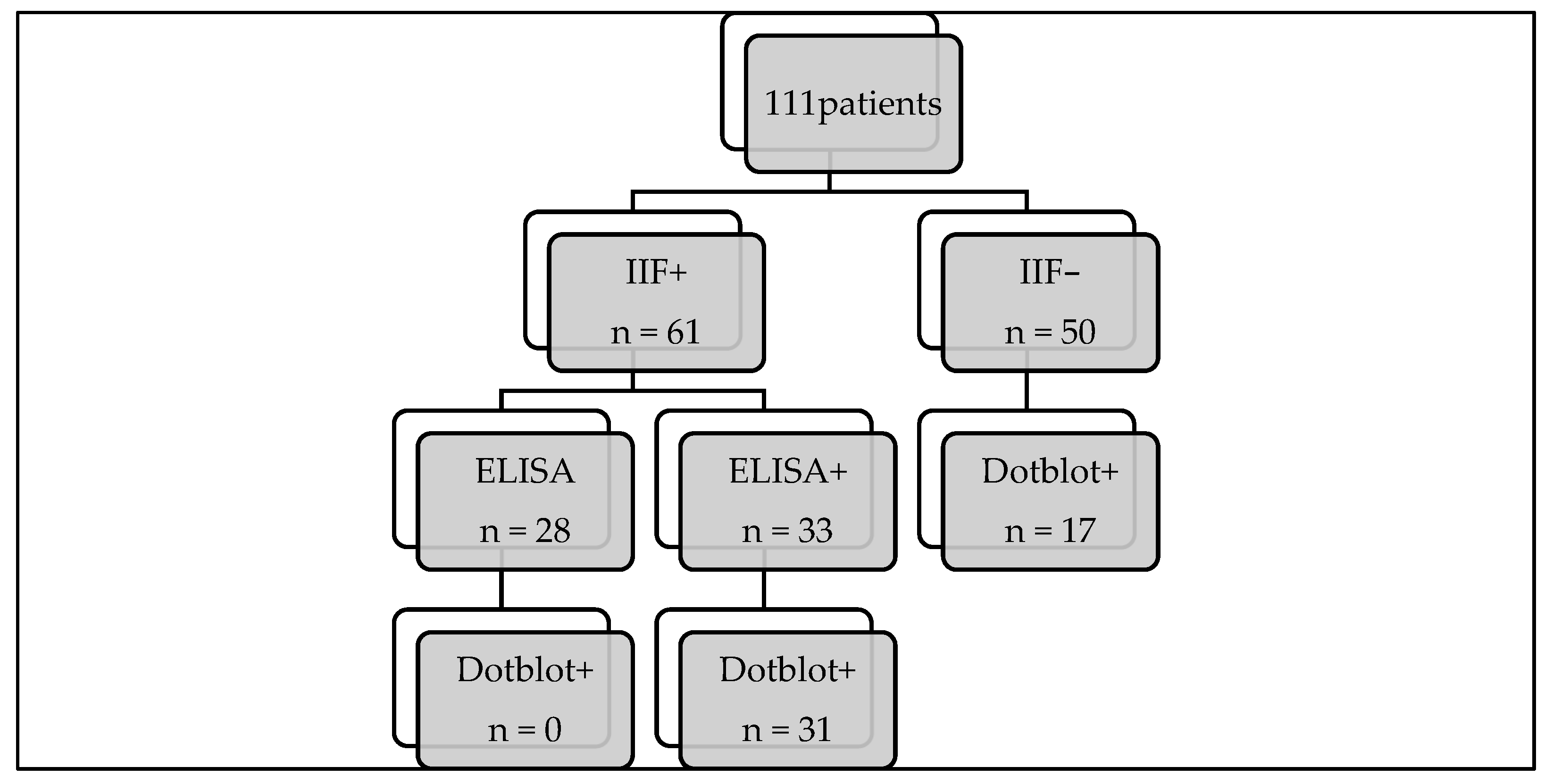
3.1. Study Population
3.2. Comparison of the “Gold Standard” with Immunodots
3.3. Immunodot Positive with Negative IIF Screening
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gatselis, N.K.; Zachou, K.; Koukoulis, G.K.; Dalekos, G.N. Autoimmune hepatitis, one disease with many faces: Etiopathogenetic, clini-co-laboratory and histological characteristics. World J. Gastroenterol. 2015, 21, 60–83. [Google Scholar] [CrossRef]
- EASL. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J. Hepatol. 2015, 63, 971–1004. [Google Scholar] [CrossRef]
- Louie, J.S.; Grandhe, S.; Matsukuma, K.; Bowlus, C.L. Primary Biliary Cholangitis: A Brief Overview. Clin. Liver Dis. 2020, 15, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Feld, J.; Heathcote, E. Epidemiology of autoimmune liver disease. J. Gastroenterol. Hepatol. 2003, 18, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Toh, B. Diagnostic autoantibodies for autoimmune liver diseases. Clin. Transl. Immunol. 2017, 6, e139. [Google Scholar] [CrossRef] [PubMed]
- Sebode, M.; Weiler-Normann, C.; Liwinski, T.; Schramm, C. Autoantibodies in Autoimmune Liver Disease-Clinical and Diagnostic Rele-vance. Front. Immunol. 2018, 9, 609. [Google Scholar] [CrossRef] [PubMed]
- Manns, M.P.; Czaja, A.J.; Gorham, J.D.; Krawitt, E.L.; Mieli-Vergani, G.; Vergani, D.; Vierling, J.M. Diagnosis and management of autoimmune hepatitis. Hepatology 2010, 51, 2193–2213. [Google Scholar] [CrossRef]
- Vergani, D.; Alvarez, F.; Bianchi, F.B.; Cançado, E.L.; Mackay, I.R.; Manns, M.P.; Nishioka, M.; Penner, E. Liver autoimmune serology: A consensus statement from the committee for autoimmune serology of the International Autoimmune Hepatitis Group. J. Hepatol. 2004, 41, 677–683. [Google Scholar] [CrossRef]
- Vanderlocht, J.; van der Cruys, M.; Stals, F.; Bakker-Jonges, L.; Damoiseaux, J. Multiplex autoantibody detection for autoimmune liver diseases and autoimmune gastritis. J. Immunol. Methods 2017, 448, 21–25. [Google Scholar] [CrossRef]
- Bonaguri, C.; Melegari, A.; Picanza, A.; Russo, A.; De Santis, E.; Trenti, T.; Parmeggiani, M.; Belloni, L.; Savi, E.; De’Angelis, G.L.; et al. Association of solid-phase assays to the indirect immunofluorescence in primary biliary cholangitis diagnosis: Results of an Italian multicenter study. Autoimmun. Rev. 2019, 18, 102389. [Google Scholar] [CrossRef]
- Gaiani, F.; Minerba, R.; Picanza, A.; Russo, A.; Melegari, A.; De Santis, E.; Trenti, T.; Belloni, L.; Peveri, S.; Aloe, R.; et al. Optimization of Laboratory Diagnostics of Primary Biliary Cholangitis: When Solid-Phase Assays and Immunofluorescence Combine. J. Clin. Med. 2022, 11, 5238. [Google Scholar] [CrossRef] [PubMed]
- Schotte, H.; Willeke, P.; Schmalhorst, J.; Schlüter, B. Diagnostic Performance of an Anti-Actin Autoantibody Binding Enzyme Immunodot Blot in Autoimmune Hepatitis Type 1. J. Clin. Lab. Anal. 2014, 30, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yang, Y.; Wang, Q.; Wang, Z.; Miao, Q.; Xiao, X.; Wei, Y.; Bian, Z.; Sheng, L.; Chen, X.; et al. The risk predictive values of UK-PBC and GLOBE scoring system in Chinese patients with primary biliary cholangitis: The additional effect of anti-gp210. Aliment. Pharmacol. Ther. 2017, 45, 733–743. [Google Scholar] [CrossRef]
- Invernizzi, P.; Podda, M.; Battezzati, P.M.; Crosignani, A.; Zuin, M.; Hitchman, E.; Maggioni, M.; Meroni, P.L.; Penner, E.; Wesierska-Gadek, J. Autoantibodies against nuclear pore complexes are associated with more active and severe liver disease in primary biliary cirrhosis. J. Hepatol. 2001, 34, 366–372. [Google Scholar] [CrossRef] [PubMed]
- I Rigopoulou, E.; Bogdanos, D.P. Role of autoantibodies in the clinical management of primary biliary cholangitis. World J. Gastroenterol. 2023, 29, 1795–1810. [Google Scholar] [CrossRef] [PubMed]
- Villalta, D.; Bizzaro, N.; Re, M.D.; Tozzoli, R.; Komorowski, L.; Villalta, D.; Bizzaro, N.; Re, M.D.; Tozzoli, R.; Komorowski, L.; et al. Diagnostic accuracy of four different immunological methods for the detection of an-ti-F-actin autoantibodies in type 1 autoimmune hepatitis and other liver-related disorders. Autoimmunity 2008, 41, 105–110. [Google Scholar] [CrossRef]
- Beretta-Piccoli, B.T.; Mieli-Vergani, G.; Vergani, D. Autoimmune Hepatitis: Serum Autoantibodies in Clinical Practice. Clin. Rev. Allergy Immunol. 2022, 63, 124–137. [Google Scholar] [CrossRef]
- Muñoz-Sánchez, G.; Pérez-Isidro, A.; de Landazuri, I.O.; López-Gómez, A.; Bravo-Gallego, L.Y.; Garcia-Ormaechea, M.; Julià, M.R.; Viñas, O.; Ruiz-Ortiz, E.; 2020 GEAI-SEI Workshop Participants. Working Algorithms and Detection Methods of Autoantibodies in Autoimmune Liver Disease: A Nationwide Study. Diagnostics 2022, 12, 697. [Google Scholar] [CrossRef]
- Villalta, D.; Sorrentino, M.C.; Girolami, E.; Tampoia, M.; Alessio, M.G.; Brusca, I.; Daves, M.; Porcelli, B.; Barberio, G.; Bizzaro, N. Autoantibody profiling of patients with primary biliary cirrhosis using a multiplexed line-blot assay. Clin. Chim. Acta 2015, 438, 135–138. [Google Scholar] [CrossRef]
- Mejdoub, S.; Hamza, Z.; Hachicha, H.; Chtourou, L.; Marzouk, S.; Feki, S.; Jerbi, A.; Makki, S.; Boukthir, S.; Bahloul, Z.; et al. Les anticorps anti-mitochondries en pratique: Concordance des techniques de détection et confrontation clinico-biologique. Rev. Méd. Interne 2024, 43, A208–A209. [Google Scholar] [CrossRef]
- Bizzaro, N.; Tozzoli, R.; Villalta, D. Autoimmune diagnostics: The technology, the strategy and the clinical governance. Immunol. Res. 2015, 61, 126–134. [Google Scholar] [CrossRef] [PubMed]



| Number of Patients | ||
|---|---|---|
| Sex | F | 79 (71%) |
| M | 32 (29%) | |
| Age (mean and range) | 37 (1–86) | |
| IIF screening | Positive | 61 (54.9%) |
| Negative | 50 (45.1%) | |
| AIH | 29 (26%) | |
| PBC | 16 (14%) | |
| Overlap variant | 7 (6.3%) |
| Comorbidities | N° Patients (%) |
|---|---|
| Autoimmune thyroiditis | 10 (9%) |
| Autoimmune diabetes | 8 (7.3%) |
| Celiac disease | 5 (4.5%) |
| IBD | 2 (1.8%) |
| Rheumatoid arthritis | 2 (1.8%) |
| Sjogren syndrome | 2 (1.8%) |
| Other | 5 (4.5%) |
| Total | 34 (30.6%) |
| Test | Screening + ELISA | DOT |
|---|---|---|
| LKM | 4 | 4 (100%) |
| M2 * | 11 | 11 (100%) |
| F-actin | 18 | 16 (88.9%) |
| DOT | Screening IIF (Triple Tissue) | ANA Screening | Confirmation | Clinical Data | |
|---|---|---|---|---|---|
| 1 | LC1: 82 (LKM+) | LKM+, LC1− | Speckled | ELISA+ | AIH 2 diagnosis |
| 2 | LC1:21 | Negative | Nucleolar | ELISA+ | AIH 1 diagnosis |
| 3 | LC1: 22 | Negative | Negative | ELISA+ | AIH 1 diagnosis |
| 4 | LKM: 9 | Negative | Speckled | ELISA+ | LKM+ AIH 2 diagnosis |
| 5 | F-actine: 10 | Negative | Speckled | ELISA+ | Post-transplant for BA |
| 6 | F-actine: 8 | Negative | Speckled | ELISA+ | AIH+PBC diagnosis |
| 7 | F-actine: 19 | Negative | Not done | ELISA+ | Suspicion of AIH 1 |
| 8 | F-actine: 18 | Negative | Speckled | ELISA+ | Hepatic cytolysis |
| 9 | F-actine: 11 | Negative | Homogeneous | ELISA+ | Previous diagnosis of AIH 1 |
| 10 | F-actine: 87 | Negative | Nucleolar | ELISA+ | AIH 1 diagnosis |
| 11 | F-actine: 44 | Negative | Speckled | ELISA+ | AI cholangitis |
| 12 | F-actine: 16 | Negative | Speckled | ELISA+ | Cholestase |
| 13 | F-actine: 62 | Negative | Speckled | ELISA+ | AIH 1 diagnosis |
| 14 | sp100: 89 (M2+) | AMA+ | Not done | ANA + (ELISA+) | PBC diagnosis |
| 15 | sp100: 99 (M2+) | AMA+ | Not done | ANA + (ELISA+) | PBC diagnosis |
| 16 | sp100: 21 SLA:100 | Negative | Not done | ANA + (ELISA+) | AIH+PBC diagnosis |
| 17 | gp210: 87 (M2+) | AMA+ | Mitochondria | ANA− for gp210 | PBC diagnosis |
| 18 | sp100: 100 | Negative | Speckled+nuclear dots | ANA+ | Unknown |
| 19 | sp100: 64, gp210: 53 (M2+) | AMA+ | Not done | ANA + (ELISA+) | PBC diagnosis |
| 20 | sp100: 19 | Negative | Speckled | ANA− | PBC diagnosis |
| 21 | sp100: 98 | Negative | Nuclear dots | ANA+ | PBC diagnosis |
| 22 | sp100: 100 | Negative | Nuclear dots | ANA+ | Previous sp100+ |
| 23 | sp100: 92 | Negative | Nuclear dots | ANA+ | AIH diagnosis |
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Overall Accuracy (%) | |
|---|---|---|---|---|---|
| Immunodot | 100 | 95.2 | 100 | 94.4 | 97.3 |
| IIF/ELISA | 71.8 | 96.8 | 100 | 75.9 | 83.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorzi, G.; Pokem, P.N.; Dahlqvist, G.; Délire, B.; Lanthier, N.; Starkel, P.; Horsmans, Y.; Aupaix, C.; Jnaoui, S.; Gruson, D. Evaluation of a Ten-Antigen Immunodot Test in Autoimmune Hepatitis and Primary Biliary Cholangitis: Lessons Learned for a Tertiary Care Academic Hospital. Diagnostics 2024, 14, 1882. https://doi.org/10.3390/diagnostics14171882
Zorzi G, Pokem PN, Dahlqvist G, Délire B, Lanthier N, Starkel P, Horsmans Y, Aupaix C, Jnaoui S, Gruson D. Evaluation of a Ten-Antigen Immunodot Test in Autoimmune Hepatitis and Primary Biliary Cholangitis: Lessons Learned for a Tertiary Care Academic Hospital. Diagnostics. 2024; 14(17):1882. https://doi.org/10.3390/diagnostics14171882
Chicago/Turabian StyleZorzi, Giulia, Perrin Ngougni Pokem, Geraldine Dahlqvist, Bénédicte Délire, Nicolas Lanthier, Peter Starkel, Yves Horsmans, Cedric Aupaix, Samia Jnaoui, and Damien Gruson. 2024. "Evaluation of a Ten-Antigen Immunodot Test in Autoimmune Hepatitis and Primary Biliary Cholangitis: Lessons Learned for a Tertiary Care Academic Hospital" Diagnostics 14, no. 17: 1882. https://doi.org/10.3390/diagnostics14171882
APA StyleZorzi, G., Pokem, P. N., Dahlqvist, G., Délire, B., Lanthier, N., Starkel, P., Horsmans, Y., Aupaix, C., Jnaoui, S., & Gruson, D. (2024). Evaluation of a Ten-Antigen Immunodot Test in Autoimmune Hepatitis and Primary Biliary Cholangitis: Lessons Learned for a Tertiary Care Academic Hospital. Diagnostics, 14(17), 1882. https://doi.org/10.3390/diagnostics14171882







