Diagnostic Performance of Serum Erythropoietin to Discriminate Polycythemia Vera from Secondary Erythrocytosis through Established Subnormal Limits
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Serum Erythropoietin Assay
2.3. Statistical Analysis
3. Results
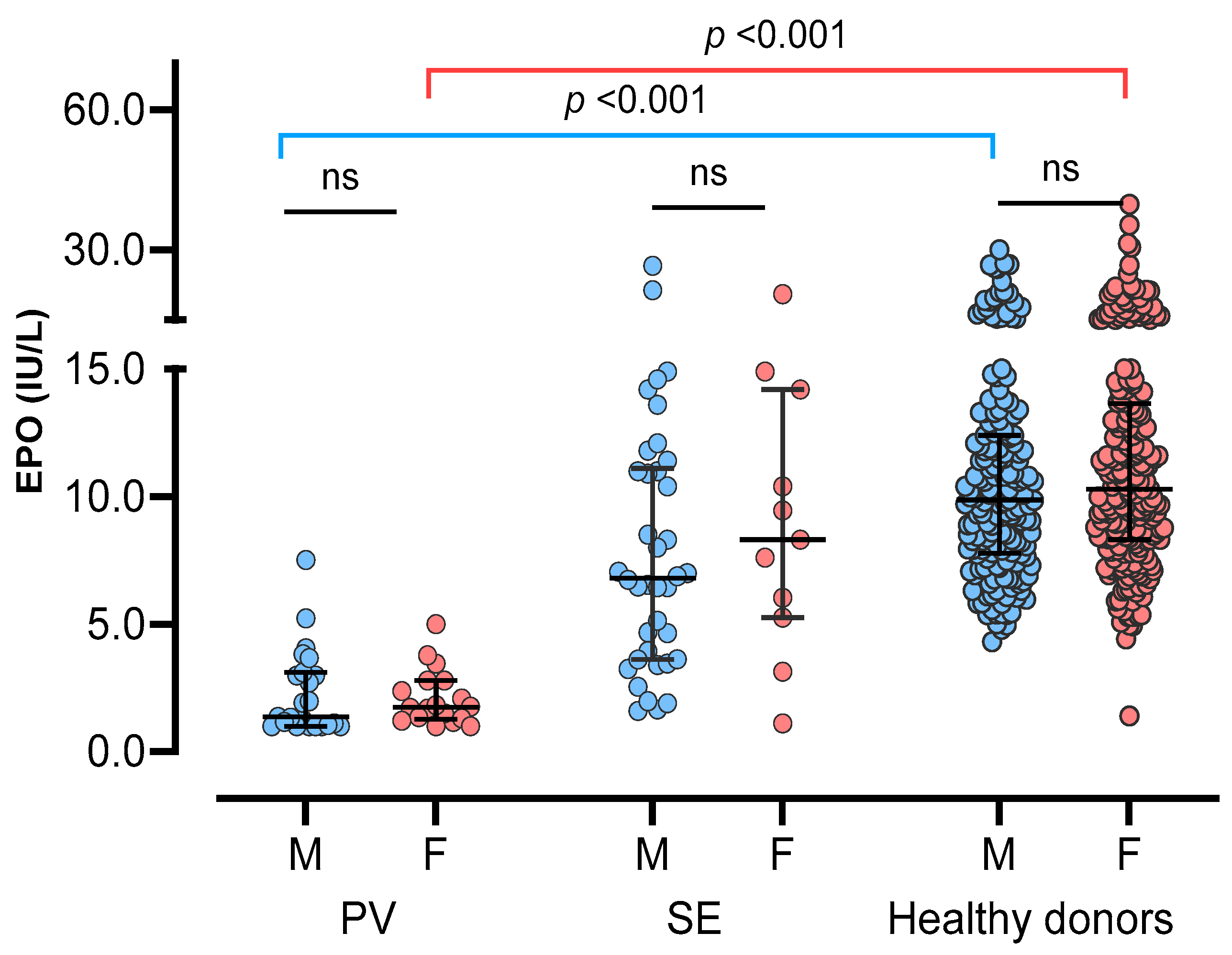
3.1. Basic Demographics of the Study Population
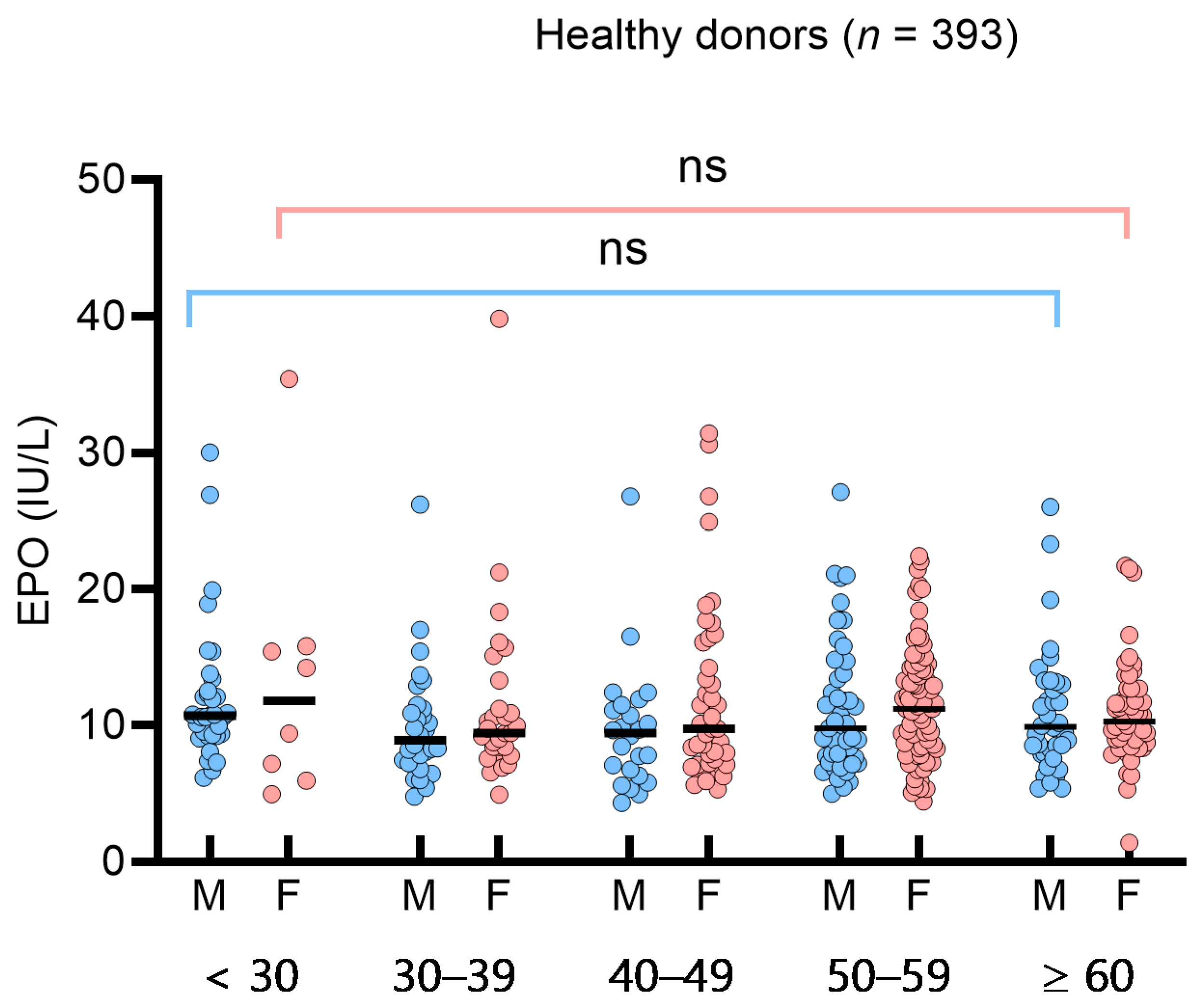
3.2. Determination of Reference Interval and Influencing Factors for Serum Erythropoietin in Healthy Donors
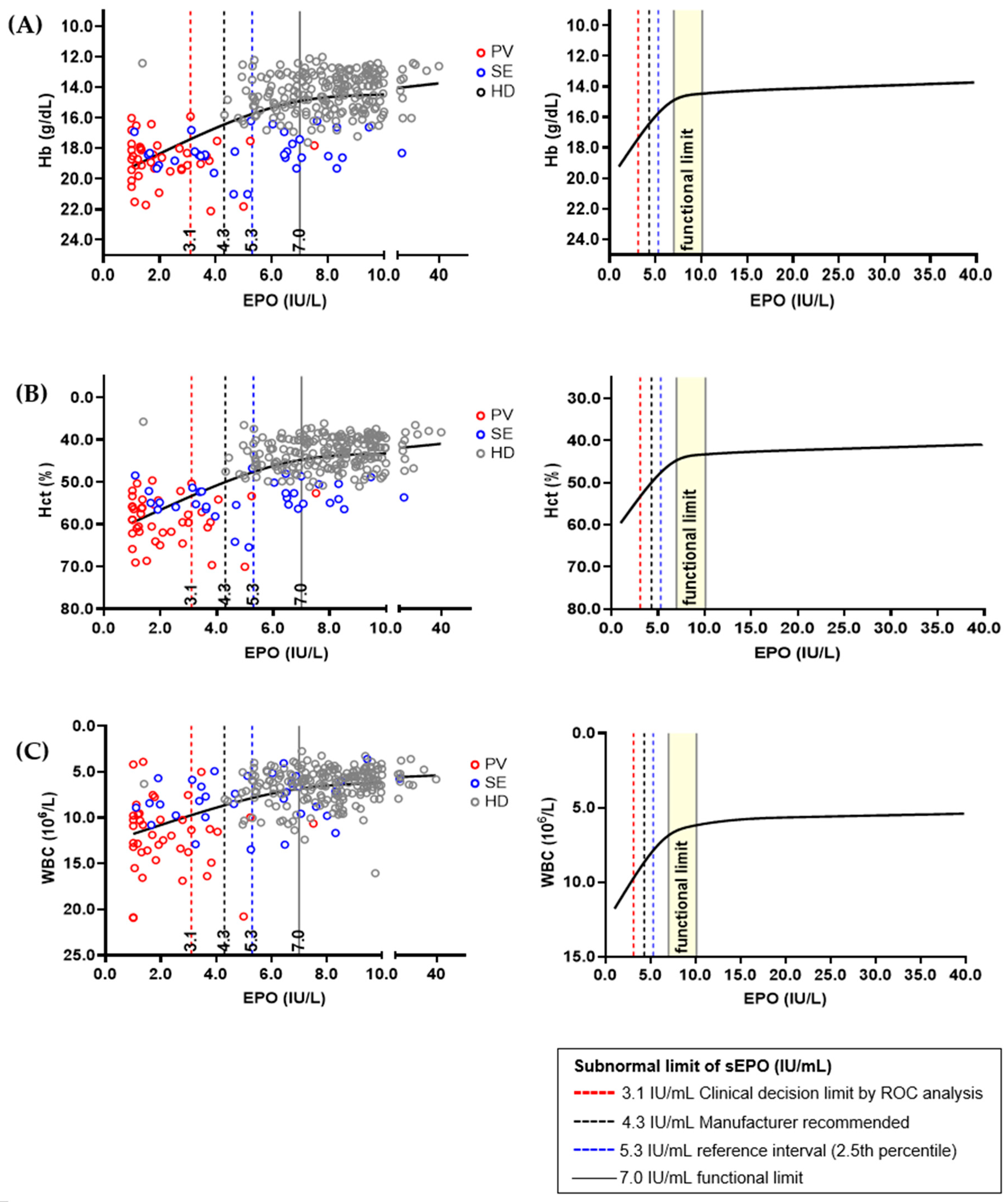
3.3. Determination of Clinical Decision Limit and Functional Reference Limit of Serum Erythropoietin
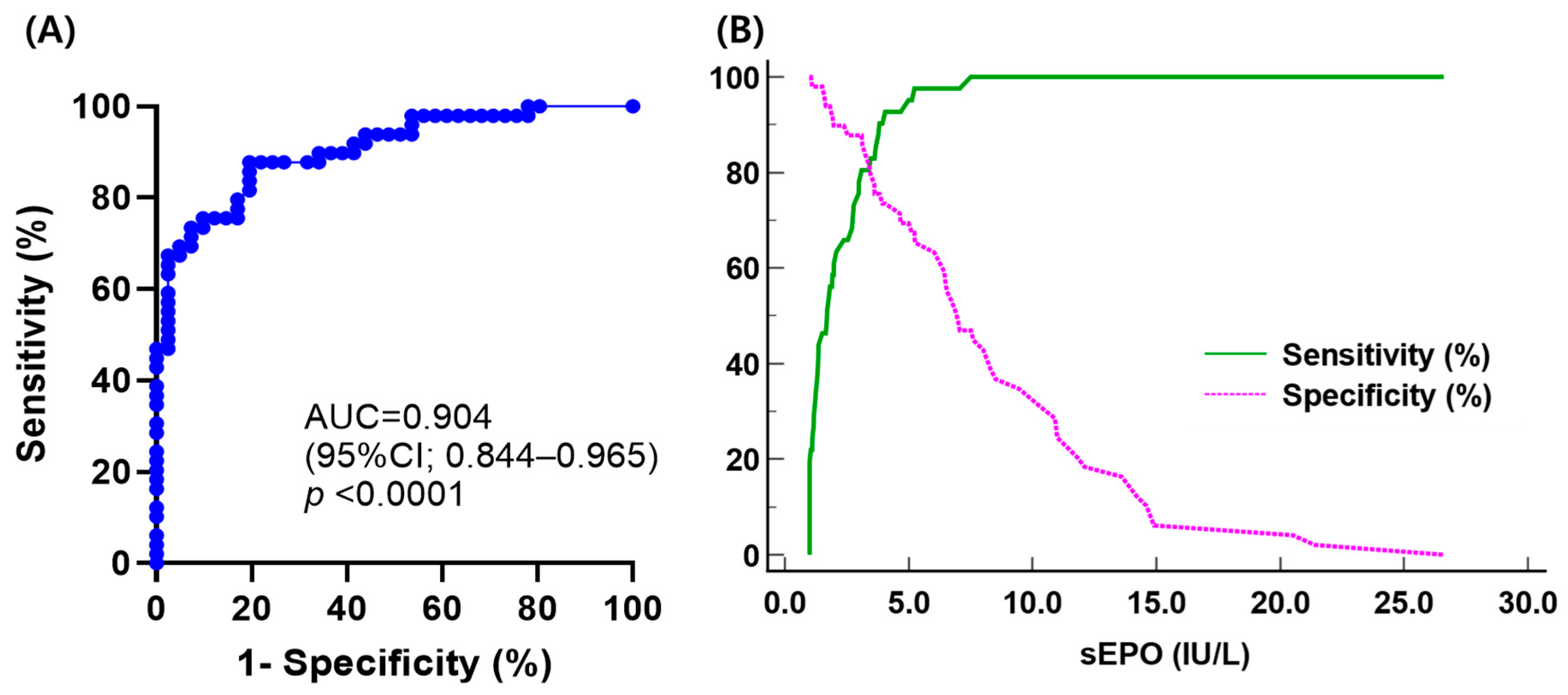
3.4. Diagnostic Performance of Serum Erythropoietin in Differential Diagnosis of Polycythemia Vera and Secondary Erythrocytosis Using Different ‘Subnormal’ Limits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shih, H.M.; Wu, C.J.; Lin, S.L. Physiology and pathophysiology of renal erythropoietin-producing cells. J. Formos. Med. Assoc. 2018, 117, 955–963. [Google Scholar] [CrossRef]
- Erslev, A.J. Erythropoietin. N. Engl. J. Med. 1991, 324, 1339–1344. [Google Scholar] [CrossRef]
- Marsden, J.T. Erythropoietin—Measurement and clinical applications. Ann. Clin. Biochem. 2006, 43, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Pearson, T.C. Apparent polycythaemia. Blood Rev. 1991, 5, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Mithoowani, S.; Laureano, M.; Crowther, M.A.; Hillis, C.M. Investigation and management of erythrocytosis. CMAJ 2020, 192, E913–E918. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, M.M.; Tefferi, A. The complete evaluation of erythrocytosis: Congenital and acquired. Leukemia 2009, 23, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Randi, M.L.; Bertozzi, I.; Cosi, E.; Santarossa, C.; Peroni, E.; Fabris, F. Idiopathic erythrocytosis: A study of a large cohort with a long follow-up. Ann. Hematol. 2016, 95, 233–237. [Google Scholar] [CrossRef]
- McMullin, M.F. Genetic Background of Congenital Erythrocytosis. Genes 2021, 12, 1151. [Google Scholar] [CrossRef]
- Krečak, I.; Holik, H.; Zekanović, I.; Morić Perić, M.; Marketin, T.; Coha, B.; Gverić-Krečak, V.; Vodanović, M.; Lucijanić, M. Thrombotic risk in secondary polycythemia resembles low-risk polycythemia vera and increases in specific subsets of patients. Thromb. Res. 2022, 209, 47–50. [Google Scholar] [CrossRef]
- Nguyen, E.; Harnois, M.; Busque, L.; Sirhan, S.; Assouline, S.; Chamaki, I.; Olney, H.; Mollica, L.; Szuber, N. Phenotypical differences and thrombosis rates in secondary erythrocytosis versus polycythemia vera. Blood Cancer J. 2021, 11, 75. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Ginzburg, Y.Z.; Feola, M.; Zimran, E.; Varkonyi, J.; Ganz, T.; Hoffman, R. Dysregulated iron metabolism in polycythemia vera: Etiology and consequences. Leukemia 2018, 32, 2105–2116. [Google Scholar] [CrossRef]
- Tefferi, A.; Vannucchi, A.M.; Barbui, T. Polycythemia vera: Historical oversights, diagnostic details, and therapeutic views. Leukemia 2021, 35, 3339–3351. [Google Scholar] [CrossRef]
- Kim, M.J.; Kwon, S.S.; Ji, Y.S.; Lee, M.-Y.; Kim, K.H.; Lee, N.; Park, S.K.; Won, J.-H.; Yoon, S.Y. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as new possible minor criteria for diagnosis of polycythemia vera. Int. J. Lab. Hematol. 2023, 45, 853–859. [Google Scholar] [CrossRef]
- Ancochea, A.; Alvarez-Larrán, A.; Morales-Indiano, C.; García-Pallarols, F.; Martínez-Avilés, L.; Angona, A.; Senín, A.; Bellosillo, B.; Besses, C. The role of serum erythropoietin level and JAK2 V617F allele burden in the diagnosis of polycythaemia vera. Br. J. Haematol. 2014, 167, 411–417. [Google Scholar] [CrossRef]
- Davila-Gonzalez, D.; Barrios-Ruiz, A.; Fountain, E.; Cheng, L.; Masarova, L.; Verstovsek, S.; Rojas-Hernandez, C.M. Diagnostic Performance of Erythropoietin Levels in Polycythemia Vera: Experience at a Comprehensive Cancer Center. Clin. Lymphoma Myeloma Leuk. 2021, 21, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Ruiz, A.; Davila-Gonzalez, D.; Fountain, E.; Cheng, L.; Verstovsek, S.; Rojas-Hernandez, C.M. Potential limitations of diagnostic standard codes to distinguish polycythemia vera and secondary erythrocytosis. Sci. Rep. 2022, 12, 4674. [Google Scholar] [CrossRef]
- Gangat, N.; Szuber, N.; Tefferi, A. JAK2 unmutated erythrocytosis: 2023 Update on diagnosis and management. Am. J. Hematol. 2023, 98, 965–981. [Google Scholar] [CrossRef]
- Mossuz, P.; Girodon, F.; Donnard, M.; Latger-Cannard, V.; Dobo, I.; Boiret, N.; Lecron, J.C.; Binquet, C.; Barro, C.; Hermouet, S.; et al. Diagnostic value of serum erythropoietin level in patients with absolute erythrocytosis. Haematologica 2004, 89, 1194–1198. [Google Scholar] [PubMed]
- Fernandez-Pombo, A.; Lorenzo, P.M.; Carreira, M.C.; Gomez-Arbelaez, D.; Castro, A.I.; Primo, D.; Rodriguez, J.; Sajoux, I.; Baltar, J.; de Luis, D.; et al. A very-low-calorie ketogenic diet normalises obesity-related enhanced levels of erythropoietin compared with a low-calorie diet or bariatric surgery. J. Endocrinol. Investig. 2024. [Google Scholar] [CrossRef] [PubMed]
- Benson, E.W.; Hardy, R.; Chaffin, C.; Robinson, C.A.; Konrad, R.J. New automated chemiluminescent assay for erythropoietin. J. Clin. Lab. Anal. 2000, 14, 271–273. [Google Scholar] [CrossRef]
- Musso, C.G.; Musso, C.A.; Joseph, H.; De Miguel, R.; Rendo, P.; Gonzalez, E.; Algranati, L.; dos Ramos Farias, E. Plasma erythropoietin levels in the oldest old. Int. Urol. Nephrol. 2004, 36, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Owen, W.E.; Roberts, W.L. Performance characteristics of a new Immulite((R)) 2000 system erythropoietin assay. Clin. Chim. Acta 2011, 412, 480–482. [Google Scholar] [CrossRef]
- Owen, W.E.; Lambert-Messerlian, G.; Delaney, C.; Christenson, R.; Plouffe, B.; Ludewig, R.; Woods, A.; Lei, J.D.; Thompson, S.; Roberts, W.L.; et al. Multisite evaluation of a monoclonal IMMULITE erythropoietin immunoassay. Clin. Biochem. 2014, 47, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Grote Beverborg, N.; Verweij, N.; Klip, I.T.; van der Wal, H.H.; Voors, A.A.; van Veldhuisen, D.J.; Gansevoort, R.T.; Bakker, S.J.; van der Harst, P.; van der Meer, P. Erythropoietin in the general population: Reference ranges and clinical, biochemical and genetic correlates. PLoS ONE 2015, 10, e0125215. [Google Scholar] [CrossRef] [PubMed]
- Eisenga, M.F.; Kieneker, L.M.; Touw, D.J.; Nolte, I.M.; van der Meer, P.; Huls, G.; Gaillard, C.; Bakker, S.J.L. Active Smoking and Hematocrit and Fasting Circulating Erythropoietin Concentrations in the General Population. Mayo Clin. Proc. 2018, 93, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.G.; Horowitz, G.L.; Ceriotti, F.; Fleming, J.K.; Greenberg, N.; Katayev, A.; Jones, G.R.; Rosner, W.; Young, I.S. Reference Intervals: Strengths, Weaknesses, and Challenges. Clin. Chem. 2016, 62, 916–923. [Google Scholar] [CrossRef]
- Suresh, S.; Rajvanshi, P.K.; Noguchi, C.T. The Many Facets of Erythropoietin Physiologic and Metabolic Response. Front. Physiol. 2019, 10, 1534. [Google Scholar] [CrossRef]
- Kang, T.; Yoo, J.; Jekarl, D.W.; Chae, H.; Kim, M.; Park, Y.-J.; Oh, E.-J.; Kim, Y. Indirect Method for Estimation of Reference Intervals of Inflammatory Markers. Ann. Lab. Med. 2023, 43, 55–63. [Google Scholar] [CrossRef]
- Chuah, T.Y.; Lim, C.Y.; Tan, R.Z.; Pratumvinit, B.; Loh, T.P.; Vasikaran, S.; Markus, C. Functional Reference Limits: Describing Physiological Relationships and Determination of Physiological Limits for Enhanced Interpretation of Laboratory Results. Ann. Lab. Med. 2023, 43, 408–417. [Google Scholar] [CrossRef]
- Sezgin, G.; Loh, T.P.; Markus, C. Functional reference limits: A case study of serum ferritin. J. Lab. Med. 2021, 45, 69–77. [Google Scholar] [CrossRef]
- Ozarda, Y.; Sikaris, K.; Streichert, T.; Macri, J. Distinguishing reference intervals and clinical decision limits—A review by the IFCC Committee on Reference Intervals and Decision Limits. Crit. Rev. Clin. Lab. Sci. 2018, 55, 420–431. [Google Scholar] [CrossRef]
- Balter, M.S.; Daniak, N.; Chapman, K.R.; Sorba, S.A.; Rebuck, A.S. Erythropoietin response to acute hypoxemia in patients with chronic pulmonary disease. Chest 1992, 102, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Andreasson, B.; Carneskog, J.; Lindstedt, G.; Lundberg, P.A.; Swolin, B.; Wadenvik, H.; Kutti, J. Plasma erythropoietin concentrations in polycythaemia vera with special reference to myelosuppressive therapy. Leuk. Lymphoma 2000, 37, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.; Andreasson, B. Hydroxyurea therapy increases plasma erythropoietin in patients with essential thrombocythaemia or polycythaemia vera. Clin. Lab. Haematol. 2006, 28, 233–236. [Google Scholar] [CrossRef]
- Gowanlock, Z.; Sriram, S.; Martin, A.; Xenocostas, A.; Lazo-Langner, A. Erythropoietin Levels in Elderly Patients with Anemia of Unknown Etiology. PLoS ONE 2016, 11, e0157279. [Google Scholar] [CrossRef]
- Panjeta, M.; Tahirovic, I.; Sofic, E.; Coric, J.; Dervisevic, A. Interpretation of Erythropoietin and Haemoglobin Levels in Patients with Various Stages of Chronic Kidney Disease. J. Med. Biochem. 2017, 36, 145–152. [Google Scholar] [CrossRef]
- Sezgin, G.; Monagle, P.; Loh, T.P.; Ignjatovic, V.; Hoq, M.; Pearce, C.; McLeod, A.; Westbrook, J.; Li, L.; Georgiou, A. Clinical thresholds for diagnosing iron deficiency: Comparison of functional assessment of serum ferritin to population based centiles. Sci. Rep. 2020, 10, 18233. [Google Scholar] [CrossRef]
- Passamonti, F.; Rumi, E.; Pietra, D.; Elena, C.; Boveri, E.; Arcaini, L.; Roncoroni, E.; Astori, C.; Merli, M.; Boggi, S.; et al. A prospective study of 338 patients with polycythemia vera: The impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia 2010, 24, 1574–1579. [Google Scholar] [CrossRef]
- Saugy, M.; Lundby, C.; Robinson, N. Monitoring of biological markers indicative of doping: The athlete biological passport. Br. J. Sports Med. 2014, 48, 827–832. [Google Scholar] [CrossRef]
- Diaz-Garzon, J.; Itkonen, O.; Aarsand, A.K.; Sandberg, S.; Coskun, A.; Carobene, A.; Jonker, N.; Bartlett, W.A.; Buño, A.; Fernandez-Calle, P. Biological variation of inflammatory and iron metabolism markers in high-endurance recreational athletes; are these markers useful for athlete monitoring? Clin. Chem. Lab. Med. (CCLM) 2024, 62, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.; Berg, R. Beyond Hemoglobin: When and How to Work Up Possible Polycythemia Vera. Clin. Med. Res. 2020, 18, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Lupak, O.; Han, X.; Xie, P.; Mahmood, S.; Mohammed, H.; Donthireddy, V. The role of a low erythropoietin level for the polycythemia vera diagnosis. Blood Cells Mol. Dis. 2020, 80, 102355. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Healthy Donor (n = 393) | Polycythemia Vera (n = 41) | Secondary Erythrocytosis (n = 49) | p-Value (PV vs. SE) | p-Value (SE vs. HD) |
|---|---|---|---|---|---|
| Males, n (%) | 172 (43.8) | 23 (56.1) | 38 (77.6) | 0.031 | <0.001 |
| Age, years, median (range) | 51 (19–77) | 56 (26–89) | 46 (18–74) | 0.001 | 0.020 |
| Body mass index 1, kg/m2 | 23.7 (22.0–26.4) | 23.5 (21.4–26.4) | 26.8 (24.4–28.9) | 0.002 | <0.001 |
| Systolic BP 1, mmHg | 122 (116–132) | 131 (124–145) | 136 (123–148) | 0.443 | <0.001 |
| Diastolic BP 1, mmHg | 76 (69–82) | 89 (78–95) | 89 (82–98) | 0.192 | <0.001 |
| Heart rate 1, /min | 74 (67–81) | 81 (75–89) | 86 (77–96) | 0.229 | <0.001 |
| Smoking history, n (%) | |||||
| Yes | 52 (13.2) | 5 (12.2) | 14 (28.6) | 0.003 | 0.001 |
| No | 95 (24.2) | 15 (36.6) | 5 (10.2) | - | - |
| Unknown | 246 (62.6) | 21 (51.2) | 30 (61.2) | - | - |
| Comorbidities, n (%) | |||||
| Hypertension | 54 (13.7) | 14 (34.1) | 16 (32.7) | - | - |
| Diabetes Mellitus | 9 (2.3) | 4 (9.8) | 6 (12.2) | - | - |
| Dyslipidemia | 44 (11.2) | 4 (9.8) | 5 (10.2) | - | - |
| Pulmonary disease | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | - |
| Others | 16 (4.1) 2 | 7 (17.1) 3 | 5 (10.2) 4 | - | - |
| None | 286 (72.8) | 18 (43.9) | 23 (46.9) | - | - |
| Laboratory data, median (IQR) | |||||
| Erythropoietin, IU/L | 10.1 (7.9–13.1) | 1.7 (1.1–3.0) | 7.0 (3.8–11.2) | <0.001 | <0.001 |
| White blood cell, ×1000/μL | 5.89 (4.82–7.03) | 11.53 (9.88–13.77) | 7.35 (5.82–9.23) | <0.001 | <0.001 |
| ANC, ×1000/μL | 3.20 (2.44–4.10) | 8.55 (6.73–10.79) | 4.55 (3.12–6.19) | <0.001 | <0.001 |
| Hemoglobin, g/dL | 14.0 (13.2–15.2) | 18.7 (17.9–19.4) | 18.4 (17.6–18.7) | 0.102 | <0.001 |
| Hematocrit, % | 42.1 (39.6–45.1) | 58.9 (54.2–61.8) | 53.7 (51.7–55.8) | <0.001 | <0.001 |
| Platelet, ×1000/μL | 256 (226–292) | 515 (352–700) | 243 (204–288) | <0.001 | 0.201 |
| Glucose, mg/dL | 96 (91–103) | 93 (87–103) | 100 (90–130) | 0.025 | 0.025 |
| HbA1C, % | 5.5 (5.3–5.7) | 5.6 (5.4–6.2) | 6.4 (5.6–7.7) | 0.161 | 0.004 |
| BUN, mg/dL | 13.2 (11.0–15.5) | 13.4 (10.9–16.0) | 12.8 (10.1–15.7) | 0.545 | 0.397 |
| Creatinine, mg/dL | 0.73 (0.64–0.85) | 0.83 (0.72–0.94) | 0.91 (0.76–1.04) | 0.073 | <0.001 |
| eGFR, mL/min/1.73 m2 | 101 (95–110) | 95 (81–103) | 99 (84–111) | 0.227 | 0.086 |
| Total cholesterol, mg/dL | 199 (172–223) | 162 (141–182) | 191 (160–218) | <0.001 | 0.304 |
| Triglyceride, mg/dL | 100 (69–149) | 101 (79–150) | 128 (82–168) | 0.307 | 0.035 |
| C-reactive protein, mg/dL | NT | 0.07 (0.04–0.16) | 0.07 (0.04–0.15) | 0.972 | - |
| Cutoff Value (IU/L) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) |
|---|---|---|---|---|
| 3.1 * | 80.5 (65.1–91.2) | 87.8 (75.2–95.4) | 84.6 (71.9–92.2) | 84.3 (74.1–91.0) |
| 4.3 † | 92.7 (80.1–98.5) | 73.5 (58.9–85.1) | 74.5 (64.5–82.4) | 92.3 (79.9–97.3) |
| 5.3 ‡ | 97.6 (87.1–99.9) | 65.3 (50.4–78.3) | 70.2 (61.5–77.6) | 97.0 (82.0–99.6) |
| 7.0 § | 97.6 (87.1–99.9) | 46.9 (32.5–61.7) | 60.6 (54.1–66.8) | 95.8 (76.4–99.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.S.; Kang, H.; Jekarl, D.W.; Lee, S.-E.; Oh, E.-J. Diagnostic Performance of Serum Erythropoietin to Discriminate Polycythemia Vera from Secondary Erythrocytosis through Established Subnormal Limits. Diagnostics 2024, 14, 1902. https://doi.org/10.3390/diagnostics14171902
Yoon JS, Kang H, Jekarl DW, Lee S-E, Oh E-J. Diagnostic Performance of Serum Erythropoietin to Discriminate Polycythemia Vera from Secondary Erythrocytosis through Established Subnormal Limits. Diagnostics. 2024; 14(17):1902. https://doi.org/10.3390/diagnostics14171902
Chicago/Turabian StyleYoon, Ji Sang, Hyunhye Kang, Dong Wook Jekarl, Sung-Eun Lee, and Eun-Jee Oh. 2024. "Diagnostic Performance of Serum Erythropoietin to Discriminate Polycythemia Vera from Secondary Erythrocytosis through Established Subnormal Limits" Diagnostics 14, no. 17: 1902. https://doi.org/10.3390/diagnostics14171902
APA StyleYoon, J. S., Kang, H., Jekarl, D. W., Lee, S.-E., & Oh, E.-J. (2024). Diagnostic Performance of Serum Erythropoietin to Discriminate Polycythemia Vera from Secondary Erythrocytosis through Established Subnormal Limits. Diagnostics, 14(17), 1902. https://doi.org/10.3390/diagnostics14171902







