Investigation of the Relationship between Vitamin D Deficiency and Vitamin D-Binding Protein Polymorphisms in Severe COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
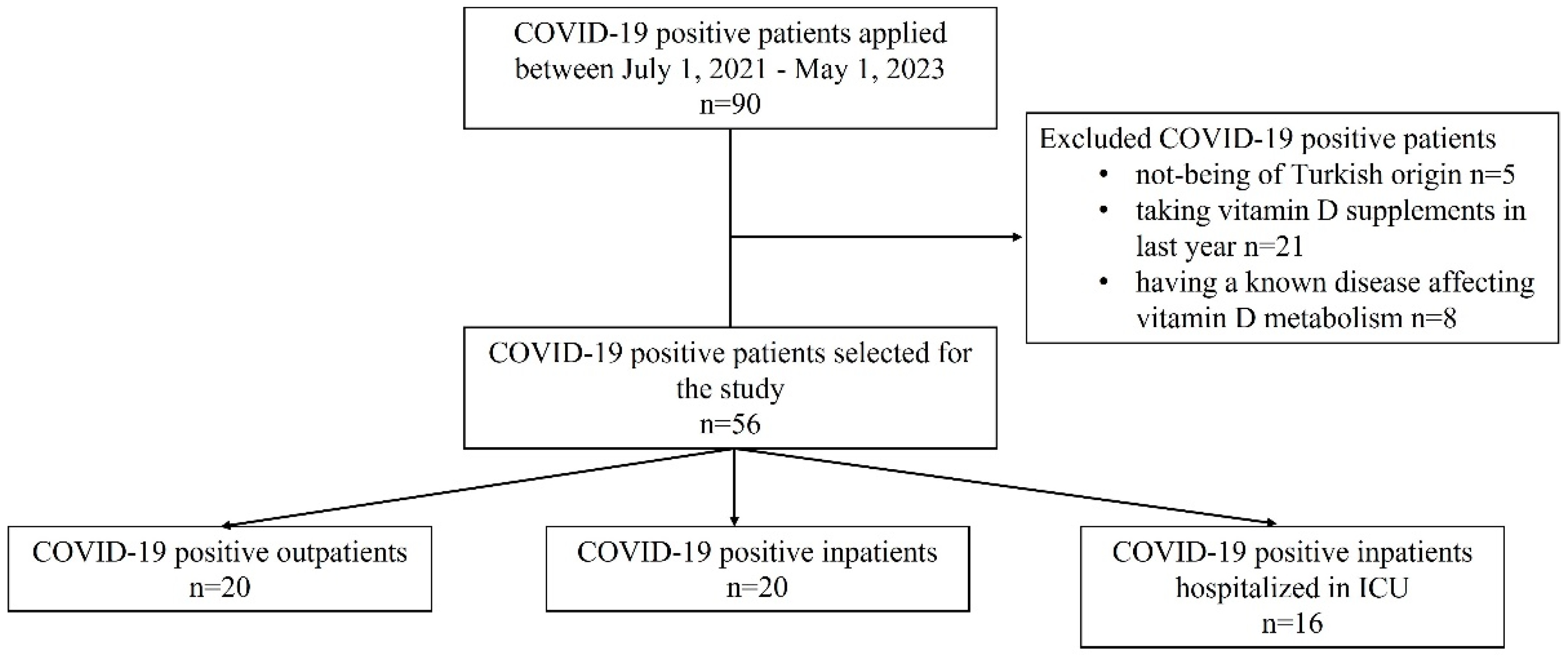
2.1. Patients, Subjects, and Setting
2.2. Data Sources and Measurement
2.3. Data Management
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. 20 April 2022. Available online: https://covid19.who.int (accessed on 12 June 2024).
- Jiang, F.; Deng, L.; Zhang, L.; Cai, Y.; Cheung, C.W.; Xia, Z. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19). J. Gen. Intern. Med. 2020, 35, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.C.; Wang, C.B.; Bernardini, S. The COVID-19 pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Schwartz, J. Vitamin D Binding Protein, Total and Free Vitamin D Levels in Different Physiological and Pathophysiological Conditions. Front. Endocrinol. 2019, 10, 317. [Google Scholar] [CrossRef]
- Hafez, W.; Saleh, H.; Arya, A.; Alzouhbi, M.; Fdl Alla, O.; Lal, K.; Kishk, S.; Ali, S.; Raghu, S.; Elgaili, W.; et al. Vitamin D Status in Relation to the Clinical Outcome of Hospitalized COVID-19 Patients. Front. Med. 2022, 9, 843737. [Google Scholar] [CrossRef]
- Rozmus, D.; Płomiński, J.; Augustyn, K.; Cieślińska, A. rs7041 and rs4588 Polymorphisms in Vitamin D Binding Protein Gene (VDBP) and the Risk of Diseases. Int. J. Mol. Sci. 2022, 23, 933. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Mohammed, A.K.; Bukhari, I.; Rikli, M.; Abdi, S.; Ansari, M.G.A.; Sabico, S.; Hussain, S.D.; Alenad, A.; Al-Saleh, Y.; et al. Efficacy of vitamin D supplementation according to vitamin D-binding protein polymorphisms. Nutrition 2019, 63–64, 148–154. [Google Scholar] [CrossRef]
- Alharazy, S.; Naseer, M.I.; Alissa, E.; Robertson, M.D.; Lanham-New, S.; Alqahtani, M.H.; Chaudhary, A.G. Association of SNPs in GC and CYP2R1 with total and directly measured free 25-hydroxyvitamin D in multi-ethnic postmenopausal women in Saudi Arabia. Saudi J. Biol. Sci. 2021, 28, 4626–4632. [Google Scholar] [CrossRef]
- Rozmus, D.; Ciesielska, A.; Płomiński, J.; Grzybowski, R.; Fiedorowicz, E.; Kordulewska, N.; Savelkoul, H.; Kostyra, E.; Cieślińska, A. Vitamin D Binding Protein (VDBP) and Its Gene Polymorphisms—The Risk of Malignant Tumors and Other Diseases. Int. J. Mol. Sci. 2020, 21, 7822. [Google Scholar] [CrossRef]
- McGrath, J.J.; Saha, S.; Burne, T.H.; Eyles, D.W. A systematic review of the association between common single nucleotide polymorphisms and 25-hydroxyvitamin D concentrations. J. Steroid Biochem. Mol. Biol. 2010, 121, 471–477. [Google Scholar] [CrossRef]
- Karcioglu Batur, L.; Hekim, N. The role of DBP gene polymorphisms in the prevalence of new coronavirus disease 2019 infection and mortality rate. J. Med. Virol. 2021, 93, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Thacher, T.D. Evaluating the Evidence in Clinical Studies of Vitamin D in COVID-19. Nutrients 2022, 14, 464. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Verde, L.; Grant, W.B.; Frias-Toral, E.; Sarno, G.; Vetrani, C.; Ceriani, F.; Garcia-Velasquez, E.; Contreras-Briceño, J.; Savastano, S.; et al. Vitamin D: A Role Also in Long COVID-19? Nutrients 2022, 14, 1625. [Google Scholar] [CrossRef]
- Batur, L.K.; Koç, S. Association between Vitamin D Status and Secondary Infections in Patients with Severe COVID-19 Admitted in the Intensive Care Unit of a Tertiary-Level Hospital in Turkey. Diagnostics 2022, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Mariani, J.; Giménez, V.M.M.; Bergam, I.; Tajer, C.; Antonietti, L.; Inserra, F.; Ferder, L.; Manuch, W. Association Between Vitamin D Deficiency and COVID-19 Incidence, Complications, and Mortality in 46 Countries: An Ecological Study. Health Secur. 2021, 19, 302–328. [Google Scholar] [CrossRef] [PubMed]
- Laird, E.; Rhodes, J.; Kenny, R.A. Vitamin D and Inflammation: Potential Implications for Severity of COVID-19. Ir. Med. J. 2020, 113, 81. [Google Scholar] [PubMed]
- Ali, N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J. Infect. Public Health 2020, 1, 1373–1380. [Google Scholar] [CrossRef]
- Little, J.; Higgins, J.P.; Ioannidis, J.P.; Moher, D.; Gagnon, F.; von Elm, E.; Khoury, M.J.; Cohen, B.; Davey-Smith, G.; Grimshaw, J.; et al. STrengthening the REporting of Genetic Association studies (STREGA)—An extension of the STROBE statement. Eur. J. Clin. Investig. 2009, 39, 247–266. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Aloia, J.; Mikhail, M.; Dhaliwal, R.; Shieh, A.; Usera, G.; Stolberg, A.; Ragolia, L.; Islam, S. Free 25(OH)D and the Vitamin D Paradox in African Americans. J. Clin. Endocrinol. Metab. 2015, 100, 3356–3363. [Google Scholar] [CrossRef]
- Mondul, A.M.; Weinstein, S.J.; Virtamo, J.; Albanes, D. Influence of vitamin D binding protein on the association between circulating vitamin D and risk of bladder cancer. Br. J. Cancer 2012, 107, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Arnaud, J.; Constans, J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP). Hum. Genet. 1993, 92, 183–188. [Google Scholar] [CrossRef]
- Wang, Z.; Joshi, A.; Leopold, K.; Jackson, S.; Christensen, S.; Nayfeh, T.; Mohammed, K.; Creo, A.; Tebben, P.; Kumar, S. Association of vitamin D deficiency with COVID-19 infection severity: Systematic review and meta-analysis. Clin. Endocrinol. 2022, 96, 281–287. [Google Scholar] [CrossRef]
- Pereira, M.; Dantas Damascena, A.; Galvão Azevedo, L.M.; de Almeida Oliveira, T.; da Mota Santana, J. Vitamin D deficiency aggravates COVID-19: Systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, H.A.; de Silva, N.L.; Sumanatilleke, M.; de Silva, S.D.N.; Gamage, K.K.K.; Dematapitiya, C.; Kuruppu, D.C.; Ranasinghe, P.; Pathmanathan, S.; Katulanda, P. Prognostic and Therapeutic Role of Vitamin D in COVID-19: Systematic Review and Meta-analysis. J. Clin. Endocrinol. Metab. 2022, 107, 1484–1502. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.O.; Pamukçu, E.; Yakar, B. The role of vitamin D deficiency on COVID-19: A systematic review and meta-analysis of observational studies. Epidemiol. Health 2021, 43, e2021074. [Google Scholar] [CrossRef]
- Gibbons, J.B.; Norton, E.C.; McCullough, J.S.; Meltzer, D.O.; Lavigne, J.; Fiedler, V.C.; Gibbons, R.D. Association between vitamin D supplementation and COVID-19 infection and mortality. Sci. Rep. 2022, 12, 19397. [Google Scholar] [CrossRef]
- Wöbke, T.K.; Sorg, B.L.; Steinhilber, D. Vitamin D in inflammatory diseases. Front. Physiol. 2014, 5, 244. [Google Scholar] [CrossRef]
- Colotta, F.; Jansson, B.; Bonelli, F. Modulation of inflammatory and immune responses by Vitamin D. J. Autoimmun. 2017, 85, 78–97. [Google Scholar] [CrossRef]
- Delanghe, J.R.; De Buyzere, M.L.; Speeckaert, M.M. Genetic polymorphisms in the host and COVID-19 infection. In Coronavirus Disease-COVID-19 2021 May 11; Springer International Publishing: Cham, Switzerland, 2021; pp. 109–118. [Google Scholar]
- Kahar, L.A.; Yusrawati, Y.; Jamsari, J.; Maskoen, T.; Aribowo, K.; Sari, W.M. Association between Polymorphism Locus rs7041 and rs4588 in VDBP Gene and Vitamin D Status with Mortality in Sepsis Patient. J. Hunan Univ. Nat. Sci. 2023, 50, 150–158. [Google Scholar] [CrossRef]
- Erasmus, R.; Maepa, S.; Machingura, I.; Davids, S.; Raghubeer, S.; Matsha, T. Vitamin D, Vitamin D-Binding Proteins, and VDR Polymorphisms in Individuals with Hyperglycaemia. Nutrients 2022, 14, 3147. [Google Scholar] [CrossRef] [PubMed]
- Apaydin, T.; Polat, H.; Dincer Yazan, C.; Ilgin, C.; Elbasan, O.; Dashdamirova, S.; Bayram, F.; Tukenmez Tigen, E.; Unlu, O.; Tekin, A.F.; et al. Effects of vitamin D receptor gene polymorphisms on the prognosis of COVID-19. Clin. Endocrinol. 2022, 96, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Speeckaert, M.M.; De Buyzere, M.L.; Delanghe, J.R. Vitamin D binding protein polymorphism and COVID-19. J. Med. Virol. 2021, 93, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.T.; Calhau, C.; Antunes, G.; Araújo, B.; Bandeira, M.; Barreira, S.; Bazenga, F.; Braz, S.; Caldeira, D.; Santos, S.C.R.; et al. Vitamin D-related polymorphisms and vitamin D levels as risk biomarkers of COVID-19 disease severity. Sci. Rep. 2021, 11, 20837. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Jaroenlapnopparat, A.; Mettler, S.K.; Grover, A. Genetic Variations of the Vitamin D Metabolic Pathway and COVID-19 Susceptibility and Severity: Current Understanding and Existing Evidence. Biomedicines 2023, 11, 400. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total (n = 56) | Outpatient (n = 20) | Inpatient (n = 20) | ICU 4 Patient (n = 16) | p-Value |
|---|---|---|---|---|---|
| Age (years), Mean ± SD 1 | 48.87 ± 22.4 | 29.4 ± 9.2 | 48.9 ± 14.7 | 73.1 ± 16.4 | <0.0001 |
| Sex, n (%) | 0.0697 | ||||
| Male | 32 (57.1%) | 13 (65%) | 10 (50%) | 9 (56.2%) | |
| Female | 24 (42.9%) | 7 (35%) | 10 (50%) | 7 (43.7%) | |
| Comorbidities, n (%) | 0.149 | ||||
| Hypertension | 13 (23.2%) | 1 (5%) | 5 (25%) | 7 (43.7%) | |
| Diabetes Mellitus | 10 (17.9%) | 0 (0%) | 4 (20%) | 6 (37.5%) | |
| CAD 2 | 7 (12.5%) | 0 (0%) | 2 (10%) | 5 (31.2%) | |
| Hypothyroidism | 3 (5.4%) | 2 (10%) | 0 (0%) | 1 (6.2%) | |
| Others | 18 (32.1%) | 4 (20%) | 6 (30%) | 8 (50%) | |
| Smoking, n (%) | 26 (46.4%) | 15 (75%) | 4 (20%) | 7 (43.7%) | 0.0022 |
| Mortality, n (%) | 9 (16.1%) | 0 (0%) | 0 (0) | 9 (56.2%) | <0.0001 |
| Total 25(OH)D (ng/mL) Median [Min–Max] | 28.9 [12.9–108.4] | 28.6 [15.6–50.7] | 31.4 [18.9–108.4] | 18.8 [12.9–77.7] a | 0.0105 |
| Free 25(OH)D (pg/mL) Median [Min–Max] | 4.5 [2.04–31.17] | 4.0 [2.04–6.54] | 5.1 [2.74–31.17] | 5.1 [3.02–14.6] | 0.106 |
| Bioavailable 25(OH)D (ng/mL) Median [Min–Max] | 16.2 [6.71–101.4] | 17.1 [8.77–29.27] | 16.7 [8.2–101.4] | 10.9 [6.71–41.35] | 0.0703 |
| Vitamin D deficient, n (%) | 12 (21.4%) | 2 (10%) | 1 (5%) | 9 (56.2%) | 0.0003 |
| VDBP (mg/L), Median [Min–Max] | 236 [19.5–316.5] | 281 [200–316] | 280 [34.8–515] | 222 [19.5–304] a,b | 0.0009 |
| Calcium (mg/dL), Mean ± SD | 8.77 ± 0.74 | 9.21 ± 0.42 | 8.70 ± 0.49 c | 8.32 ± 1.01 d | 0.0007 |
| Albumin (g/dL), Mean ± SD | 36.93 ± 9.36 | 45.52 ± 2.82 | 36.62 ± 4.61 d | 26.58 ± 8.44 d,e | <0.0001 |
| iPTH 3 (pg) Median [Min–Max] | 35.1 [11.32–873.7] | 30.1 [12.66–62.06] | 34.0 [11.32–191] | 49.0 [12.83–873.7] b,f | 0.0021 |
| Phosphor (mg/dL), Median [Min–Max] | 3.5 [2.18–54.53] | 3.4 [2.4–4.44] | 3.7 [2.44–54.53] | 4.0 [2.18–6.8] | 0.124 |
| Variables | Total (n = 56) | Outpatient (n = 20) | Inpatient (n = 20) | ICU 1 Patient (n = 16) | p-Value |
|---|---|---|---|---|---|
| rs7041 SNP 2 | 0.0301 | ||||
| Wild type | 10 (17.9%) | 0 (%) | 6 (30%) | 4 (25%) | |
| Homozygote (T > G) | 12 (21.4%) | 6 (30%) | 1 (5%) | 5 (31.3%) | |
| Heterozygote (T > G) | 34 (60.%) | 14 (70%) | 13 (65%) | 7 (43.7%) | |
| rs4588 SNP | 0.424 | ||||
| Wild type | 33 (58.9%) | 14 (70%) | 11 (55%) | 8 (50%) | |
| Homozygote (C > A) | 1 (1.8%) | 0 (0%) | 0 (0%) | 1 (6.3%) | |
| Heterozygote (C > A) | 22 (39.3%) | 6 (30%) | 9 (45%) | 7 (43.7%) |
| rs7041 | rs4588 | ||||||
|---|---|---|---|---|---|---|---|
| Variables | WT (n = 10) | Homozygous (n = 12) | Heterozygous (n = 34) | p-Value | WT (n = 33) | Homozygous + Heterozygous (n = 23) | p-Value |
| Total 25(OH)D (ng/mL), Median [Min–Max] | 26.9 [18.9–77.7] | 28.1 [14.6–50.7] | 29.5 [12.9–108.4] | 0.534 | 28.9 [14.6–108.4] | 29 [12.9–77.7] | 0.696 |
| Free 25(OH)D (pg/mL), Median [Min–Max] | 4.43 [2.74–14.6] | 4.28 [3.18–7.4] | 4.85 [2.04–31.2] | 0.784 | 4.34 [2.04–31.17] | 4.53 [2.43–14.6] | 0.973 |
| Bioavailable 25(OH)D (ng/mL), Median [Min–Max] | 15.82 [8.2–41.4] | 16.12 [6.7–29.3] | 16.64 [7.9–101.4] | 0.659 | 16.45 [6.71–101.4] | 16.05 [7.89–41.35] | 0.474 |
| Vitamin D deficient N (%) | 1 (10%) | 5 (41.7%) | 6 (17.6%) | 0.136 | 7 (21.2%) | 5 (21.7%) | 0.962 |
| VDBP (mg/L), Median [Min–Max] | 269 [42–515] | 260.5 [19.5–307] | 250 [34.8–420] | 0.570 | 283 [19.5–420] | 237.5 [80–515] | 0.150 |
| Calcium (mg/dL), Mean ± SD | 8.55 ± 0.98 | 8.79 ± 0.95 | 8.83 ± 0.58 | 0.590 | 8.82 ± 0.84 | 8.71 ± 0.58 | 0.572 |
| Albumin (g/dL), Mean ± SD | 33.4 ± 8.79 | 36.03 ± 11.52 | 38.29 ± 8.64 | 0.291 | 37.84 ± 9.62 | 35.62 ± 9.01 | 0.383 |
| iPTH 1 (pg) Median [Min–Max] | 30.35 [12.83–75.66] | 41.84 [20.08–873.7] | 35.09 [11.32–191] | 0.308 | 36.06 [11.3–873.7] | 34.75 [12.8–179.6] | 0.527 |
| Phosphor (mg/dL), Median [Min–Max] | 4.1 [3.07–4.71] | 3.76 [2.49–5.33] | 3.48 [2.18–54.53] | 0.188 | 3.81 [2.51–3.81] | 3.52 [2.18–6.8] | 0.479 |
| Variables | Outpatients and Inpatients | ICU 2 Patients | ||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | WT (n = 6) | Homozygous (n = 7) | Heterozygous (n = 27) | p-Value | WT (n = 4) | Homozygous (n = 5) | Heterozygous (n = 7) | p-Value |
| Total 25(OH)D (ng/mL) Median [Min–Max] | 26.9 [18.9–36.6] | 32.9 [27.3–50.7] | 29.5 [15.6–108.4] | 0.268 | 29.6 [21.8–77.7] | 17.4 [14.6–17.7] | 19.9 [12.9–53.9] | 0.072 |
| Free 25(OH)D (pg/mL) Median [Min–Max] | 3.94 [2.74–4.58] | 4.34 [3.77–6.54] | 4.53 [2.04–31.17] | 0.282 | 10.31 [3.51–14.60] | 3.47 [3.18–7.40] | 5.40 [3.02–9.41] | 0.170 |
| Bioavailable 25(OH)D (ng/mL) Median [Min–Max] | 14.74 [8.2–17.55] | 18.38 [15.12–29.27] | 16.85 [8.77–101.4] | 0.078 | 17.08 [11.24–41.35] | 8.33 [6.71–16.88] | 10.56 [7.89–30.86] | 0.113 |
| Vitamin D deficient n (%) | 1 (16.7) | 0 (0) | 2 (7.4) | 0.523 | 1 (25) | 0 (0) | 2 (28.6) | 0.428 |
| VDBP (mg/L) Median [Min–Max] | 294 [248–515] | 289 [251–307] | 275 [34.8–420] | 0.113 | 232 [42–304] | 200 [19.5–248] | 221 [80–278] | 0.794 |
| Calcium (mg/dL) Mean ± SD | 8.93 ± 0.64 | 9.06 ± 0.32 | 8.93 ± 0.55 | 0.845 | 7.99 ± 1.2 | 8.42 ± 1.42 | 8.44 ± 0.59 | 0.445 |
| Albumin (g/dL) Mean ± SD | 37.92 ± 4.28 | 44.89 ± 3.31 | 40.78 ± 6.28 | 0.091 | 26.63 ± 9.95 | 23.62 ± 4.32 | 28.67 ± 10.19 | 0.383 |
| iPTH 1 (pg) Median [Min–Max] | 27.72 [17.07–44.22] | 29.30 [20.07–45.59] | 34.69 [11.32–191] | 0.413 | 38.96 [12.83–75.66] | 146.79 [38.50–873.7] | 48.98 [26.88–179.6] | 0.128 |
| Phosphor (mg/dL) Median [Min–Max] | 3.95 [3.07–4.39] | 3.71 [2.74–4.44] | 3.44 [2.40–54.53] | 0.365 | 4.17 [4.05–4.71] | 3.95 [2.49–5.33] | 3.73 [2.18–6.80] | 0.683 |
| Outpatients and Inpatients | ICU 2 Patients | ||||||
|---|---|---|---|---|---|---|---|
| Variables | WT (n = 25) | Heterozygous (n = 15) | p-Value | WT (n = 8) | Homozygous (n = 1) | Heterozygous (n = 7) | p-Value |
| Total 25(OH)D (ng/mL) Median [Min–Max] | 30.6 [15.6–108.4] | 29.0 [16.4–42.2] | 0.214 | 17.45 [14.6–53.9] | 23.30 | 31.20 [12.9–77.7] | 0.511 |
| Free 25(OH)D (pg/mL) Median [Min–Max] | 4.34 [2.04–31.17] | 4.26 [2.43–6.98] | 0.576 | 4.21 [3.18–14.03] | 3.51 | 5.69 [3.02–14.60] | 0.802 |
| Bioavailable 25(OH)D (ng/mL) Median [Min–Max] | 16.85 [8.77–101.4] | 16.57 [8.2–24.85] | 0.241 | 8.94 [6.71–30.86] | 40.8 | 41.41 [12.83–179.6] | 0.020 |
| Vitamin D deficient n (%) | 1 (4%) | 2 (13.3%) | 0.642 | 5 (62.5%) | 0 (0%) | 3 (42.9%) | 0.440 |
| VDBP (mg/L) Median [Min–Max] | 289 [34.8–420] | 247 [210.5–515] | 0.029 | 181.5 [19.5–248] | 304 | 223.75 [80–237.5] | 0.262 |
| Calcium (mg/dL) Mean ± SD | 9.01 ± 0.56 | 8.86 ± 0.45 | 0.381 | 8.22 ± 1.28 | 7.9 | 8.56 ± 0.77 | 0.613 |
| Albumin (g/dL) Mean ± SD | 42.16 ± 5.26 | 39.25 ± 6.57 | 0.157 | 24.34 ± 7.29 | 34.3 | 27.78 ± 10.76 | 0.476 |
| iPTH 1 (pg) Median [Min–Max] | 33.39 [11.32–191] | 31.64 [14.72–47.93] | 0.696 | 80.21 [26.88–873.7] | 40.08 | 39.62 [12.83–179.6] | 0.312 |
| Phosphor (mg/dL) Median [Min–Max] | 3.59 [2.51–54.53] | 3.32 [2.40–4.83] | 0.118 | 4.08 [2.49–5.33] | 4.05 | 4.21 [2.18–6.80] | 0.915 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karcıoğlu Batur, L.; Dokur, M.; Koç, S.; Karabay, M.; Akcay, Z.N.; Gunger, E.; Hekim, N. Investigation of the Relationship between Vitamin D Deficiency and Vitamin D-Binding Protein Polymorphisms in Severe COVID-19 Patients. Diagnostics 2024, 14, 1941. https://doi.org/10.3390/diagnostics14171941
Karcıoğlu Batur L, Dokur M, Koç S, Karabay M, Akcay ZN, Gunger E, Hekim N. Investigation of the Relationship between Vitamin D Deficiency and Vitamin D-Binding Protein Polymorphisms in Severe COVID-19 Patients. Diagnostics. 2024; 14(17):1941. https://doi.org/10.3390/diagnostics14171941
Chicago/Turabian StyleKarcıoğlu Batur, Lutfiye, Mehmet Dokur, Suna Koç, Mehmet Karabay, Zeyneb Nur Akcay, Ezgi Gunger, and Nezih Hekim. 2024. "Investigation of the Relationship between Vitamin D Deficiency and Vitamin D-Binding Protein Polymorphisms in Severe COVID-19 Patients" Diagnostics 14, no. 17: 1941. https://doi.org/10.3390/diagnostics14171941






